Analysis of Preoperative Factors lnfluencingHypoglossal-facial ‘Side’-to-side Neurorrhaphy for Facial Paralysis after Excision of Acoustic Neuroma*
SU Di Ya, WAN Hong, LI De Zhi, QIAO Hui, SCHUMACHER Michael, and LIU Song,,,#
1. Beijing Neurosurgical Institute and Beijing Key Laboratory of Central Nervous System Injury, Capital Medical University, Beijing 100070, China; 2. Dalian University Affiliated Xinhua Hospital, Dalian 116000, Liaoning,China; 3. Department of Neurosurgery and China National Clinical Research Center for Neurological Diseases,Beijing Tiantan Hospital, Capital Medical University, Beijing 100070, China; 4. U 1195, INSERM and University Paris-Sud and University Paris Saclay, 94276 Le Kremlin-Bicêtre, France
Abstract
Objective Hypoglossal nerve-facial nerve ‘side’-to-side neurorrhaphy is a new method for the treatment of potential incomplete facial paralysis after acoustic neuroma. However, there are differences in postoperative outcomes among patients. This study analysed preoperative factors that may influence the treatment outcomes of neurorrhaphy.
Methods We performed a retrospective study of 53 patients who were treated by neurorrhaphy for facial paralysis after acoustic neuroma resection. After a one-year follow-up period, the patients were divided into two groups according to facial functional outcome: better recovery or ordinary recovery.We analysed the following factors: gender, age, tumour size, and characteristics, tumour adhesion to the facial nerve, the duration of facial paralysis (DFP) and F wave appearance prior to neurorrhaphy (F wave).
Results Univariate analysis showed significant differences between the two groups in DFP (P = 0.0002),tumour adhesion to the facial nerve (P = 0.0079) and F waves (P = 0.0048). Logistic regression analysis of these factors also showed statistical significance with P values of 0.042 for the DFP, 0.043 for F waves,and 0.031 for tumour adhesion to the facial nerve.
Conclusions Tumour adhesion to the facial nerve, F waves appearance and DFP prior to neurorrhaphy are the predominant factors that influence treatment outcomes.
Key words: Facial nerve injury; Nerve regeneration; Preoperative factors analysis
INTRODUCTION
Facial nerve injury is one of the main complications following the surgical removal of acoustic neuroma at the cerebellopontine angle area[1]. When treating facial nerve injury, end-to-end neurorrhaphy between the facial nerve stumps is the most effective method with regard for the recovery of facial natural function and the axonal regrowth pathway. However, this approach is impossible in most cases because it is often not feasible to regain the proximal stump of the injured facial nerve. Thus, other facial dynamic reanimation methods that use the nerve transferring technique have been employed with the aim of offering alternative sources of axons to innervate the denervated facial muscles; these include using the hypoglossal nerve, spinal accessory nerve, and masseteric nerve[2,3]. Among the nerves used, the hypoglossal nerve, as a nerve donor, has its own advantages for such purposes. Because it provides a sufficient axonal source and more powerful facial reanimation even if the hypoglossal nerve is only hemisectioned for neurorrhaphy, the existing shared innervation of the facial and hypoglossal nuclei within the brainstem allows intense facial exercises and the rebypromotes central plasticity and facilitates the development of new functions for hypoglossal motoneurons to improve facial function[4]and prevent synkinesis of the doubly innervated facial muscles[5-7].In neurosurgery, the development of microsurgical techniques allows us to anatomically conserve the affected facial nerve in a large number of clinical cases, although to different extents, and provide the possibility of the patient’s facial nerve recovering on its own. The definition of this condition is potential incomplete facial paralysis, the incidence of which is relatively higher in patients with facial nerve injury[8,9].For treating these patients, we improved the classic method of hypoglossal-facial end-to-end neurorrhaphy with ‘side’-to-side neurorrhaphy with the aim of preserving the facial nerve without compromising the remnant axons and allowing the possibility of spontaneous recovery[10]. A main outcome is the double innervation of paralyzed facial mimic muscles by both hypoglossal and facial motoneurons. However, in clinical practice, we have observed variability among patients treated with facial functional recovery after ‘side’-to-side neurorrhaphy. Because all neurorrhaphy procedures were performed by the same neurosurgeon and with the same and standardized method, we suspect that preoperative factors would have major influence on treatment outcomes.
In this study, we retrospectively assessed 53 patients who were treated with hypoglossal nervefacial ‘side’-to-side neurorrhaphy for facial paralysis resulting from acoustic neuroma resection but with anatomical preservation of the injured facial nerve in our neurosurgical department. Possible preoperative influencing factors were collected and analysed. We used univariate and logistic analyses to analyse the impacts of these factors on postoperative functional outcomes and to obtain useful information for clinical practice.
SUBJECTS AND METHODS
This was a retrospective study performed with 53 patients who experienced facial paralysis due to the removal of acoustic tumours and were surgically treated by hypoglossal-facial nerve ‘side’-to-side neurorrhaphy in our Department of Neurosurgery between June 2011 and September 2016. The inclusion criteria were as follows: (1) the patient developed facial paralysis after acoustic neuroma resection and had normal nerve function before the operation, (2) the patient’s age ranged from 16 to 70 years old, (3) at least one side of the sural nerve function was normal, (4) no contraindications for general anaesthesia, and (5) voluntary treatment via hypoglossal-facial ‘side’ -to-side neurorrhaphy.Patients who did not meet the above criteria were excluded from the study. Before the removal of the acoustic tumour, no facial function deficit was observed in any of these patients. After surgery, the patients developed serious facial paralysis with a House-Brackmann (H-B) scale grade V or VI, even though their facial nerve was anatomically preserved during tumour removal. The patients were followedup for one year after the neurorrhaphy. This study was approved by the local Ethics Committee of Beijing Tiantan Hospital, Capital Medical University,China (KY2017-006-02).
Neurorrhaphy Treatment
The principal indication for hypoglossal-facial nerve ‘side’-to-side neurorrhaphy was significant incomplete facial paralysis due to facial nerve injury.The preservation of facial nerve anatomical structure after injury could allow some remnant facial axons to be conserved and/or lead to spontaneous regeneration. The standard hypoglossal-facial nerve‘side’-to-side neurorrhaphy was performed using a predegenerated nerve autograft (PSNG) in each patient by the same neurosurgeon[11]. The use of a PSNG was based on the aim of improving axonal regeneration given the proliferation of its Schwann cells due to axotomies. This concept has been demonstrated in previous studies by other investigators as well as our own study[11]. Briefly, the surgical operation was performed under general anaesthesia. The ipsilateral hypoglossal nerve was exposed and identified using an electrostimulator while recording the active potential in the tongue muscle at the ipsilateral side through two inserted electrodes[12]. One half of the hypoglossal nerve was cross-sectioned at a site closely distal to the descending branch. The proximal extremity of the PSNG that was predegenerated one week prior to neurorrhaphy and removed from the ipsilateral sural nerve was surgically bridged end-to-‘side’ to the hypoglossal nerve at the partial cross-section site.The injured facial nerve was exposed from its main trunk to the bifurcation area as well as its two main branches within the parotid gland. An epineurium window was created using microsurgical scissors on the exposed facial nerve at each of the two main branches closely caudal to the bifurcation while carefully preserving the tissue structure. The distal extremity of the PSNG was divided into two ends that were then surgically bridged to the facial nerve,end-to-side, at each of the epineurium windows.Figure 1 illustrates the hypoglossal-facial nerve‘side’-to-side neurorrhaphy. All patients underwent intense rehabilitation exercise after neurorrhaphy.
Clinical Data
The clinical data of the 53 patients are shown above. Tumour-related clinical data were obtained from detailed surgical records of acoustic neuroma resection. Patients were included in the study only when their surgical record regarding the acoustic neuroma resection was complete and detailed.Among the included patients, 29 were female and 23 were male. The patients’ ages ranged from 19 to 68 years old with a mean ± standard deviation (SD) of 42.47 ± 13.18 years old. In the clinic, we followed the traditional route of repair and generally recommended patients undergo 3−6 months of observation after facial paralysis. The period from the onset of facial paralysis to the neurorrhaphy ranged from 1 to 84 months with a mean ± SD of 12.86 ± 16.26 months (only one patient insisted on undergoing the surgery 1 month after facial paralysis). To obtain accurate results, we did not exclude these data from calculations of significant differences. We further divided the patients into 3 subgroups according to the duration of facial paralysis to analyse the time pointsat which neurorrhaphy was performed. The patients were divided into short (≤ 6 months), medium (7−12 months) or long (≥ 13 months) period groups.According to the records, a solid tumour was found in 34 patients, and tight adhesion between the tumour and facial nerve was observed in 35 patients.The greatest tumour diameters ranged from 1.5 to 6.0 cm with a mean ± SD of 4.00 ± 1.10 cm. Before neurorrhaphy, electrophysiological examination was performed using electromyography (Nicolet EDX,VIASYS Health Care Inc., Madison, Wisconsin, USA)to detect F wave appearances in all patients. F waves are one of the late responses produced by the antidromic activation of motoneurons via supramaximal stimulation of the nerve trunk and indicates nerve conduction from the motoneuron cell body to the motor endplate, which has a persistence of typically 80%–100% (or at least above 50%) in intact muscles[13]. We found 19 patients with positive and 34 with negative responses for F waves.The H-B facial nerve scale was used as the main indicator to assess facial function Supplementary Table S1 (available in www.besjournal.com).
Statistical Analysis
Age, the duration of facial paralysis and the greatest tumour diameter are expressed as the mean ± SD. GraphPad Prism 6.02 software was used for single-factor statistical analysis and one-way ANOVA. Continuous variables such as age, the duration of facial paralysis and greatest tumour diameter were analysed byunpaired T test. Other factors, including, F wave appearance, tumour adhesion to the facial nerve and tumour characteristics, were binary variables and were analysed by the chi-squared test. SPSS 19.0 software was used for logistic statistical analysis. P values ≤0.05 were considered statistically significant. For the logistic statistical analysis, separate odds ratios (ORs)and 95% CIs were also calculated. In addition, the patients were also divided into three subgroups according to the duration of facial paralysis for neurorrhaphy timing analysis. The three subgroups were analysed by one-way ANOVA followed by Tukey’s posthoc test for between-group comparisons.
RESULTS
Analysis of Preoperative Clinical Data Using Univariate Analysis
In this study, 36/53 patients showed better recovery of facial function, and 17/53 patients showed ordinary recovery. Clinical data were analysed using univariate analysis to determine which preoperative factors influenced treatment prognosis after neurorrhaphy.
The age distributions were 41.69 ± 2.19 years old in the better recovery group and 44.35 ± 3.37 years old inthe ordinary recovery group. There wasno significant difference between the two groups (P =0.50). There was also no significant difference between the two groups (P = 0.57) in the gender distribution. The duration of facial paralysis before neurorrhaphy significantly differed between the better recovery group (9.29 ± 0.81 months) and the ordinary recovery group (24.24 ± 6.01 months; P =0.0002). In the better recovery group, 25 patients had solid tumours, and 11 patients had cystic tumours. In the ordinary recovery group, 9 patients had solid tumours, and 8 patients had cystic tumours. No significant difference was established between the two groups intumour characteristics (P= 0.24). However, tumour adhesion to the facial nerve was observed in 19/36 patients in the better recovery group and in 16/17 patients in the ordinary group (P = 0.0079). A significant difference was also found between the two groups in F wave recordings(P = 0.0048). Before neurorrhaphy, 18/36 patients had positive F waves in the better recovery group. In contrast, only 1/17 patients had a positive F wave response in the ordinary recovery group.
Analysis of Preoperative Clinical Data Using Logistic Regression Analysis
Univariate analysis of the duration of facial paralysis, F wave appearance and tumour adhesion to the facial nerve revealed significant differences between the better recovery and ordinary recovery groups. These factors were further analysed by logistic regression analysis, as shown in Table 1. The P values of the logistic regression analysis were 0.042 for the duration of facial paralysis (OR = 1.129,95% CI = 1.004–1.270), 0.043 for F wave appearance(OR = 0.095, 95% CI = 0.010–0.924), and 0.031 for tumour adhesion to the facial nerve (OR = 0.063,95% CI = 0.005–0.779), indicating that they influenced neurorrhaphy treatment prognoses.
The Timing of Performing Neurorrhaphy
Patients were divided into subgroups based on facial paralysis duration: 26/53 in the short-period subgroup, with facial paralysis durations of 4.54 ±1.24 months ( ≤ 6 months); 12/53 in the mediumperiod subgroup with facial paralysis durations of 9.92 ± 1.98 months (7–12 months); and 15/53 in the long-period subgroup with facial paralysis durations of 29.67 ± 22.76 months ( ≥ 13 months). Statistical analysis performed using one-way ANOVA followed by Tukey’s post hoc test was performed according to the postoperative functional recovery of H-B grade and showed that there were significant differences among the three subgroups (P ≤ 0.0001). Upon analysis of the sets of two subgroups (Figure 2), a significant difference was established between the short- and long-period subgroups (P < 0.0001),although there was no difference between the shortand medium-period subgroups (P = 0.053) or between the medium- and long-period subgroups(P = 0.111).
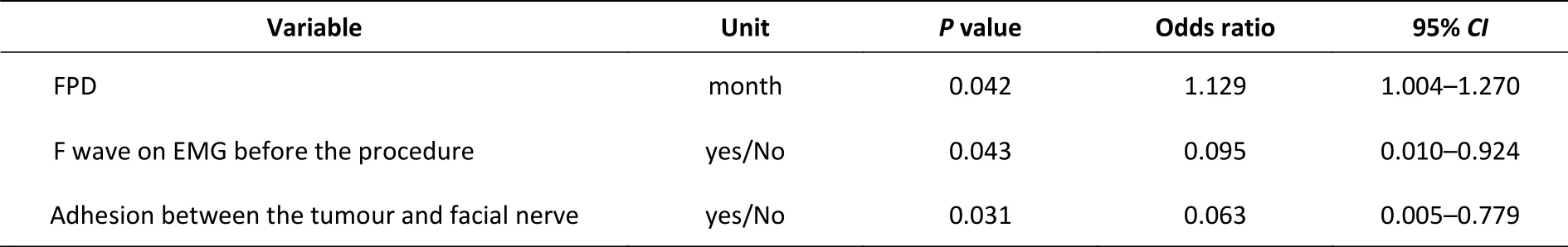
Table 1. Logistic regression analysis results for neurorrhaphy
DISCUSSION
Both internal and external factors have been shown to influence the treatment prognosis after hypoglossal-facial nerve ‘side’-to-side neurorrhaphy for incomplete facial paralysis resulting from the removal of acoustic tumour at the cerebellopontine angle area[14,15]. In this study, the surgical neurorrhaphy procedure was considered an invariant factor for the extent of facial function recovery as the surgeries were performed by the same neurosurgeon with the same method. We found that tumour adhesion to the facial nerve, the duration of facial paralysis and F wave appearance prior to neurorrhaphy played much more important roles in treatment prognosis than was found for the other preoperative factors, indicating that both the preservation of facial axons and their microenvironment are pivotal for postoperative facial function recovery after ‘side’-to-side
neurorrhaphy[16,17].
Preservation of the anatomical structure of the facial nerve was a prerequisite for the patients enrolled in this study, as confirmed by a review of surgical recordings after removing their acoustic tumour as well clinical and electrophysiological evaluations. Based on our clinical experience,patients who underwent facial nerve injury with preservation of anatomical structure can have some remnant and/or spontaneously regenerated facial axons even though they experienced complete facial paralysis during the early period after injury that may have persisted for several months.
Hypoglossal-facial nerve ‘side’-to-side
neurorrhaphy can effectively preserve facial nerve fibres and promote double innervation of the paralyzed facial muscles by both hypoglossal and facial motoneurons[8,11]. The concept of side-to-side neurorrhaphy was introduced by Dr. Terzis in 1984 as a part of the ‘babysitter’ procedure for facial reanimation[18]. Since then, few studies have reported using side-to-side neurorrhaphy to treat facial paralysis, particularly in cases with significant incomplete facial paralysis with hypoglossal-facial nerve side-to-side neurorrhaphy. However, the disadvantages of this technique potentially include that it requires two coaptation sites between the hypoglossal and facial nerve when using a nerve graft, and this is likely to reduce the number of regenerated axons that enter the repaired facial nerve, most likely because scar formation at the coaptation sites obstruct the passage of the regenerating axons and lead to axonal escaping from the sites and a longer pathway for axonal elongation.These points remain to be further investigated.Limitations of the study may include eintrinsic and extrinsic factors related to nerve regeneration abilities, which are most likely related to the patient’s psychological state and quality, individual physical conditions, the extent of intensive postoperative facial exercise, large variation in age, and other unknown factors.
After ‘side’ -to-side neurorrhaphy, the participation of facial axons in postoperative facial functional recovery involves the remnant and/or spontaneous regeneration of axons within the original facial nerve pathway. Undoubtedly, tumour adhesion to the facial nerve results in nerve damage during its removal at the cerebellopontine angle area, which occurs inproportion to the amount of tumour adhesion[1,8,19]. Thus, careful preservation of the facial nerve or restoration of the anatomic continuity when the nerve is sectioned during removal of the acoustic tumour is very important for the postoperative prognosis. In addition, F wave appearance prior to neurorrhaphy is an effective indicator of the extent of nerve damage[20].
Double innervation of facial muscles by both hypoglossal and facial axons may result in the reliable restoration of resting facial symmetry and tone. Their innervation by hypoglossal motoneurons allows powerful movement, whereas reinnervation by facial motoneurons improves physiological recovery[21]. In addition, the incidence of synkinesis maybe reduced if the facial muscles are innervated not only by transposed hypoglossal axons but also by remnant and/or spontaneous regenerating facial axons. Indeed, shared innervation of facial muscles by the motoneurons of the facial and hypoglossal brainstem nuclei may prevent synkinesis[5-7,22]. The respective contribution of remnant facial axons and regenerated hypoglossal axons to facial functional recovery and the underlying mechanisms remain to be investigated.
The delay between facial palsy and nerve reconstruction is referred to as facial paralysis duration and has also been found to be an important factor for functional recovery. The duration of facial paralysis may result in unfavourable changes in the axonal microenvironment within the facial nerve.During prolonged facial paralysis, Schwann cell tubules within the distal nerve stump and motor endplates progressively degenerate and may even disappear, whereas distal target muscles undergo atrophy[23-25]. Moreover, the functional reorganization of central nervous system sensorimotor areas after long-term facial paralysis also plays an important role in functional recovery,particularly after nerve transfer treatment performed using an ectopic axonal source[26-28].
Therefore, we stress that early facial nerve repair is necessary to achieve a better functional recovery.In this study, the best results were obtained in patients who had experienced facial paralysis for less than 6 months without any apparent facial muscle atrophy. These results are consistent with the work of Guntinas-Lichius et al.[29], who showed that a denervation period not exceeding 112 d allowed better functional recovery after facial nerve injury.We thus propose that hypoglossal-facial nerve ‘side’-to-side neurorrhaphy should be performed as early as possible in patients with significant facial nerve injury if the absence of spontaneous innervation is observed within 3 months after the onset of facial paralysis[30].
CONCLUSION
In this study, we reviewed 53 patients who experienced facial nerve injury due to the removal of an acoustic tumour at the cerebellopontine angle area and then underwent hypoglossal-facial nerve ‘side’ -to-side neurorrhaphy to treat significant facial paralysis in our neurosurgical department from June 2011 to September 2016. To analyse the impact of factors evaluated prior to neurorrhaphy on the postoperative recovery of facial function, we assessed preoperative factors,including gender, age, tumour size and characteristics, tumour adhesion to the facial nerve, the duration of facial paralysis and F wave appearance, using univariate as well as logistic analyses. We found that tumour adhesion to the facial nerve, F wave appearance and the duration of facial paralysis prior to neurorrhaphy are the predominant factors that influence neurorrhaphy treatment outcomes. Tumour adhesion to the facial nerve and F wave appearance are indicators of the extent of facial nerve injury, whereas the duration of facial paralysis affects the microenvironment in the nerve distal stump and target muscles, suggesting that the careful preservation of the facial nerve and early neurorrhaphy are very important for treatment outcomes.
ACKNOWLEDGEMENTS
The authors thank Ms. WANG Ming Ran and LI Ping (Beijing Neurosurgical Institute) for their excellent assistance in the electrophysiological evaluations.
The authors report no conflicts of interest concerning the materials or methods used in this study or the findings specified in this paper.
Received: March 20, 2019;
Accepted: December 4, 2019
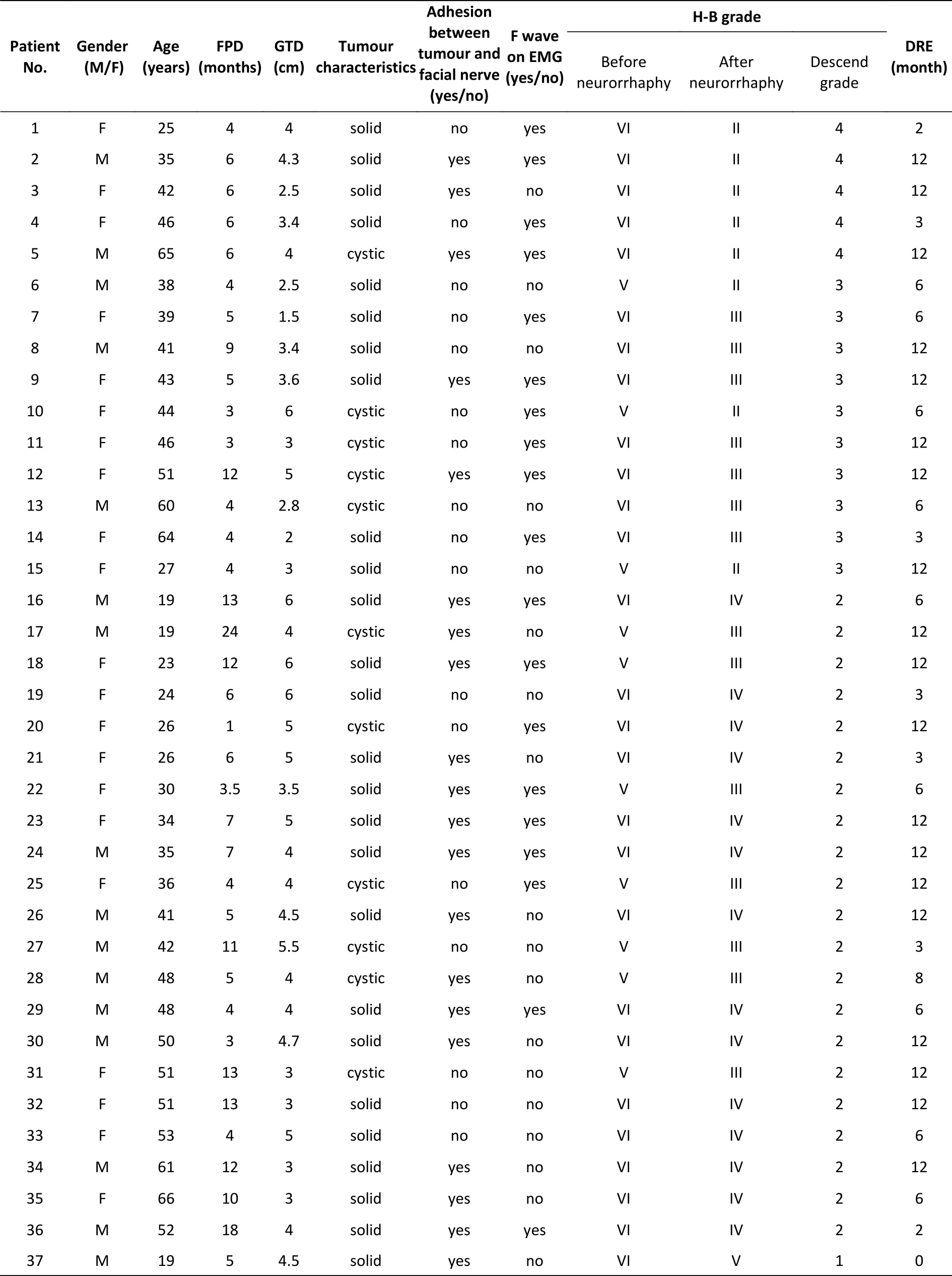
Supplementary Table S1. Basic clinical data for the 53 patients in the research
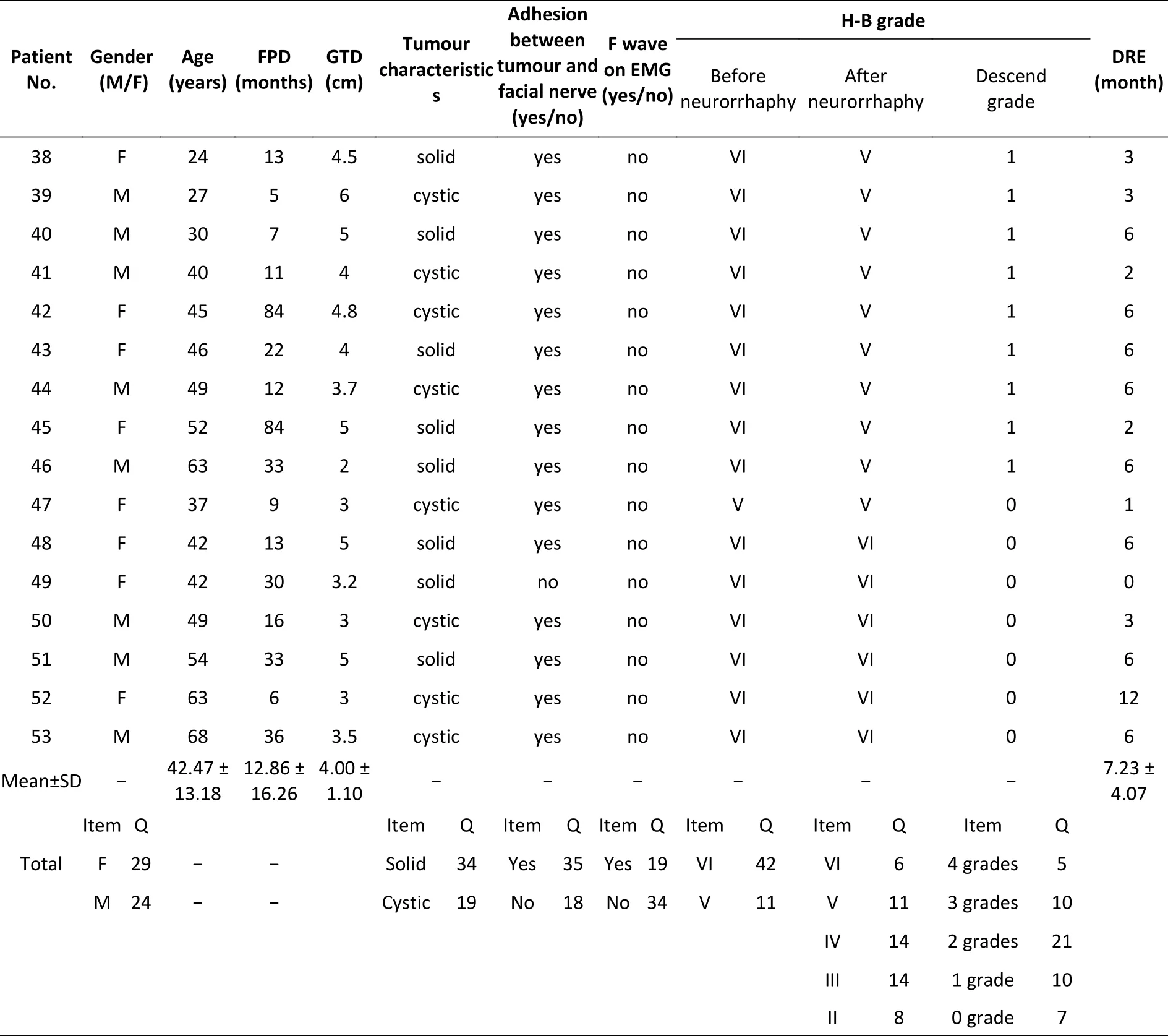
Continued
 Biomedical and Environmental Sciences2020年1期
Biomedical and Environmental Sciences2020年1期
- Biomedical and Environmental Sciences的其它文章
- Burden of Cirrhosis and Other Chronic Liver Diseases Caused by Specific Etiologies in China, 1990−2016:Findings from the Global Burden of Disease Study 2016
- Effect of Body Mass lndex on the Associations between Parity and Metabolic Syndrome and its Components among Northern Chinese Women*
- Association of Red Meat Usual lntake with Serum Ferritin and the Risk of Metabolic Syndrome in Chinese Adults: A Longitudinal Study from the China Health and Nutrition Survey*
- Effects of lncretin-based Therapies on Weight-related lndicators among Patients with Type 2 Diabetes: ANetwork Meta-analysis*
- Analysis of Cardiovascular Risk Factors in Newly Defined Stage 1 Hypertension among Chinese on the Basis of the 2017 ACC/AHA Hypertension Guidelines*
- Characteristics of Hypertension Death in Low-income Regions of lnner Mongolia, China*
