3,5-二(2′,5′-苯二羧酸)苯甲酸构筑的金属有机配合物的荧光识别及磁性
高玲玲 牛晓燕 杜意恩*, 胡拖平*,
(1晋中学院化学化工学院,晋中 030619)
(2中北大学理学院化学系,太原 030051)
0 Introduction
More and more heavy metal ions discharged result in serious pollution to the water resources and cause a threat to human health[1].Therefore,it is very necessary to design a new material for detecting heavy metal ions in aqueous solution.
Over the past decades,metal-organic complexes(MOCs)as a new type of functional crystalline materials have been extensively investigated in luminescence sensing and molecular magnets,presenting the advantages of high sensitivity,low cost,light weight and strong magnetic[2-3].
The structural diversity of MOCs is always influenced by many factors,such as the organic ligands,central metal ions,synthetic method,solvent,pH value and reaction temperature[4-6].Among all of them,the organic ligands and central metal ions play decisive role in constructing MOCs with the various configurations and directed functions[7-10].Recent studies have found that the symmetric polycarboxylic acid with multiple coordination sites and strong coordination ability is used widely in the constructive ofcomplicated MOCs[11-12].Many fluorescence MOCs based on the symmetric polycarboxylic acid ligand have been designed to detect heavy metal ions and toxic anion,such as Fe3+,Al3+,Cr2O72−,CrO42−,MnO4−.Yet,most of them are performed in organic solvents instead of aqueous solution,which is not desirable in practical application[13].Therefore,it is very meaningful to synthetize water stable MOCs,which serves as fluorescence probes for the detection for heavy metal ions in aqueous solution.
According to the above analysis,a rigid symmetric V-pattern polycarboxylic acid of 3,5-di(2′,5′-dicarboxylphenyl)benozoic acid(H5L)was introduced to build the title MOCs based on the following reasons:(ⅰ)five carboxylate groups of H5L ligand have multiple coordination modes,which will construct various structures of MOCs;(ⅱ) H5L ligand has abundantπ-electron,which is beneficial to synthesize fluorescence materials;(ⅲ)the rigid of H5L ligand helps to enhance the stability of MOCs.
Here,through a“mixed-ligand”strategy,three new MOCs,{[Pb2(HL)(phen)]·2H2O}n(1),{[Ni(H3L)(4,4′-bipy)1.5(H2O)4]·6H2O}n(2)and{[Ni2(HL)(1,4-bibb)(H2O)]· (CH3CN)·H2O}n(3)(phen=1,10-phenanthroline,4,4′-bipy=4,4′-bipyridine,1,4-bibb=1,4-bis(benzimidazole)benzene),were constructed by the reaction of H5L and Pb (Ⅱ)/Ni (Ⅱ) ions under solvothermal method(Scheme 1).Furthermore,the fluorescence of 1 and the variable-temperature magnetic susceptibility of 3 were studied in detail.
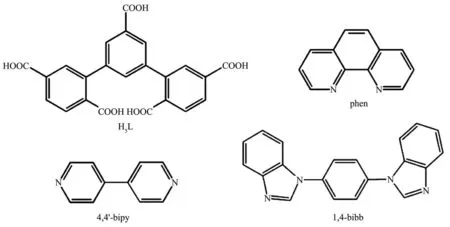
Scheme 1 Structures of the organic ligands
1 Experimental
1.1 Materials and physical measurements
All the chemicals were of reagent grade and used without further purification.Elemental analyses(C,N,and H)were confirmed by elemental analyzer with a Vario MACRO cube.The infrared spectra were detected in a range from 4 000 to 400 cm−1by a FTIR-8400s spectrometer.The X-ray powder diffraction patterns were recorded using Rigaku D/Max-2500 PC diffractometer with a 2θrange of 5°~50°(MoKα,λ=0.071 073 nm).The TG analyses were obtained using a METTLER TGA analyzer under a N2atmosphere.Magnetic susceptibility data were investigated using a Quantum Design MPMS XL-7 SQUID instrument in a range of 2~300 K.
1.2 Syntheses of the complexes
1.2.1 Synthesis of{[Pb2(HL)(phen)]·2H2O}n(1)
H5L(0.01 mmol,4.5 mg),phen(0.01 mmol,1.98 mg),Pb(NO3)2(0.06 mmol,19.9 mg)and H2O(1 mL)were placed in a glass tube,sealed and heated to 130℃for 72 h.After the glass tube was cooled to 25℃,the colorless block crystals were obtained(Yield:52%,based on Pb).Anal.Calcd.for C35H22N2O12Pb2(%):C,39.00;H,2.04;N,2.60.Found(%):C,39.37;H,2.35;N,2.01.IR(KBr pellet,cm−1):549(s),725(s),845(vs),900(s),1 108(w),1 240(w),1 382(m)1 514(vs),1 670(vs),1 735(s),2 339(vs),3 646(w)(Fig.S1).
1.2.2 Synthesis of{[Ni3/2(H2L)(4,4′-bipy)1.5(H2O)4]·6H2O}n(2)
H5L(0.02 mmol,9 mg),4,4′-bipy(0.02 mmol,3.12 mg),Ni(NO3)2·6H2O(0.06 mmol,18.4 mg)and H2O(10 mL)were transferred into a 25 mL Teflonlined stainless-steel vessel and heated to 140℃for 96 h.After the Teflon-lined stainless-steel vessel was cooled to 25℃,the blue block crystals were gained(Yield:37%,based on Ni).Anal.Calcd.for C76H82N6Ni3O38(%):C,48.93;H,4.40;N,4.51.Found(%):C,48.98;H,4.23;N 4.49.IR(KBr pellet,cm−1):673(vs),819(s),1 185(w),1 364(w),1 457(w),1 503(vs),1 659(vs),1 735(s),2 350(s),3 551(m)(Fig.S1).
1.2.3 Synthesis of{[Ni2(HL)(1,4-bibb)(H2O)]·(CH3CN)·H2O}n(3)
A mixture of H5L(0.01 mmol,4.5 mg),1,4-bibb(0.005 mmol,1.55 mg)and Ni(NO3)2·4H2O(0.015 mmol,4.36 mg),and CH3CN/H2O(1 mL,1∶3,V/V)was transferred into a pressure-resistant glass tube,sealed and heated at 130℃for 83.33 h.Green block crystals were collected after being cooled to 25℃(Yield:53%,based on Ni).Anal.Calcd.for C65H48Ni2N9O14(%):C,60.16;H,3.71;N,9.72.Found(%):C,60.98;H,3.18;N,9.31.IR(KBr pellet,cm−1):549(vs),611(vs),743(vs),858(vs),989(vs),1 097(vs),1 329(s),1 415(vs),1 523(vs),1 648(vs),1 863(w),2 344(w),3 103(w),3 426(m)(Fig.S1).
1.3 Single crystal X-ray diffraction
X-ray single-crystal diffraction data of MOCs 1,2 and 3 were performed on a Bruker ApexⅡCCD diffractometer(MoKα,λ=0.071 073 nm)at 298,200 and 100 K,respectively.The structures of three complexes were solved by OLEX-2 and refined by full-matrix least-squares procedure based onF2by the SHELXL-2015[14].All non-hydrogen atoms were refined anisotropically.All hydrogen atoms were calculated and added at ideal positions.The relevant crystallographic data,selected bond lengths and bond angles are listed in Table 1 and Table S2.

Table 1 Crystallographic data of 1~3

Continued Table 1
CCDC:1879614,1;1899101,2;1899100,3.
2 Results and discussion
2.1 Descriptions of crystal structures
2.1.1 Crystal structure of{[Pb2(HL)(phen)]·2H2O}n(1)Complex 1 crystallizes in the monoclinic system withP21/nspace group.There are two Pb (Ⅱ)ions,one partly deprotonated HL4−ligand,a phen molecule in the asymmetric unit of 1(Fig.1).The Pb1 (Ⅱ)ion is sevencoordinated by seven O-atoms(Pb1-O1 0.280 43(134)nm,Pb1-O2 0.237 99(138)nm,Pb1-O3A 0.271 35(116)nm,Pb1-O4A 0.241 89(120)nm,Pb1-O6B 0.300 75(133)nm,Pb1-O9C 0.252 6(14)nm,Pb1-O10C 0.255 52(122)nm)from four different HL4−ligand,presenting a distorted{PbO7}pentagonal bipyramid geometry.The Pb2 (Ⅱ)ion is bridged by five oxygen atoms from two HL4−ligands(Pb2-O3 0.285 70(122)nm,Pb2-O4 0.298 25(120)nm,Pb2-O5 0.247 76(132)nm,Pb2-O8 0.237 30(128)nm,Pb2-O9C 0.286 58(138)nm)and two nitrogen atoms from a phen molecule(Pb2-N1 0.259 09(143)nm,Pb2-N2 0.259 94(145)nm),presenting a distorted octahedral geometry.The angles of O-Pb-N and O-Pb-O are from 46.946(373)°to 102.720(409)°.

Fig.1 Coordination environment of Pb (Ⅱ)in 1
The carboxylate groups of H5L in 1 are partially deprotonated and adopt chelating(η2)and chelatingbridging(μ2-η2∶η1)coordination modes(Scheme 2a).The four carboxylate groups of H5L ligand connect Pb ions to form 1D chains based on[Pb2(μ2-COO)2(μ1-COO)4]SBUs(Ni…Ni 0.426 99(9)nm)(Fig.2a,2b),which are further expanded into a 3D structure by hydrogen bonds(C26…O5 0.287 34(126)nm,C8…O8 0.278 04 nm)andπ…πinteractions(centroid…centroid 0.338 93 nm)(Fig.2c,2d).
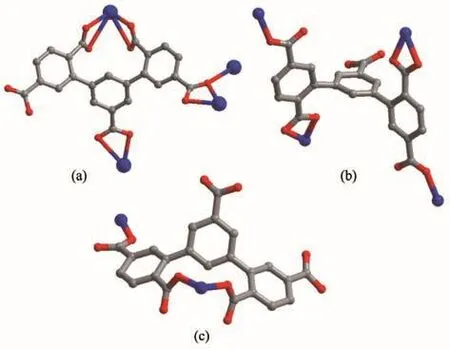
Scheme 2 Coordination configurations of partially deprotonated H5L ligand
2.1.2 Crystal structure of{[Ni3/2(H2L)(4,4′-bipy)1.5(H2O)4]·6H2O}n(2)

Fig.2 (a)[Pb2(μ2-COO)2(μ1-COO)4]SBUs of 1;(b)1D chain of 1;(c)3D framework of 1 viewed along b axis;(d)π…π interaction between molecules of complex 1
Complex 2 crystallizes in the triclinic system withPspace group.The asymmetric unit of 2 is shown in Fig.3,which contains two independent Ni (Ⅱ)ions,one H2L3−ligand,one and a half of 4,4′-bipy linkers,four coordination water molecules and six lattice water molecules.
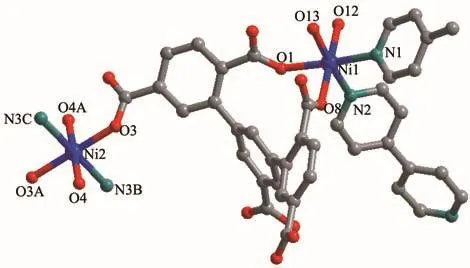
Fig.3 Coordination environment of Ni (Ⅱ)in 2
The Ni1 (Ⅱ)ion is bridged by four O-atoms from a H2L3−ligand(Ni1-O1 0.211 67(34)nm,Ni1-O8 0.206 04(31)nm)and two coordinated water molecules(Ni1-O12 0.207 95(32)nm,Ni1-O13 0.206 13(30)nm),and two N-atoms from two 4,4′-bipy linkers(Ni1-N1 0.212 56(43)nm,Ni1-N2 0.207 33(32)nm),forming a slightly distorted[NiN2O4]octahedral geometry.The angles of O-Ni-N and O-Ni-O are from 82.943(110)°to 174.913(177)°.Ni2 (Ⅱ) ion is surrounded by four O-atoms(Ni2-O3 0.209 32(28)nm,Ni2-O3A 0.209 32(28)nm,Ni2-O4 0.205 19(32)nm,Ni2-O4A 0.205 19(32)nm)from two H2L3−ligands and two water molecules,two N-atoms(Ni2-N3B 0.212 60(35)nm,Ni2-N3C 0.212 60(35)nm)from two 4,4′-bipy linkers,showing a[NiN2O4]octahedron geometry.The carboxylate groups of H5L in 2 are partially deprotonated and adopt bridging(η1)coordination modes(Scheme 2b).Two kinds of H2L3−ligands bridge three Ni (Ⅱ) ions to form a[Ni3(H2L3−)2]supramolecular structure(Ni…Ni 1.122 64(9)nm)(Fig.4a).At the same time,the 4,4′-bipy connects with two Ni (Ⅱ) ions to yield 1D zigzag[Nin(4,4′-bipy)]polymeric chain(Fig.4b),where the distances of Ni-Ni are from 1.120 37(9)to 1.126 45(11)nm.A 2D layer structure with 1D square channels(1.034 75(58)nm×2.158 51(65)nm)is obtained by sharing the central Ni (Ⅱ)ions(Fig.4c),which is further extended to a 3D network framework through hydrogen bond interactions.(O4…O7 0.191 35(12)nm,C21…O11 0.225 53(44)nm,O12…O14 0.193 50(41)nm,C10…O17 0.199 24(75)nm,O15…O16 0.192 45(73)nm)(Fig.5a).Topologically[15],both H2L3−ligands and Ni ions can be regarded as 4-connected nodes,and the whole 3D structure is simplified as a 2-nodal 4,4-connected topology with the Schläfli symbol{4.62}2{42.62.82}(Fig.5a,5b).
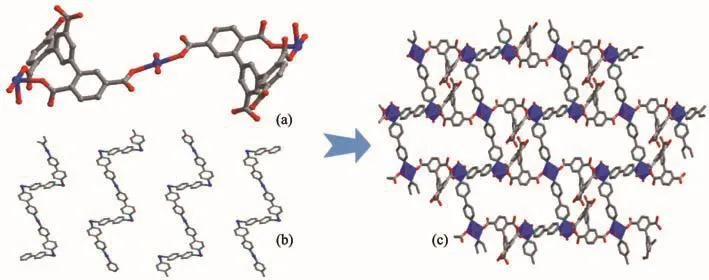
Fig.4 Structure of 2:(a)0D structure based on H5L and Ni ions;(b)1D zigzag chain formed by 4,4′-bipy and Ni ions;(c)2D structure
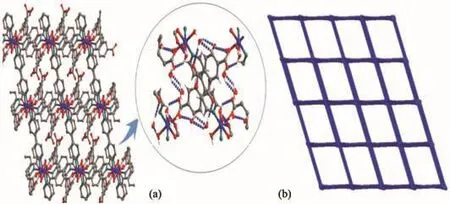
Fig.5 (a)Three-dimensional structure of 2 by hydrogen bond interactions;(b)Schematic view of the 4,4-connected net of 2
2.1.3 Crystal structure of{[Ni2(HL)(1,4-bibb)(H2O)]·(CH3CN)·H2O}n(3)
The structural analysis indicated that 3 crystallizes in the monoclinic system withP2/cspace group.The asymmetric unit contains two independent Ni (Ⅱ)ions,half of HL4−ligands,a 1,4-bibb molecule,two coordination water molecules,a lattice acetonitrile molecule and a lattice water molecule.As shown in Fig.6,Ni1 (Ⅱ)ion presents a slightly distorted[NiN2O4]octahedral geometry and is coordinated by two N-atoms from two 1,4-bibb linkers(Ni1-N3 0.206 45(46)nm,Ni1-N3A 0.206 45(46)nm),four O-atoms from two HL4−ligands(Ni1-O1 0.206 44(38)nm,Ni1-O1A 0.206 44(38)nm)and two coordinated water molecules(Ni1-O6 0.212 26(47)nm,Ni1-O6A 0.212 26(47)nm),respectively.Ni2 (Ⅱ)is surrounded by two N-atoms from two 1,4-bibb linkers(Ni2-N1 0.203 09(48)nm,Ni2-N1B 0.203 09(48)nm),four O-atoms from two different HL4−ligands(Ni2-O3 0.228 92(35)nm,Ni2-O3B 0.228 92(35)nm,Ni2-O4 0.201 53(42)nm,Ni2-O4B 0.201 53(42)nm),showing a distorted octahedral geometry.

Fig.6 Coordination environment of Ni (Ⅱ)in 3
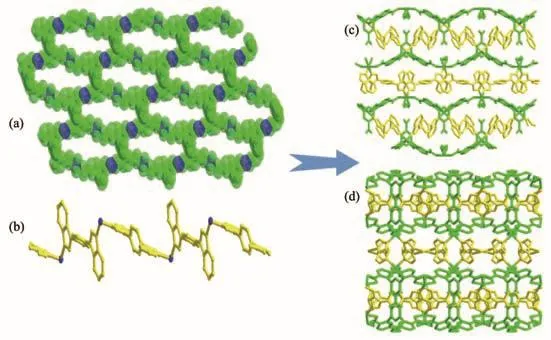
Fig.7 (a)Two-dimensional layer structure of 3 along b axis;(b)1D[Nin(1,4-bibb)]polymeric chain obtained by 1,4-bibb linker and Ni (Ⅱ)ion;(c),(d)3D framework of 3 along a and c axes
In the assembly of 3,H5L ligands are partly deprotonated and connect Ni ions by adopting chelating(η2)and bridging(μ2-η1∶η1)mode(Scheme 2c).The carboxylate groups of H5L ligand connect Ni (Ⅱ)ions to generate an infinite 2D layer structure with 1D hexagon channels of 0.860 56(72)nm×2.099 63(92)nm(Fig.7a,7b),which is further expanded by a 1D[Nin(1,4-bibb)]polymeric chain formed by Ni (Ⅱ)ions and 1,4-bibb into a 3D network structure(Fig.7c,7d).Topologically,the 3D structure of 3 is simplified as a 3-nodal 4,4,4-connected topology with the Schläfli symbol of{62.84}{64.82}2,in which the HL4−,1,4-bibb and Ni (Ⅱ) ion can be considered as 4-c nodes,4-c nodes and 4-c nodes,respectively(Fig.8).

Fig.8 Topology of 3
2.2 IR analysis,thermal analyses and X-ray powder diffraction analyses
The IR measurements of 1~3 have been carried out to confirm their structure(Fig.S1).The thermal stabilities of 1~3 were investigated(Fig.S2),and it is found that 1~3 have high thermal stability.Complex 1 had no weight loss between 30 and 447℃,and then the structure of 1 began to decompose.For 2,the weight loss of 10.4%(Calcd.9.7%)below 120℃is due to the release of four coordinated water molecules and six lattice water molecules.When the temperature exceeded 440℃,the framework of 2 began to collapse.In the temperature range of 30~200 ℃ ,complex 3 showed a weight loss of 7.3%(Calcd.6.4%),which is attributed to the release of two coordinated water molecules,a lattice acetonitrile and a lattice water molecule.Furthermore,the experimental PXRD patterns of 1~3 were characterized,which are good consistent with simulated ones,presenting the good phase purity of MOCs(Fig.S3).
2.3 Luminescent properties
The solid state luminescence emission spectra of complex 1,H5L and phen were investigated at room temperature(Fig.9).The maximum emission peaks of H5L and phen were at 370 and 374 nm(λex=270 nm),respectively.The emission peak of 1 was at 381 nm and had a slightly red-shift(Δλ=11 nm),which may be assigned to the metal perturbed emission of ligand[16].
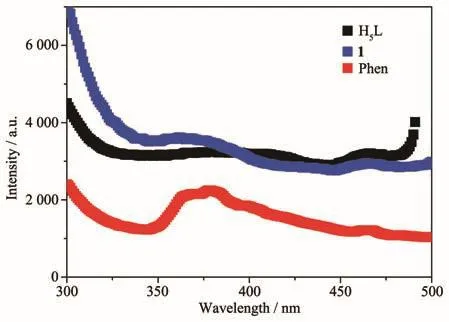
Fig.9 Solid-state fluorescence emissions spectra of the ligands and complex 1 at room temperature
Because complex 1 has good fluorescent performance in the solid state and can remain stable in aqueous solution,we further explored the luminescent sensing properties of 1 to different metal ions in aqueous solution.Complex 1(2 mg)was dispersed in 2 mL different nitrate salts aqueous solutions(0.01 mol·L−1).As shown in Fig.10,the fluorescence intensities of 1 depended on different metal ions.When 1 was dispersed in Ca(NO3)2aqueous solutions,its fluorescence intensity was basically unchanged.When dispersed in aqueous solutions containing Zn2+,Ag+,Ba2+,Mg2+,Pb2+,K+,Cd2+and Al3+,the fluorescence intensities of 1 were reduced to varying degrees.Especially,when 1 was dispersed in Fe3+aqueous solutions,the fluorescence intensity of 1 was almost completely quenched and the quenching rate of Fe3+to 1 was 98.1%,which means that 1 can serve as a fluorescence probe for sensing Fe3+.Meanwhile,the anti-interference experiment was also carried out to study the effect of mixed cations on fluorescence intensity of 1.Equal amounts of Fe3+and other cations were added into the suspension of 1@H2O(2 mL),respectively.The results show that the quenching effect of Fe3+is not affected by other cations(Fig.S4).
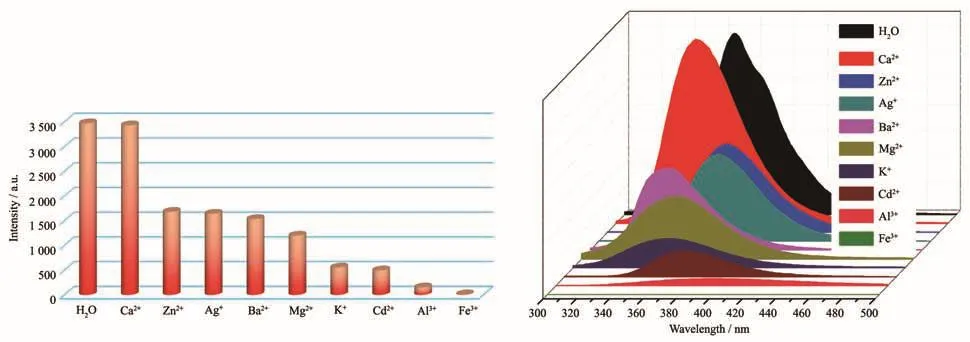
Fig.10 Photoluminescence intensities of 1 dispersed in different cations aqueous solutions
To better test the luminescent sensing sensitivity of complex 1 for Fe3+ions,titration experiments were carried out by gradually increasing the concentration of Fe3+to 1@H2O suspension.As shown in Fig.11a,the luminescent intensity of 1 gradually decreased with an increasing of Fe3+concentration.The luminescence intensity of 1 was completely quenched when the concentration of Fe3+came up to 1.07 mmol·L−1.The fluorescence quenching efficiency of Fe3+was well-fitted by using the exponential equations:I0/I=0.675 28exp(cM/0.277 15)+0.928 21,wherecMis the concentration(mmol·L−1)of Fe3+;I0andIstand for luminescent intensities of 1@H2O suspension in the presence and absence of Fe3+,respectively.The Stern-Volmer plots for Fe3+were almost linear at lower concentration range of Fe3+,and followed the Stern-Volmer equation ofI0/I=1+KsvcM(Fig.11,Inset).The quenching constant(Ksv)of 1 for Fe3+was calculated to be 7.24×104L·mol−1based on the linear part.The detection limit(DL)of 1 for Fe3+was calculated to be 1.65×10−5mol·L−1by 3σ/Ksv,whereσis the standard deviations for blank solutions(Table S3).
To further study the sensitivity of 1 for different anions,2 mg samples of 1 were immersed in 2 mL KmX aqueous solution (0.01 mol·L−1,X=Br−,Cl−,I−,CH3COO−,HPO42−,CO32−,HCO3−,C2O42−,SCN−and Cr2O72−).As exhibited in Fig.12,the luminescence of Cr2O72−anions for 1@H2O suspension presented an obviously quenching effect,and the quenching efficiency was as high as 95.5%.
The Stern-Volmer plot for Cr2O72−showed a linear behavior at low concentration and bends upwards at high concentration(Fig.13).According to the linear part of the S-V plot,theKsvvalue of Cr2O72−was calculated to be 2.17×103L·mol−1.Based on the data of blank tests and titration tests,the DL of 1 for CrO2−27calculated to be 7.52×10−4mol·L−1(Table S4).The low DL shows that 1 can serve as a fluorescent sensitive probe for Fe3+/Cr2O72−in aqueous system[17].

Fig.11 (a)Luminescent emission spectra of 1 dispersed in aqueous solution upon incremental addition of Fe3+;(b)Plot of I0/I vs concentration of Fe3+
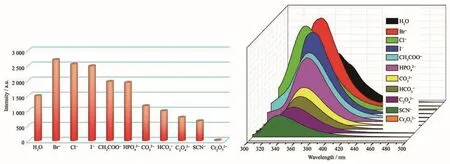
Fig.12 Photoluminescence intensities of complex 1 dispersed in different anion
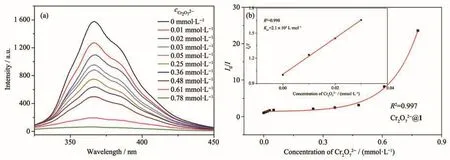
Fig.13 (a)Luminescent emission spectra of 1 dispersed in aqueous solution upon incremental addition of Cr2O72−;(b)Plot of I/I vs concentration of CrO2−027
The sensing mechanisms of 1 for Fe3+and Cr2O72−can be illuminated by the following experiments.Firstly,the PXRD patterns of 1 after being immersed in Fe3+and Cr2O72−solutions are almost consistent with the original(Fig.S5),which indicates that the structural collapse of 1 is not the cause of fluorescence quenching.Secondly,inductively coupled plasma(ICP)tests were measured for samples after luminescence test,which indicates that no Pb2+ions were detected in the suspension(Table S5,S6).Lastly,as can be seen from Fig.S6,the absorption spectra of Fe3+and Cr2O72−overlapped the excitation spectra of 1 to a greater extent,which shows that the competitive absorption between the analytes and complex 1 is the main cause for fluorescence quenching[18-20].In addition,the weak interaction between the framework of complex and metal ion is also an important factor.
2.4 Magnetic properties
The variable-temperature magnetic susceptibility of 3 was measured in a range of 2~300 K under a static field of 1 000 Oe(Fig.14).For 3,theχMTvalue was 2.1 cm3·K·mol−1at ambient temperature,which was larger than the value for two isolated Ni (Ⅱ) ions of 2.0 cm3·K·mol−1(g=2,s=1),indicating the orbital contribution in octahedron Ni (Ⅱ)[21-22].With the decreasing of temperature,theχMTvalue increased continuously to the maximum value of 2.24 cm3·K·mol−1at about 18 K,and then decreased sharply to 1.51 cm3·K·mol−1at 2 K.The plot of the reciprocal magnetic susceptibility vsTfollowed the Curie-Weiss Law(χM=C/(T−θ))from 25 to 300 K,yieldingC=2.16 cm3·K·mol−1,θ=−1.44 K for 3.The reducingχMTvalue and the negativeθvalue show that MOC 3 has the antiferromagnetic coupling interactions between the adjacent Ni (Ⅱ)ions.

Fig.14 χMT and χMvs T plots of 3
3 Conclusions
In summary,three new MOCs have been synthesized by mixed-ligand strategy under solvothermal method.Structural analysis indicates that the structural difference of the title MOCs is mainly attributed to the coordination modes of the carboxylate groups and the use of different auxiliary ligands.MOC 1 has good water stability,sensitive and selective sensing for Fe3+and Cr2O72−in aqueous solutions,which is ascribed to the competitive adsorption between the analytes and the framework of 1.While MOC 3 shows the antiferromagnetic coupling interactions between the adjacent Ni (Ⅱ)ions.
Acknowledgments:The authors gratefully acknowledge the financial support of this work by the fund for the Jinzhong University“1331 Project”Key Innovation Team(Grant No.jzxycxtd2019005)and the National Natural Science Foundation of China(Grant No.21676258)and the support of innovative research team of inorganic-organic hybrid functional materials in North University of China.
Supporting information is available at http://www.wjhxxb.cn