Construction of Novel S/CdS-Au Composite for Improved Activity of Hydrogen Evolution Under Visible Light
DOU Renhao,ZHANG Meiyu,LIN Haili
(School of Chemistry and Materials Science,Huaibei Normal University,235000,Huaibei,Anhui,China)
Abstract:Novel ternary S/CdS-Au composite was constructed by loading Au and S on the surface of CdS nanorods.Under visible light(λ>420 nm),S/CdS-Au displayed excellent photocatalytic activity for H2 evolution(4.38 mmol·g-1·h-1)in comparison with the binary CdS-Au(2.56 mmol·g-1·h-1)and S/CdS(1.86 mmol·g-1·h-1). The photoin⁃duced electrons of CdS were efficiently separated by the synergetic effect of S and Au,which endowed the photocata⁃lytic activity of S/CdS-Au for H2 generation,ensured by transient photocurrent and electrochemical impedance.The S/CdS-Au composite is a highly efficient and stable photocatalyst for H2 production from water.
Key words:semiconductors;composite;water splitting;hydrogen production
0 Introduction
Water splitting for clean energy H2through photocatalysis is very potential for solving the tough problem of energy crisis[1]. CdS is considered as one of the potential photocatalysts for H2evolution under visible light,however,its photocarriers separation efficiency and activity are still far away from the practical applica⁃tion[2]. Therefore,necessary modifications are needed for improving the performance of CdS[3-5].
Construction of CdS-based heterojuction composite is a very important method to improve the separation efficiency of photocarriers on the basis of the matching energy band structure[6-7]. Also,loading metal cocata⁃lyst can further enhance the activity of CdS-based heterojuction[4,8-9],so,it is useful to design ternary CdSbased composite for acquiring high activity[10-12]. Our formerly reported ternary S/CdS-Pt compositea chieved impressive photocatalytic activity of H2evolution[13]. However,Pt is rare and quite expensive,which impels us to replace Pt by other metals.Comparatively,Au also shows strong electron trapping effect like Pt,especially,it is much cheaper than Pt.Therefore,it is worthwhile to construct a novel ternary S/CdS-Au composite for H2production from water splitting.
In this paper,the novel S/CdS-Au composite showed much higher activity than binary S/CdS and CdSAu for H2evolution under visible light(λ>420 nm). The photocarriers separation of CdS were discussed on the synergistic effect of S and Au.This study provides a guideline for designing ternary CdS-based composite for efficient water splitting to produce H2.
1 Experimental
All chemicals were obtained from Sinopharm Chemical Reagent Co. ,Ltd.They were of analytical purity and used without further purification.Deionized water was employed in all experiments.CdS nanorods were synthesized via solvothermal process[12]. CdS-Au was prepared by photoreduction process(The theoretical weight percentage of Au to CdS was 2.07%). S/CdS-Au was fabricated using solvent-evaporation-depositionprecipitation method(The theoretical weight percentage of S to CdS was 1.50%).
A BRUKER D8 ADVANCE X-ray diffractometer with a scanning speed of 10(°)/min and Cu Kα radia⁃tion(λ=0.15406 nm)was used to investigate the crystal phase of the samples.A JEOL-2011 transmission electron microscope was used to obtain the TEM and HRTEM images,in which the accelerating voltage was set as 200 kV.The XPS data of the samples were analyzed by a Thermo ESCALAB 250 with Al Kα(1486.6 eV)line at 150 W.At liquid nitrogen temperature(77.3 K),the BET specific surface area of the powders was measured on an ASAP2020 physical adsorption instrument(Micromeritics). A TU-1901 UV-vis spectro⁃photometer was employed to detect the optical absorption properties of the samples.
The photocatalytic activity of H2production was evaluated on CEL-SPH2N photocatalytic system(Bei⁃jing China Education Au-light co.,Ltd.). 50 mg photocatalyst was dispersed in 30 mL mixture(0.75 mol/L Na2S and 1.05 mol/L Na2SO3). After vacuuming the reaction system for 30 min at 6oC,the reactor was contin⁃uously irradiated by a 300 W Xe lamp with a 420 nm cut-off filter for 3 h.The H2produced from water was analyzed by a gas chromatograph(GC-7900)with N2as carrier gas.
2 Results and discussion
The XRD pattern(Fig.1)shows that CdS is indexed to the hexagonal phase structure(JCPDS file No.65-3414)and elemental S belongs to orthorhombic structure(JCPDS file No.08-0247). In S/CdS,CdSAu and S/CdS-Au samples,only weak S(222)peak can be found but Au cannot be discovered due to its low content.
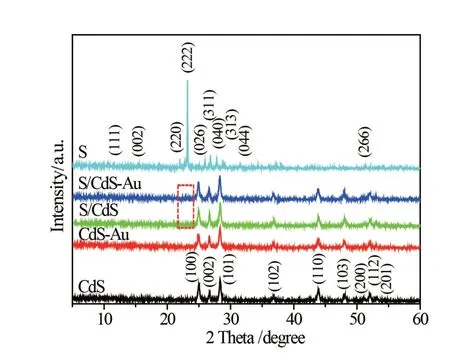
Fig.1 XRD patterns of CdS,CdS-Au,S/CdS,S/CdS-Auand S

Fig.2 XPS spectra of fresh and used S/CdS-Au composites: (a)survey spectra,(b)Cd 3d,(c)S 2p and(d)Au 4f
The XPS spectra(Fig.2a)present that the fresh S/CdS-Au consists Cd,S,Au,C and O,in which ele⁃mental S(Fig.2c)and metallic Au(Fig.2d)can be ensured.The O and C perhaps come from the adsorbed gaseous molecules and adventitious hydrocarbon,respectively.Furthermore,the used S/CdS-Au has the simi⁃lar XPS pattern as the fresh one,suggesting S/CdS-Au possesses excellent stability in the photocatalytic process.
The TEM(Fig.3a)shows that many nano-particles adhere on the surface of CdS nanorods,those are de⁃termined to be CdS(002),S(535)and Au(111)crystal planes(Fig.3b~3c)in S/CdS-Au sample on the ba⁃sis of the lattice spacings of 0.337,0.175 and 0.235 nm.Moreover,the BET surface areas are measured to be 26.70,23.60,23.61 and 22.83 m2·g-1for CdS,CdS-Au,S/CdS and S/CdS-Au,respectively.
The effect of S and Au on the light absorption of CdS was investigated in detail(Fig.4a). S/CdS-Au composite displays the mixed light absorption characteristic of S,CdS and Au.The band gap energies(Eg)of CdS and S(Fig.4b)were estimated to be 2.41 and 2.79 eV,respectively,according to equationahv=A(hv-Eg)n/2[14]. Furthermore,the conduction band potentials(ECB)of CdS and S were further calculated to be -0.51 and -0.09 V separately,on the basis of the Mott-Schottkyplot(Fig.4c).
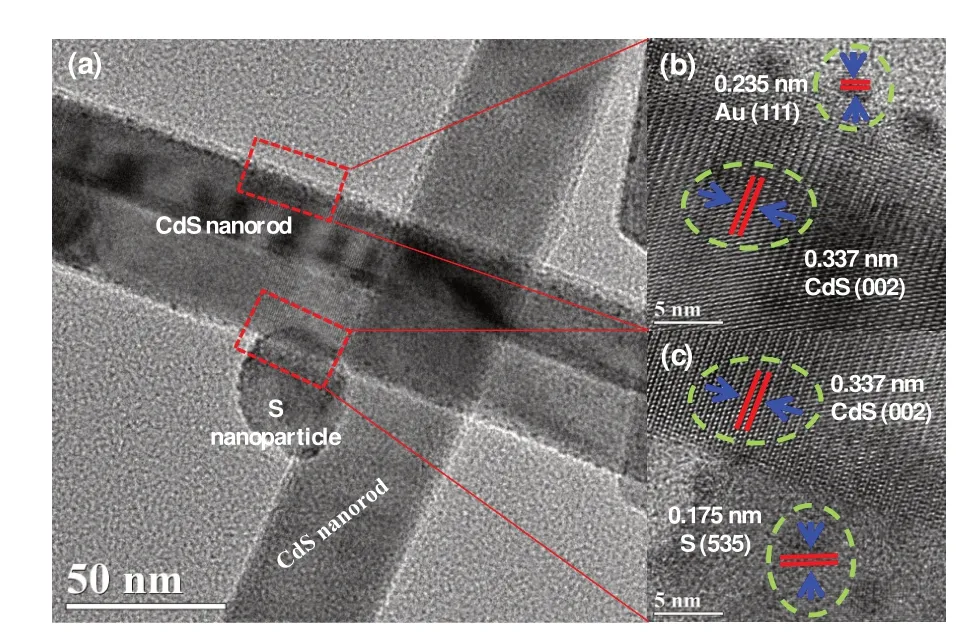
Fig.3 (a)TEM and(b-c)HRTEM image of S/CdS-Au composite

Fig.4 (a)UV-vis diffuse reflectance spectra of CdS,CdS-Au,S/CdS,S/CdS-Au,S.(b)the corresponding band gap energies and(c)Mott-Schottky curves of CdS and S
Under visible light(λ>420 nm),the photocatalytic activity of H2evolution was evaluated in a mixed Na2S/Na2SO3aqueous solution.From Fig.5a,pure CdS only generates 5.3 mmol·g-1H2after 3 h irradation,meanwhile no activity can be found for the single S.

Fig.5 (a)Photocatalytic activity of H2 evolution and(b)recycling test of S/CdS-Au composite under visible light(λ>420 nm)
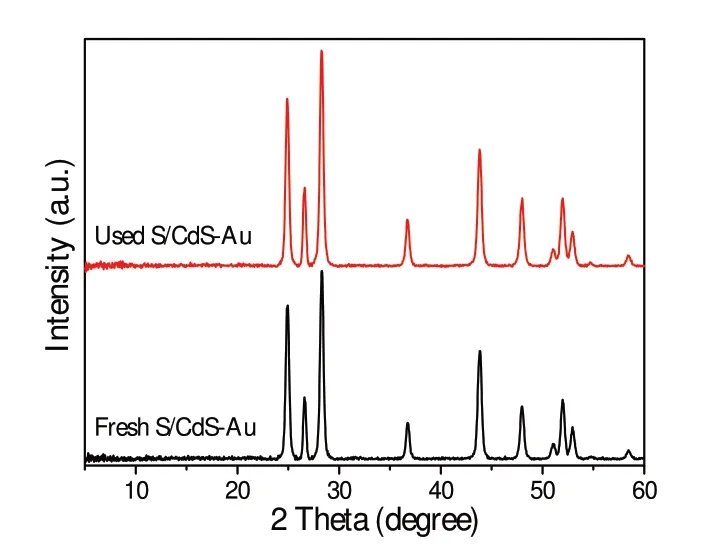
Fig.6 XRD patterns of the fresh and used S/CdS-Au
Little amount of Au or S,in contrast,improves the activity of CdS.Especially,S/CdS-Au exhibits the best activity with 13.1 mmol·g-1H2after 3 hirradation.The corresponding H2evolution rates were calculated to be 1.76,2.56,1.86 and 4.38 mmol·g-1·h-1for CdS,CdS-Au,S/CdS and S/CdS-Au separately.This result mainly results from the synergetic effect of S and Au on the activity enhancement of CdS.Furthermore,apparent quantum efficiency(AQE)[15]of S/CdSAu is 0.78% at 420 nm. After 6 cycles,S/CdS-Au still keeps excel⁃lent activity(Fig.5b)due to the structural stability on the basis of the XRD(Fig.6)and XPS(Fig.2)results.
Generally speaking,the separation efficiency of photocarriers restricts the activity of photocatalysts[16].So,the high activity of S/CdS-Au composite pushes us to understand the intrinsic separation of photocarriers.Fig.7a shows pure CdS has weak transient photocurrent(TPC)intensity owing to its fast recombination of photocarriers.Binary S/CdS displays higher TPC intensity because of the heterojunction interface effect mean⁃while CdS-Au also has TPC response improvement due to the strong electron trapping capacity of Au nanoparticles.Particularly,ternary S/CdS-Au composite reveals the highest TPC intensity,indicating it has the best separation of photocarriers[17]. This is a matter of course from the synergetic effect of S and Au on the efficient photocarriers separation of CdS through constructing a novel S/CdS-Au composite.The lowest electrochemical impedance(Fig.7b)also proves the best photocarriers separation of S/CdS-Au composite[18].

Fig.7 (a)Transient photocurrent spectra and(b)electrochemical impendence spectra of S/CdS-Au composite
Taking the energy structure into consideration,the fundamental separation process of photocarriers over S/CdS-Au composite was de⁃scribed in Fig.8.CdS is activated by visible light to produce elec⁃trons and holes.The electrons on the conduction band of CdS is sepa⁃rated through two ways.They can transfer to the conduction band of S due to the type II energy structure of S/CdS,meanwhile move to the Au nanoparticles owing the electron trapping role of Au.After separation,the electrons will react with H+to generate H2.Additional⁃ly,the holes on the valence band of S will migrate to that of CdS and then are consumed by Na2S/Na2SO3.As a result,S/CdS-Au com⁃posite possesses outstanding photocatalytic activity of H2evolution based on the synergetic effect of S and Au on the photocarriers separation of CdS.
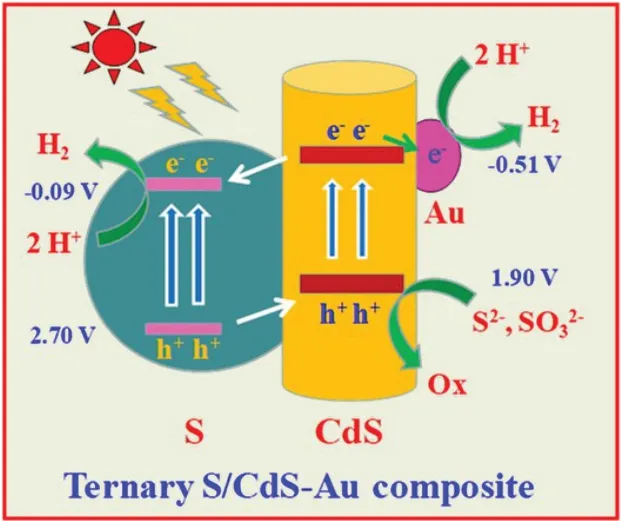
Fig.8 Photocarriers separation mechanism of S/CdS-Au composite under visible light(λ>420 nm)
3 Conclusions
In this study,novel ternary S/CdS-Au composite was reported to produce H2from water splitting under visible light.S/CdS-Au displays excellent photocatalytic activity and exhibits good stability.This finding guides us to greatly boost the photocatalytic activity of CdS via constructing ternary composite for efficient photocarriers separation.

