牛妊娠相关糖蛋白6基因密码子优化及其在HEK293细胞中的表达
刘长彬 卢春霞 石国庆 倪建宏 卢守亮

摘要:【目的】解決天然牛妊娠相关糖蛋白(bPAG)不易分离纯化的问题,获得高效异源表达且具有良好免疫反应性的bPAG6,为国产bPAG检测产品的研发提供技术支持。【方法】根据宿主细胞对密码子的偏好性,利用MaxCodonTM在不改变氨基酸序列的前提下,对bPAG6基因密码子进行优化,采用全基因合成技术扩增优化后的bPAG6基因,构建proEM-bPAG6重组载体,并转染至HEK293细胞中进行高效表达;然后通过SDS-PAGE电泳、Western blotting和ELISA检测重组蛋白的表达效果及免疫反应性,并利用在线生物信息学分析软件对重组蛋白的结构及功能进行预测。【结果】优化后bPAG6基因的密码子适用指数(CAI)由0.80提高到0.96,G+C含量由49.4%提高到58.8%,其蛋白编码区(CDS)长度为1137 bp,共编码379个氨基酸残基。将全基因合成技术扩增获得的bPAG6基因插入proEM载体构建proEM-bPAG6重组载体,经EcoR I和Hind III双酶切鉴定可获得2条与预期结果相符的片段[proEM载体(4369 bp)和bPAG6基因(1176 bp)],测序结果也显示其碱基序列与优化后的bPAG6基因完全一致。proEM-bPAG6重组载体转染HEK293细胞表达获得的重组蛋白bPAG6分子量约46 kD。重组蛋白bPAG6可被抗bPAG6抗体识别,即具有良好的免疫反应性。重组蛋白bPAG6信号肽由N端的前15个氨基酸残基组成;存在5个N-糖基化位点,其N-糖基化修饰主要位于57Asn、73Asn、102Asn、124Asn和181Asn位点处;重组蛋白bPAG6为分泌性蛋白,无跨膜螺旋区,其二级结构由无规则卷曲(42.74%)、延伸链(31.93%)、α-螺旋(19.26%)和β-转角(6.07%)组成。【结论】通过基因优化及真核表达获得的重组蛋白bPAG6具有良好免疫反应性,可作为抗原应用于牛早期妊娠快速诊断产品的研发。
关键词: 牛妊娠相关糖蛋白6(bPAG6);早期妊娠诊断;密码子优化;真核表达
中图分类号: S823.41 文献标志码: A 文章编号:2095-1191(2020)11-2597-10
Codon optimization of bovine pregnancy associated glycoprotein 6 gene and its expression in human embryonic kidney
293(HEK293) cell
LIU Chang-bin1, LU Chun-xia2*, SHI Guo-qing1, NI Jian-hong1, LU Shou-liang1
(1State Key Laboratory of Sheep Genetic Improvement and Healthy Production/Xinjiang Academy of Agriculture and Reclamation Science, Shihezi, Xinjiang 832000, China; 2School of Advanced Agriculture and Bioengineering,
Yangtze Normal University, Chongqing 408100, China)
Abstract:【Objective】This study was to solve the problem that natural bovine pregnancy-associated glycoproteins (bPAG) was not easy to isolate and purify, and to obtain a recombinant bPAG6 with high heterogeneous expression and good immunoreactivity. The research provided the technical support for the research of domestic bPAG testing products. 【Method】In this study, the bPAG6 gene was optimized using MaxCodon software according to codon usage preference of host cells without changing the amino acid sequence. The optimized bPAG6 gene was synthesized by gene-synthesis techniques, and inserted into the expression plasmid proEM to construct fusion protein expression plasmids proEM-bPAG6. Subsequently, the proEM-bPAG6 recombinant vector was transfected into HEK293 cells. SDS-PAGE electrophoresis, Western blotting and ELISA were performed to evaluate the expression and immunoreactivity of the recombinant protein. The structure and function of recombinant protein was predicted and analyzed by bioinformatics software. 【Result】After codon optimization, the codon adaptation index (CAI) of the bPAG6 gene raised from 0.80 to 0.96 and its G+C content changed from 49.4% to 58.8%, protein coding region(CDS) was 1137 bp and encoding 379 amino acid residues. The bPAG6 gene was inserted into the proEM vector to construct proEM-bPAG6 recombinant vector, two fragments consistent with the expected results were obtained by EcoR I and Hind III double enzyme digestion [proEM vector(4369 bp) and bPAG6 gene(1176 bp)], sequencing results also showed that the base sequence was completely consistent with the optimized bPAG6 gene. The molecular weight of recombinant protein bPAG6 obtained from proEM-bPAG6 recombinant vector transfected HEK293 cells was about 46 kD. Recombinant protein bPAG6 could be recognized by anti bPAG6 antibodies. That is, it had good immunoreactivity. Recombinant protein bPAG6 signal peptide consisted of the first 15 amino acid residues at the N end; five N-glycosylation sites existed, N-glycosylation modification was mainly located at sites 57Asn, 73Asn, 102Asn, 124Asn and 181Asn. bPAG6 recombinant protein was secretory protein, with no transmembrane helix. The secondary structure consisted of random coil(42.74%), extended strand(31.93%),α-helix(19.26%) and β-turn(6.07%), respectively. 【Conclusion】In this paper, recombinant bPAG6 with good immunoreactivity are successfully obtained by gene optimization and prokaryotic expression systems, which can be used in dairy cattle early pregnancy diagnosis as antigen.
1. 2 试验方法
1. 2. 1 bPAG6基因优化与合成 根据NCBI已公布的bPAG6基因序列(GenBank登录号NM_176617.2),利用密码子优化软件MaxCodonTM对bPAG6基因进行优化。根据宿主细胞HEK293对密码子的偏好性,在不改变氨基酸序列的前提下,将bPAG6基因的低频密码子替换为宿主细胞高频率使用的密码子,提高密码子适用指数(CAI),降低序列中碱基重复结构;在优化后的bPAG6基因5'端和3'端分别设计EcoR I、Hind III酶切位点及His标签序列,采用Primer Premier 5.0设计18条引物(bPAG6-1~bPAG6-18),其中,首引物bPAG6-1为5'-AGTTTAAACGGATCTCTAG CGAATTCCCGCCGCCACCATGAAGTGGCTGG TGCTCCTGGGACTCTGGTGGCTTTCAGCGAG TGCATC-3'(下划线处为EcoR I酶切位点),尾引物bPAG6-18为5'-TTGCCGGCCTCGAGCGGCCGCTA GCAAGCTTATCAGTGGTGGTGATGATGGTGCA CTGCTCTGGCCAGTCCGATTCTGTC-3'(下划线处为Hind III酶切位点),然后进行第一轮PCR扩增,反应体系50.0 μL:引物bPAG6-1~bPAG6-18各0.5 μL,Pfu DNA聚合酶(5 U/μL)0.5 μL,5×PCR Buffer 0.5 μL,dNTP(10 mmol/L)1.0 μL,ddH2O补足至50.0 μL。扩增程序:95 ℃预变性3 min;95 ℃ 25 s,62 ℃ 20 s,72 ℃ 40 s,进行25个循环;72 ℃延伸1 min,4 ℃保存。第二轮PCR扩增体系只添加引物bPAG6-1和bPAG6-18,其余条件及扩增程序同第一轮PCR扩增。PCR产物经1.0%琼脂糖凝胶电泳检测后以Axygen DNA凝胶回收试剂盒回收目的片段。bPAG6基因优化及合成委托德泰生物科技(南京)有限公司完成。
1. 2. 2 proEM-bPAG6重组载体构建 proEM载体采用EcoR I和Hind III進行双酶切,酶切体系50.0 μL:5.0 μL proEM载体,2.5 μL EcoR I(10 U/μL),2.5 μL Hind III(10 U/μL),5.0 μL 10×FD Buffer,35.0 μL ddH2O;37 ℃酶切1 h。酶切产物经1.0%琼脂糖凝胶电泳检测后进行回收,并与目的基因片段在T4 DNA连接酶作用下(22 ℃)连接2 h,连接体系20.0 μL:4.0 μL目的基因片段,3.5 μL双酶切proEM载体,1.0 μL T4 DNA连接酶(5 U/μL),2.0 μL 10×T4 Buffer,9.5 μL ddH2O。
1. 2. 3 proEM-bPAG6重组载体转化与筛选 采用热激法将连接产物转化至DH5a感受态细胞中,摇床培养30 min。取20.0 μL菌液涂抹至含有氨苄青霉素(100 μg/mL)的LB固体培养基上,37 ℃培养过夜,再挑取单个菌落接种至LB液体培养基中,37 ℃下摇床(200 r/min)培养8 h,取2.0 μL菌液为模板进行PCR鉴定。以bPAG6-1和bPAG6-18为引物,PCR反应体系50.0 μL:2.0 μL菌液模板,0.5 μL引物bPAG6-1,0.5 μL引物bPAG6-18, 0.5 μL Pfu DNA聚合酶(5 U/μL),10.0 μL 5×PCR Buffer,1.0 μL dNTP(10 mmol/L),ddH2O补足至50.0 μL。扩增程序同1.2.1中的第一轮PCR扩增。PCR扩增产物经1.0%琼脂糖凝胶电泳检测后,将阳性转化子接种至新鲜的LB液体培养基中,37 ℃培养16 h,收集菌液,离心去上清液,提取质粒并进行双酶切鉴定,酶切体系及条件同1.2.2。同时将阳性转化子送至德泰生物科技(南京)有限公司测序,验证插入序列正确无误后采用质粒抽提试剂盒进行质粒大量抽提,-20 ℃保存备用。
1. 2. 4 proEM-bPAG6重组载体诱导表达 在37 ℃下复苏HEK293细胞,将其转移至10 mL Gibco? FreeStyleTM 293表达培养基中,800 r/min离心5 min弃上清液,重悬HEK293细胞并转移到培养瓶中,使其密度达3.0×105~4.0×105 Cells/mL,活力大于95%。在37 ℃、5% CO2条件下培养2 d,细胞密度达2.0×106 Cells/mL时进行传代。转染前1 d将HEK293细胞悬浮培养,使其接种密度为1.0×106 Cells/mL,proEM-bPAG6重组载体与转染试剂混匀后加入到HEK293细胞中,在37 ℃、5% CO2条件下培养5~6 d。
1. 2. 5 重组蛋白纯化 收集细胞培养物,12500 r/min离心10 min,收集上清液过0.22 μm滤膜,过滤后的上清液置于10 mmol/L PBS中透析过夜(4 ℃)。透析液采用Ni-IDA柱进行纯化:首先采用10 mmol/L PBS平衡Ni-IDA柱,流速为1 mL/min,取透析液以同样的流速上柱,然后采用10 mmol/L PBS冲洗Ni-IDA柱,再以含有不同浓度咪唑(50和500 mmol/L)的洗脱液进行梯度洗脱,流速为2 mL/min。分别收集上清液、流出液和洗脱液进行SDS-PAGE电泳,通过考马斯亮蓝染色检测重组蛋白的纯化效果,BCA蛋白定量试剂盒检测其浓度。
1. 2. 6 重组蛋白Western blotting鉴定 重组蛋白经SDS-PAGE电泳后,取下凝胶,采用半干式电转印法将其转印至PVDF膜上。取下PVDF膜,以5%脱脂奶粉室温封闭2 h,用1×PBST(含0.05% Tween-20的PBS)漂洗5次,每次5 min;加入按1∶2000稀释的小鼠抗6×His单克隆抗体,室温孵育1 h,漂洗后采用ECL化学发光底物试剂盒进行发光,并拍照。
1. 2. 7 重组蛋白免疫反应性鉴定 采用牛快速可视怀孕检测试剂盒对重组蛋白bPAG6进行检测,具体步骤按照产品说明进行操作。
1. 2. 8 重组蛋白生物信息学分析 通过SignalP 4.1 Server(http://www.cbs.dtu.dk/services/SignalP/)预测重组蛋白bPAG6的信号肽,TMHMM Server 2.0(http://www.cbs.dtu.dk/services/TMHMM/)预测其跨膜结构,NetNGlyc 1.0 Server(http://www.cbs.dtu.dk/services/#opennewwindow)预测其糖基化修饰位点;采用SOPMA(https://npsa-prabi.ibcp.fr/cgi-bin/npsa_ automat.pl?page=npsa_sopma.html)预测重组蛋白bPAG6二级结构。
2 结果与分析
2. 1 bPAG6基因优化与合成效果
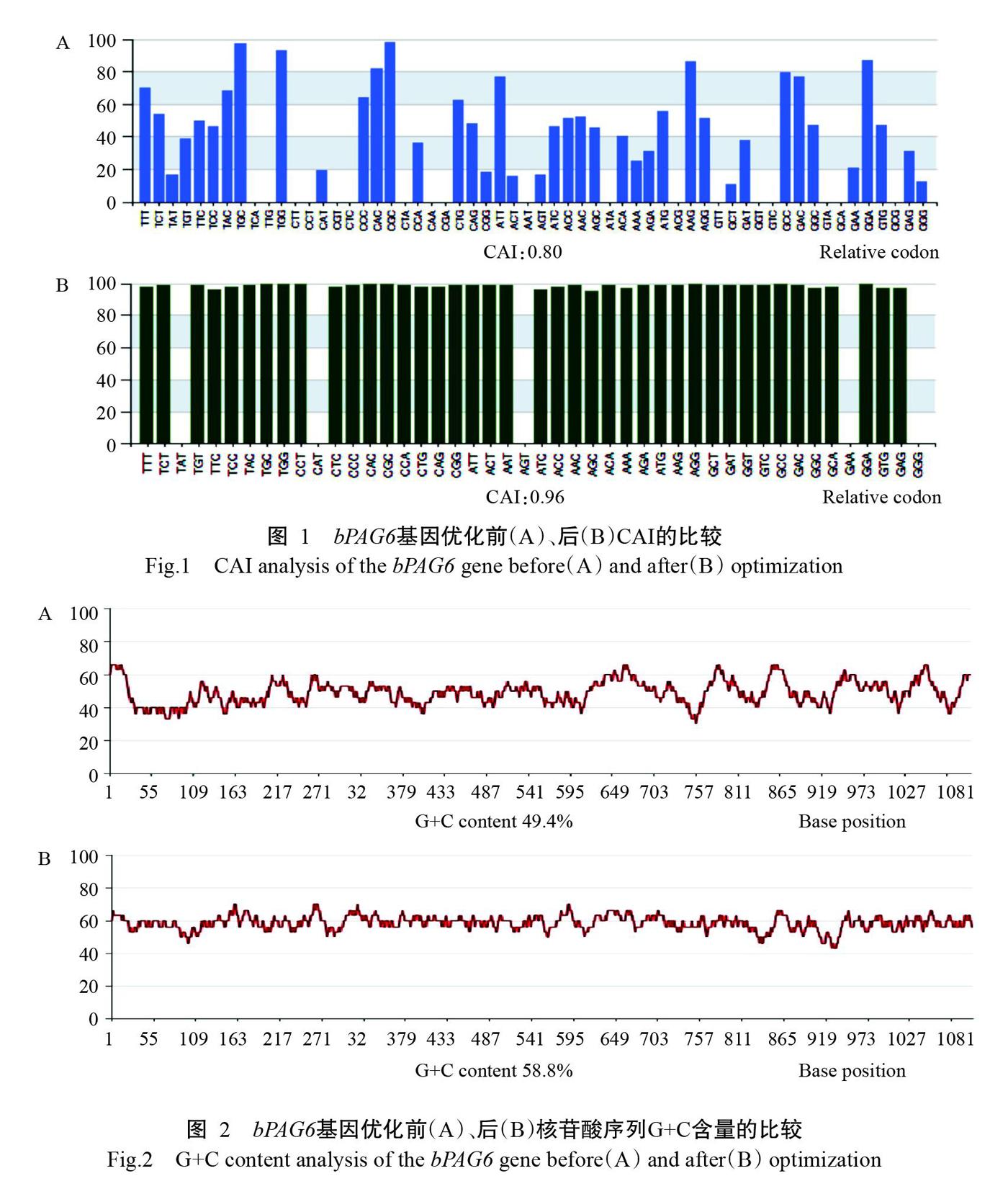
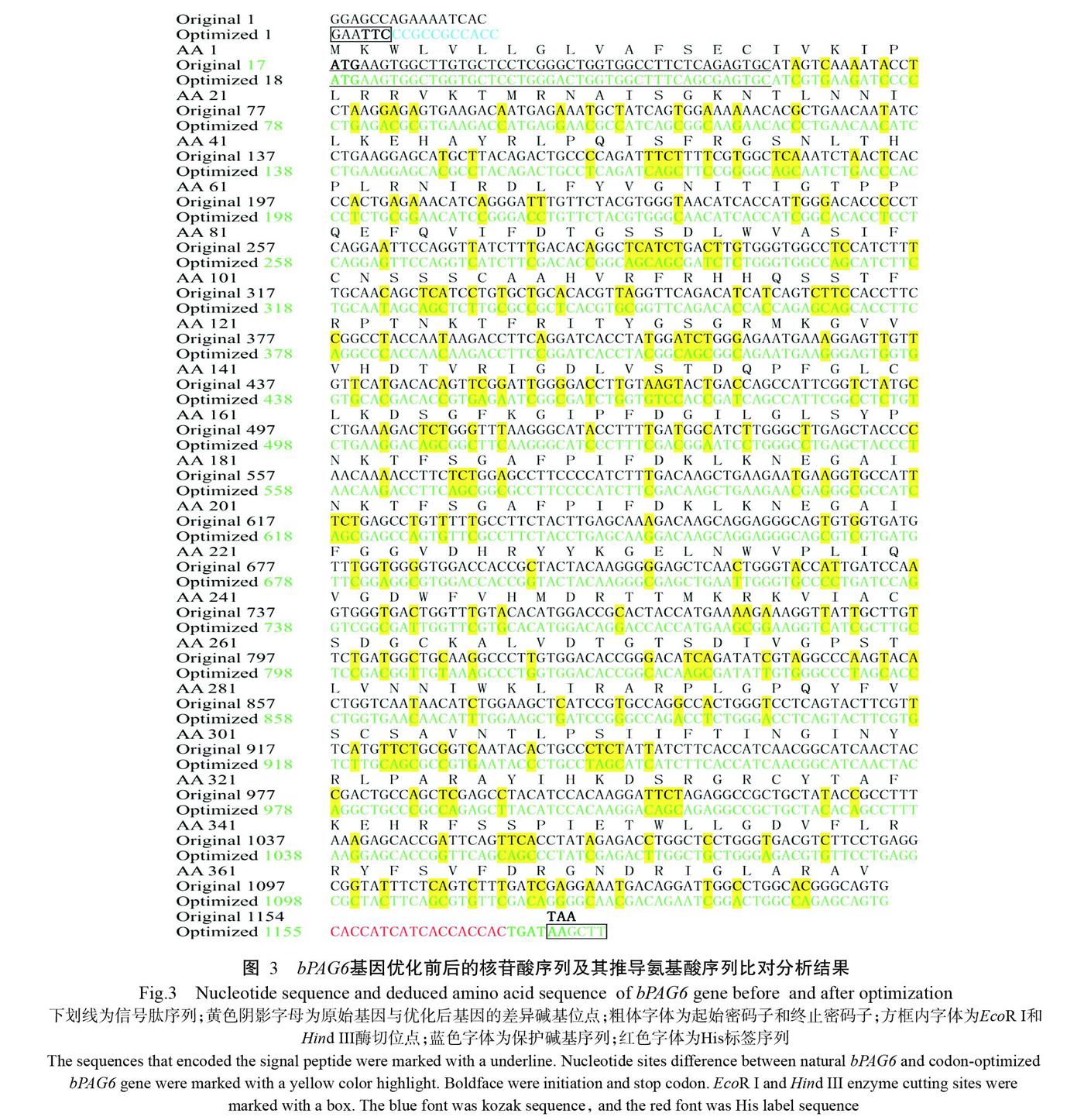
为提高bPAG6基因在HEK293细胞中的表达水平,将bPAG6基因CDS序列中的低频密碼子同义突变为宿主细胞基因组中高频率使用的密码子。由图1和图2可知,优化后bPAG6基因的CAI由0.80提高到0.96,G+C含量由49.4%提高到58.8%。优化前后bPAG6基因的核苷酸序列及其推导氨基酸序列如图3所示,优化后的bPAG6基因核苷酸序列全长1182 bp,其编码区(CDS)长度为1137 bp,包括信号肽45 bp、目标序列1092 bp,共编码379个氨基酸残基。优化后的bPAG6基因与原始基因的核苷酸序列相似性为78.0%,而推导氨基酸序列完全一致。采用全基因合成技术扩增优化的bPAG6基因,PCR扩增产物经1.0%琼脂糖凝胶电泳检测,结果(图4)显示目的条带单一清晰,无拖尾现象,且片段大小与预期结果(1182 bp)相符。
2. 2 proEM-bPAG6重组载体构建及阳性转化子筛选结果
将带有EcoR I和Hind III酶切位点的优化bPAG6基因插入proEM载体,构建获得proEM-bPAG6重组载体,转化DH5α感受态细胞,挑选单克隆进行PCR鉴定,鉴定结果如图5-A所示,泳道1~8均出现明亮、单一的目的条带,且片段大小与预期结果一致。proEM-bPAG6重组载体经EcoR I和Hind III双酶切鉴定,鉴定结果如图5-B所示,泳道1为proEM-bPAG6重组载体的环状、线性和超螺旋3种状态,泳道2为双酶切获得的proEM载体(4369 bp)和bPAG6基因(1176 bp),其片段大小与预期结果一致,说明已成功构建获得proEM-bPAG6重组载体。将阳性转化子的测序结果与优化的bPAG6基因进行核苷酸序列比对分析,结果显示二者的核苷酸序列完全一致,表明优化的bPAG6基因正确插入proEM载体。
2. 3 重组蛋白诱导表达及鉴定结果
收集诱导表达的HEK293细胞上清液,经Ni-IDA柱层析纯化后以SDS-PAGE电泳检测重组蛋白bPAG6的纯化效果,结果(图6-A)显示,以500 mmol/L咪唑的洗脱效果较优,约在46 kD处出现预期的目的蛋白条带。由于重组蛋白bPAG6的C端包含1个His标签,因此利用小鼠抗6×His单克隆抗体对其进行Western blotting鉴定,结果(图6-B)显示,约在46 kD处出现特异性杂交条带,与SDS-PAGE电泳分析结果一致,进一步验证重组蛋白bPAG6在HEK293细胞中成功高效表达。
2. 4 重组蛋白免疫反应性鉴定结果
为了验证重组蛋白bPAG6是否具有免疫反应性,采用牛快速可视怀孕检测试剂盒进行检测,结果如图7所示。重组蛋白bPAG6的反应液呈蓝色,说明其可被抗bPAG6抗体识别,即重组蛋白bPAG6具有良好的免疫反应性。
2. 5 重组蛋白生物信息学分析结果
重组蛋白bPAG6信号肽由N端的前15个氨基酸残基组成(图8-A);存在5个N-糖基化位点,其N-糖基化修饰主要位于57Asn、73Asn、102Asn、124Asn和181Asn位点处(图8-B);重组蛋白bPAG6为分泌性蛋白,无跨膜螺旋区(图8-C)。SOPMA预测结果(图9)显示,重组蛋白bPAG6二级结构主要由无规则卷曲(Random coil,占42.74%)、延伸链(Extended strand,占31.93%)、α-螺旋(α-helix,占19.26%)和β-转角(β-turn,占6.07%)组成。
3 讨论
基于免疫分析的bPAG检测已成为奶牛养殖生产中应用最广泛的早期妊娠诊断技术(Ricci et al.,2015;Commun et al.,2016;薄小辉,2017)。Ricci等(2015)分别收集人工受精后25和32 d的奶牛血液和牛奶样品,采用ELISA测定bPAG含量,并以超声波检测进行确认,结果显示血液和牛奶样品的准确率分别为92%和89%;Commun等(2016)采用相同的方法测定人工受精后奶牛血液和牛奶中bPAG含量,也获得较高的准确率,且2种样品的检测结果无明显差异。目前,已成功开发出基于ELISA的商品化PAG检测试剂盒,如美国IDEXX公司开发的牛快速可视怀孕检测试剂盒,可对人工受精后28d的奶牛进行早期妊娠快速诊断,且具有较高的准确率(Kaya et al.,2016;Dufour et al.,2017)。市场上销售的PAG检测试剂盒产品虽然具有较高的准确率及特异性,但其价格较高,每头奶牛的检测成本需30.00~40.00元。因此,亟需研发出简便、经济的bPAG检测产品,为我国规模化牛场的早期妊娠批量诊断提供技术支持,而获取高纯度的bPAG蛋白是研发bPAG检测产品的关键。由于bPAG种类繁多,传统的分离纯化方法很难获得高纯度、高产量的bPAG蛋白,限制了其功能研究及推广应用。
体外重组蛋白技术的不断发展与成熟,为PAG的功能研究及应用提供了新思路,但外源蛋白的表达水平受多种因素影响,包括启动子、信号肽(Kim et al.,1997)、宿主细胞(Patel et al.,2004)、密码子使用偏好性(Zhou et al.,2016)及转染条件(邓晓芬等,2019)等,其中密码子的偏好使用程度与其表达水平呈正相关。已有研究表明,对基因的同义密码子进行优化使其与表达宿主的密码子偏好性相匹配,可显著提高外源基因的表达水平(Mansouri et al.,2013;Kianmehr et al.,2016)。其中,CAI常被用于评估外源基因在宿主细胞内的表达水平。一般情况下,CAI≥0.80被认为是预测重组蛋白高效表达的基本标准。本研究利用MaxCodonTM在不改变氨基酸序列的前提下,通过消除稀有密码子及平衡G+C含量等策略对bPAG6基因进行优化,优化后bPAG6基因的CAI大幅提高,G+C含量调整至59.0%左右,同时减少A+T和G+C富集区。然后通过全基因合成技术扩增优化后的bPAG6基因,将其插入proEM载体构建proEM-bPAG6重组载体,并转染HEK293细胞,成功实现了bPAG6基因在HEK293细胞中的高效表达,进一步佐证基于全基因合成技术的密码子优化具有良好的时效性和实用性。
本研究结果表明,优化后的bPAG6基因CDS区为1137 bp,共编码379个氨基酸残基,其信号肽区域位于第1~15位氨基酸残基处,蛋白质理论分子量为41.82 kD,理论等电点(pI)为9.76。通过HEK293细胞表达获得的重组蛋白bPAG6表观分子量约46 kD,与理论分子量存在一定差异,与Patel等(2004)、刘长彬等(2019a)的研究结果相似,究其原因可能与PAG在表达过程中的糖基化修饰有关(Xie et al.,1997;Garbayo et al.,2000;Klisch et al.,2005)。已有研究表明,各种偶蹄动物的PAG氨基酸序列存在不同数目的N-糖基化位点。其中,山羊PAG氨基酸序列(CaPAG)存在2~5个N-糖基化位点(Garbayo et al.,2000),绵羊PAG氨基酸序列(OvPAG)存在2~7个N-糖基化位点(Xie et al.,1997;Garbayo et al.,2008),牛BoPAG氨基酸序列存在1~6个N-糖基化位点(Xie et al.,1997;Klisch et al.,2005;Garbayo et al.,2008)。本研究的NetNGlyc 1.0 Server预测结果显示,重组蛋白bPAG6存在5个N-糖基化位点,可能是导致其蛋白在表达分泌时分子量增大的主要原因。
4 结论
通过基因优化及真核表达获得的重组蛋白bPAG6具有良好免疫反应性,可作为抗原应用于牛早期妊娠快速诊断产品的研发。
参考文献:
薄小辉. 2017. 妊娠相关糖蛋白在奶牛早期妊娠诊断上的应用研究[D]. 北京:中国农业科学院. [Bo X H. 2017. The application of pregnancy-associated glycoproteins in early pregnancy diagnosis of dairy cows[D]. Beijing:Chinese Academy of Agricultural Sciences.]
鄧晓芬,杨晓佳,易天红,冯英,柯潇,赖维莉. 2019. 融合蛋白基因与抗体基因电转染CHO-S细胞的条件摸索优化[J]. 生物技术通报,35(4):223-228. [Deng X F,Yang X J,Yi T H,Feng Y,Ke X,Lai W L. 2019. Optimization of electrotransfection conditions of genes for fusion protein and antibody to CHO-S cells[J]. Biotechnology Bulletin,35(4):223-228.]
黄浩,王阳,堵国成,康振. 2019. 重组蛋白微生物表达系统的研究进展[J]. 生物产业技术,(3):36-43. [Huang H,Wang Y,Du G C,Kang Z. 2019. Research progress in microbial expression systems for recombinant protein production[J]. Biotechnology & Business,(3):36-43.]
金海,赵拴平,徐磊,贾玉立,贾玉堂. 2018. 肉牛妊娠初期血、尿中孕酮、妊娠相关糖蛋白、早孕因子的变化[J]. 中国牛业科学,44(6):8-11. [Jin H,Zhao S P,Xu L,Jia Y L,Jia Y T. 2018. Changes of progesterone,pregnancy-associated glycoprotein and early pregnancy factors in blood and urine of beef cattle during early pregnancy[J]. China Cattle Science,44(6):8-11.]
李艳艳,胡建宏,雷静,曲立立,赵健玲. 2013. 牛妊娠相关糖蛋白ELISA对早期妊娠诊断效果的影响[J]. 家畜生态学报,34(3):54-57. [Li Y Y,Hu J H,Lei J,Qu L L,Zhao J L. 2013. Effect of bovine pregnancy-associated glycoprotein ELISA on early pregnancy diagnosis[J]. Journal of Domestic Animal Ecology,34(3):54-57.]
劉长彬,卢春霞,杨华,张云生,石国庆. 2019a. 牛妊娠相关糖蛋白1(bPAG1)的真核表达及纯化[J]. 西南农业学报,32(8):1944-1949. [Liu C B,Lu C X,Yang H,Zhang Y S,Shi G Q. 2019a. Eukaryotic expression and purification of recombinant bovine pregnancy associated glycoprotein-1[J]. Southwest China Journal of Agricultural Sciences,32(8):1944-1949.]
刘长彬,石国庆,卢春霞. 2019b. 牛妊娠相关糖蛋白9(bPAG9)的真核表达及纯化[J]. 新疆农业科学,56(8):1552-1559. [Liu C B,Shi G Q,Lu C X. 2019b. Eukaryo-tic expression and purification of bovine pregnancy associated glycoprotein-9(bPAG9)[J]. Xinjiang Agricultural Sciences,56(8):1552-1559.]
卢春霞,刘长彬,石国庆,杨华. 2020. 基于密码子优化策略的牛妊娠相关糖蛋白9(bPAG9)的原核表达及纯化[J]. 江苏农业学报,36(1):122-129. [Lu C X,Liu C B,Shi G Q,Yang H. 2020. Prokaryotic expression and purification of recombinant bovine pregnancy associated glycoprotein-9(bPAG9) based on codon optimization[J]. Jiangsu Journal of Agricultural Sciences,36(1):122-129.]
祁文婧,张帅,赵鑫,张红星,谢远红,金君华. 2019. 牛妊娠相关糖蛋白基因boPAG4的原核表达及单克隆抗体的制备[J]. 河北农业大学学报,42(6):103-108. [Qi W J,Zhang S,Zhao X,Zhang H X,Xie Y H,Jin J H. 2019. Prokaryo-tic expression of bovine pregnancy-associated glycoprotein gene boPAG4 and preparation of monoclonal antibo-dies[J]. Journal of Hebei Agricultural University,42(6):103-108.]
史秀芬,王苏瑶,张雅飞,刘月琴,张英杰. 2017. 绵羊妊娠相关糖蛋白PAG3的制备[J]. 中国兽医学报,37(7):1408-1413. [Shi X F,Wang S Y,Zhang Y F,Liu Y Q,Zhang Y J. 2017. Preparation of sheep pregnancy-associated glycoprotein[J]. Chinese Journal of Veterinary Science,37(7):1408-1413.]
钟旭,薛立群,田见晖. 2011. 妊娠相关糖蛋白(PAG)在奶牛早期妊娠诊断上的应用现状[J]. 中国畜牧兽医,38(11):140-143. [Zhong X,Xue L Q,Tian J H. 2011. Early pregnancy diagnosis by pregnancy associated glycoprotein in dairy cattle[J]. China Animal Husbandry & Veteri-nary Medicine,38(11):140-143.]
Bella A,Sousa N M,Dehimi M L,Watts J,Beckers J F. 2007. Western analyses of pregnancy-associated glycoprotein family(PAG) in placental extracts of various mammals[J]. Theriogenology,68(7):1055-1066.
Chaves C M E S,da Costa D R L,Duarte K M R,Machado D C,de Paz C C P,Beltrame R T. 2017. Visual ELISA for detection of pregnancy-associated glycoproteins (PAGs) in ewe serum[J]. Theriogenology,97:78-82.
Commun L,Velek K,Barbry J B,Pun S,Rice A,Mestek A,Eqli C,Leterme S. 2016. Detection of pregnancy-associa-ted glycoproteins in milk and blood as a test for early pregnancy in dairy cows[J]. Journal of Veterinary Diagnostic Investigation,28(3):207-213.
Dufour S,Durocher J,Dubuc J,Dendukuri N,Hassan S,Buc-zinski S. 2017. Bayesian estimation of sensitivity and specificity of a milk pregnancy-associated glycoprotein-based ELISA and of transrectal ultrasonographic exam for diagnosis of pregnancy at 28-45 days following breeding in dairy cows[J]. Preventive Veterinary Medicine,140:122-133.
El-Amiri B,Remy B,de Sousa N M,Beckers J F. 2004. Isolation and characterization of eight pregnancy-associated glycoproteins present at high levels in the ovine placenta between day 60 and day 100 of gestation[J]. Reproduction Nutrition Development,44(3):169-181.
El-Battawy K A,Sousa N M,Szenci O,Beckers J F. 2009. Pregnancy-associated glycoprotein profile during the first trimester of pregnancy in Egyptian buffalo cows[J]. Reproduction in Domestic Animals,44(2):161-166.
Friedrich M,Holtz W. 2010. Establishment of an ELISA for measuring bovine pregnancy-associated glycoprotein in serum or milk and its application for early pregnancy detection[J]. Reproduction in Domestic Animals,45(1):142-146.
Garbayo J M,Green J A,Manikkam M,Beckers J F,Kiesling D O,Ealy A D,Roberts R M. 2000. Caprine pregnancyassociated glycoproteins(PAG):Their cloning,expression,and evolutionary relationship to other PAG[J]. Molecular Reproduction & Development,57(4):311-322.
Garbayo J M,Serrano B,Lopez-Gatius F. 2008. Identification of novel pregnancy associated glycoproteins(PAG) expressed by the peri-implantation conceptus of domestic ruminants[J]. Animal Reproduction Science,103(1-2):120-134.
Green J A,Parks T E,Avalle M P,Telugu B P,McLain A L,Peterson A J,McMillan W,Mathialagan N,Hook R R,Xie S C,Roberts R M. 2005. The establishment of an ELISA for the detection of pregnancy-associated glycoproteins (PAGs) in the serum of pregnant cows and heifers[J]. Theriogenology,63(5):1481-1503.
Green J A,Xie S,Quan X,Bao B,Gan X,Mathialagan N,Beckers J F,Roberts R M. 2000. Pregnancy-associated bovine and ovine glycoproteins exhibit spatially and temporally distinct expression patterns during pregnancy[J]. Biology of Reproduction,62(6):1624-1631.
Karen A,de Sousa N M,Beckers J F,Bajcsy ? C,Tibold J,Mádl I,Szenci O. 2015. Comparison of a commercial bovine pregnancy-associated glycoprotein ELISA test and a pregnancy-associated glycoprotein radiomimmunoassay test for early pregnancy diagnosis in dairy cattle[J]. Animal Reproduction Science,159:31-37.
Kaya M S,K?se M,Bozkaya F,Mutlu H,U?ar E H,Atli M O. 2016. Early pregnancy diagnosis using a commercial ELISA test based on pregnancy-associated glycoproteins in Holstein-Friesian heifers and lactating cows[J]. Tur-kish Journal of Veterinary & Animal Sciences,40:694-699.
Kianmehr A,Golavar R,Rouintan M,Mahrooz A,Fard-Esfa-hani P,Oladnabi M,Khajeniazi S,Mostafavi S S,Omidi-nia E. 2016. Cloning and expression of codon-optimized recombinant darbepoetin alfa in Leishmania tarentolae T7-TR[J]. Protein Expression and Purification,118:120-125.
Kim C H,Oh Y,Lee T H. 1997. Codon optimization for high-level expression of human erythropoietin(EPO) in mammalian cells[J]. Gene,199(1-2):293-301.
Klisch K,de Sousa N M,Beckers J F,Leiser R,Pich A. 2005. Pregnancy associated glycoprotein-1,-6,-7,and -17 are major products of bovine binucleate trophoblast giant cells at midpregnancy[J]. Molecular Reproduction and Deve-lopment,71(4):453-460.
Mansouri M,Mousavy S J,Ehsaei Z,Nazarian S,Zali M R,Moazzeni S M. 2013. The codon-optimization of cfaE gene and evaluating its high expression capacity and conserved immunogenicity in Escherichia coli[J]. Biologicals,41(3):169-175.
Nagappan M,Michael M,Robert S. 2009. Methods for early detection of pregnancy in cows:PAT-US7604950[P]. U.S. Patent.
Patel O V,Takahashi T,Imai K,Hashizume K. 2004. Generation and purification of recombinant bovine pregnancy associated glycoprotein[J]. Veterinary Journal,168(3):328-335.
Ricci A,Carvalho P D,Amundson M C,Fourdraine R H,Vincenti L,Fricke P M. 2015. Factors associated with pregnancy-associated glycoprotein (PAG) levels in plasma and milk of Holstein cows during early pregnancy and their effect on the accuracy of pregnancy diagnosis[J]. Journal of Dairy Science,98(4):2502-2514.
Wooding F B P,Roberts R M,Green J A. 2005. Light and electron microscope immunocytochemical studies of the distribution of pregnancy associated glycoproteins(PAGs) throughout pregnancy in the cow:Possible functional implications[J]. Placenta,26(10):807-827.
Xie S,Green J,Bao B,Beckers J F,Valdez K E,Hakami L,Roberts R M. 1997. Multiple pregnancy-associated glycoproteins are secreted by day 100 ovine placental tissue[J]. Biology of Reproduction,57(6):1384-1393.
Zhou Z P,Dang Y K,Zhou M,Li L,Yu C H,Fu J J,Chen S,Liu Y. 2016. Codon usage is an important determinant of gene expression levels largely through its effects on transcription[J]. Proceedings of the National Academy of Sciences of the United States of America,113(41):E6117-E6125.
Zoli A P,Guilbault L A,Delahaut P,Ortiz W B,Beckers J F. 1992. Radioimmunoassay of a bovine pregnancy-associa-ted glycoprotein in serum:Its application for pregnancy diagnosis[J]. Biology of Reproduction,46(1):83-92.
(責任编辑 兰宗宝)
刘长彬(1977-),博士,研究员,主要从事动物繁育新技术研究工作。先后主持国家自然科学基金项目、国家引智示范推广项目、国家科技支撑计划项目、兵团创新人才培养示范基地计划项目、兵团中青年科技创新领军人才项目、院士基金项目等科研项目12项;作为课题核心成员参与国家级或省部级科研项目10余项。在《Analytical and Bioanalytical Chemistry》《Microchimica Acta》《南方农业学报》《西南农业学报》《西北农业学报》《江苏农业学报》等期刊上发表学术论文35篇;以副主编或参编出版专著2部。获得授权发明和实用新型专利16项;获软件著作权6项;获兵团科技进步奖一等奖2项,二等奖2项;2020年获兵团中青年科技创新领军人才项目支持,并获优秀科技特派员荣誉称号。
收稿日期:2020-03-30
基金项目:国家自然科学基金项目(31860647)
作者简介:*为通讯作者,卢春霞(1978-),博士,副教授,主要从事食品质量安全研究工作,E-mail:shzlcx2002@163.com。刘长彬(1977-),博士,研究员,主要从事动物繁育新技术研究工作,E-mail:xlchangbin@163.com

