Non-invasive prediction of persistent villous atrophy in celiac disease
Barbora Packowa, Petra Kovaldikova, Zdenek Pavlovsky, Daniel Bartusek, Jika Prokesova, Jiri Dolina, Radek Kroupa
Abstract
Key words: Celiac disease; Villous atrophy; Anti-tissue transglutaminase antibodies; Antideamidated gliadin peptide antibodies; Abdominal ultrasound; Gluten-free diet
INTRODUCTION
Celiac disease (CD) is an immune-mediated enteropathy triggered by gluten in genetically susceptible individuals. The only therapy for CD is a gluten-free diet (GFD). Mucosal healing (Marsh 0 or 1 on follow-up biopsy) is the main endpoint of this therapy; however, this goal has been achieved in approximately 60% of patients after one year of GFD, especially in cases of CD diagnosed in adulthood[1,2]. In contrast, some recent studies state that up to 81% of patients achieved mucosal healing, as seen on long-term follow-ups[3].

Currently, duodenal biopsy is the only way to evaluate mucosal healing. There is no reliable widely available non-invasive marker of persistent villous atrophy (VA), which is one of the core pathological signs of CD. Many authors regard anti-tissue transglutaminase antibodies (aTTG) as a poor predictor of persistent VA[2,4], with a low sensitivity 0.50 [95% confidence interval (CI): 0.41-0.60] and a relatively high level of specificity 0.83 (95%CI: 0.79-0.87) for TTG IgA assay[5]. However, there is not much data on anti-deamidated gliadin peptide antibodies (aDGP). There is one study evaluating aDGP as a reliable marker of persistent VA[6], while another study found only 48% sensitivity and 91% specificity of aDGP IgA for predicting persistent VA[7]. Currently, to the best of our knowledge, there are no studies indicating the absolute necessity of routine follow-up biopsy[8,9]; however, many centers recommend its implementation[9,10]and itis considered as an important tool in the follow-up of symptomatic patients with CD, based on the recommendations by the American Gastroenterology Association[11]. A personalized approach with respect to risk factors is essential. Together with factors such as the advanced age at diagnosis, the male sex, and untreated CD, even asymptomatic persistent VA is considered to be a risk factor for lymphoproliferative malignancy[12]and possibly higher mortality rates[13]. Other parameters potentially related to VA may be available in the standard clinical care process[14]. Besides counseling with a qualified dietitian, only few objective methods to assess persistent gluten intake are available. There might be a clinical advantage in using non-invasive methods for the detection of patients with high risks of VA, independent of improvement in symptoms after at least 1 year on GFD. Abdominal ultrasound is a widely available method, and several studies have reported specific abnormalities on small bowel imaging that could be related to CD[15,16]. Persistence of these findings might indicate the absence of mucosal healing. Awareness of the risk factors is essential for the selection of patients indicated for thorough follow-up.
Our aim was to evaluate the ability of non-invasive markers (aTTG, aDGP, small bowel ultrasonography, and clinical and laboratory parameters) to predict persistent VA determined using histology in patients with CD who had been on a GFD for at least one year.
MATERIALS AND METHODS
Patient selection
The records of 190 patients with CD from 2014 to 2018 were available in the hospital database at the Department of Gastroenterology and Internal Medicine, University Hospital Brno.The initial diagnosis of CD was based on the presence of VA on an intestinal biopsy, positivity of aTTG and/or aDGP, or the clinical effect of a GFD in cases of seronegative CD. Adherence to a GFD was evaluated by an experienced dietitian. Follow-up duodenal biopsy and ultrasound examination at least after 1 year of GFD was proposed to all patients, independent of symptoms. Patients who had agreed to undergo follow-up biopsy were selected for further evaluation. In our retrospective cohort study, we included patients who had been on complete GFD for at least one year and for whom data on follow-up duodenal biopsy and quantitative evaluation of aTTG and/or aDGP using the enzyme-linked immunosorbent assay (ELISA) method were available as well. Abdominal ultrasonography focused on bowel imaging within 30 d from when duodenal biopsy was performed.
The patients included in the study were divided in two subgroups: (1) The study group with patients with persistent VA on follow-up duodenal biopsy; and (2) Control group with patients classified as Marsh 0 or Marsh 1 on follow-up duodenal biopsy. All patients signed an informed consent regarding anonymous data collection, and the study protocol was approved by the multicentric ethical committee of the University Hospital Brno (No. 03-180919/EK).
Duodenal sampling and assessment of histological findings
All the selected patients underwent esophagogastroscopy with biopsies from the second part of the duodenum and one from duodenal bulb; at least four biopsy specimens were fixed in 40 g/L formaldehyde. Paraffin-embedding blocks were created for basic hematoxylin-eosin staining and special staining. The Marsh classification modified by Oberhuber was used for microscopic evaluation[17]. Mucosal architecture (villus height, crypt depth), intraepithelial lymphocytes, inflammatory cell infiltrate, and level of epithelial differentiation were evaluated. The pathologist was blinded to the clinical and antibody results.
Methods of serologic testing
Serum samples were collected within 30 d after duodenal biopsy. Sera were assayed for aTTG IgA and IgG and aDGP IgA and IgG using ELISA. Cutoff values over 18 U/mL and 20 U/mL for aTTG and aDGP, respectively, were regarded as positive by the kit manufacturer. Lab kits for analyses were provided by TestLine Clinical Diagnostics Ltd., Brno, Czech Republic.
Ultrasonography evaluation
Ultrasonography examinations of the intestine within 30 d from duodenal biopsy were available for 66 patients. The remainder of the patients underwent ultrasonography at longer periods from duodenal biopsy; therefore, these results were excluded from the analysis. Using a high-frequency linear probe, it was possible to evaluate the intestinal wall, intestinal folds, surrounding mesentery, mesenteric lymph nodes, and other characteristics. The main ultrasound findings in patients with active CD were decreased numbers of jejunal folds, increased numbers of ileal folds and thickening of bowel folds, dysmotility, jejunal dilatation, and intermittent intussusception[15,16]. Mostly non-enlarged mesenteric lymph nodes were detected. As a positive result, persistent ultrasound abnormalities usually related to CD were assessed by an experienced physician who was blinded to serology and biopsy results.
Clinical and laboratory parameters
Clinical symptoms typical of active CD, such as diarrhea, abdominal pain, and weight loss, were reviewed. Laboratory signs of nutritional deficiency, such as anemia (hemoglobin level less than 135 g/L in men and less than 120 g/L in women), sideropenia (ferritin level less than 30 µg/Lin men and less than 13 µg/L in women), and vitamin D deficiency (less than 50 nmol/L), were evaluated.
Statistical analysis
The mean, standard deviation, median, minimum, and maximum were used to analyze quantitative parameters. Absolute and relative frequency were used to analyze qualitative parameters. Sensitivity, specificity, and positive and negative predictive value were calculated from frequency of VA and positivity ofaTTG and aDGP and were reported with their 95%CI. To quantify antibody titers (aTTG IgA, aTTG IgG, aDGP IgA, aDGP IgG) receiver operating characteristic analysis was used to evaluate the best cutoff values with highest total sensitivity and specificity. Sensitivity, specificity, and positive and negative predictive value were calculated for these new cutoff values. The Mann–Whitney test or Fisher’s exact test were used for comparison of aTTG and aDGP positive and negative patients as well as for comparison of patients according to the persistence of VA. APvalue < 0.05 was considered statistically significant. SPSS software version 23.0 for Windows (SPSS Inc., Chicago, IL, United States) was used for the statistical analyses.
RESULTS
Eighty-two patients fulfilled the inclusion criteria and were further analyzed. In this group, 67 (81.7%) patients were women and the mean age at diagnosis was 33.8 ± 17.4 years. Mean length of the disease at the time of follow-up biopsy was 9.1 years, and mean age at follow-up biopsy was 42.1 ± 13.4 years. Seventy patients (85.4%) were on a GFD longer than 2 years. All patients had CD that was initially properly diagnosed, with positive duodenal biopsy graded according tothe Marsh classification modified by Oberhuber (2× Marsh 2, 17× Marsh 3a, 30× Marsh 3b, 33× Marsh 3c) and either positivity of aTTG and/or aDGP (74×) or clinical effect of GFD in case of seronegative CD (8×). No seronegative patient was in the persistent VA group, as other diagnoses needed to be considered in such cases.
The most frequent clinical symptoms and laboratory signs of malnutrition at the time of follow-up biopsy were diarrhea (23.2%), abdominal pain (20.7%), weight loss (9.8%), sideropenia (26.8%), vitamin D deficiency (20.7%), and anemia (11.0%). Autoantibodies for aTTG were positive (cutoff value 18 U/mL recommended by manufacturer) in 18 cases (22.2%); those of aDGP were positive (cutoff value 20 U/mL determined by laboratory) in 29 cases (37.2%) at the time of follow-up biopsy. Ultrasonography was available in 66 patients with signs correlating with active CD found in 24 (29.3%) cases (details in Table 1).
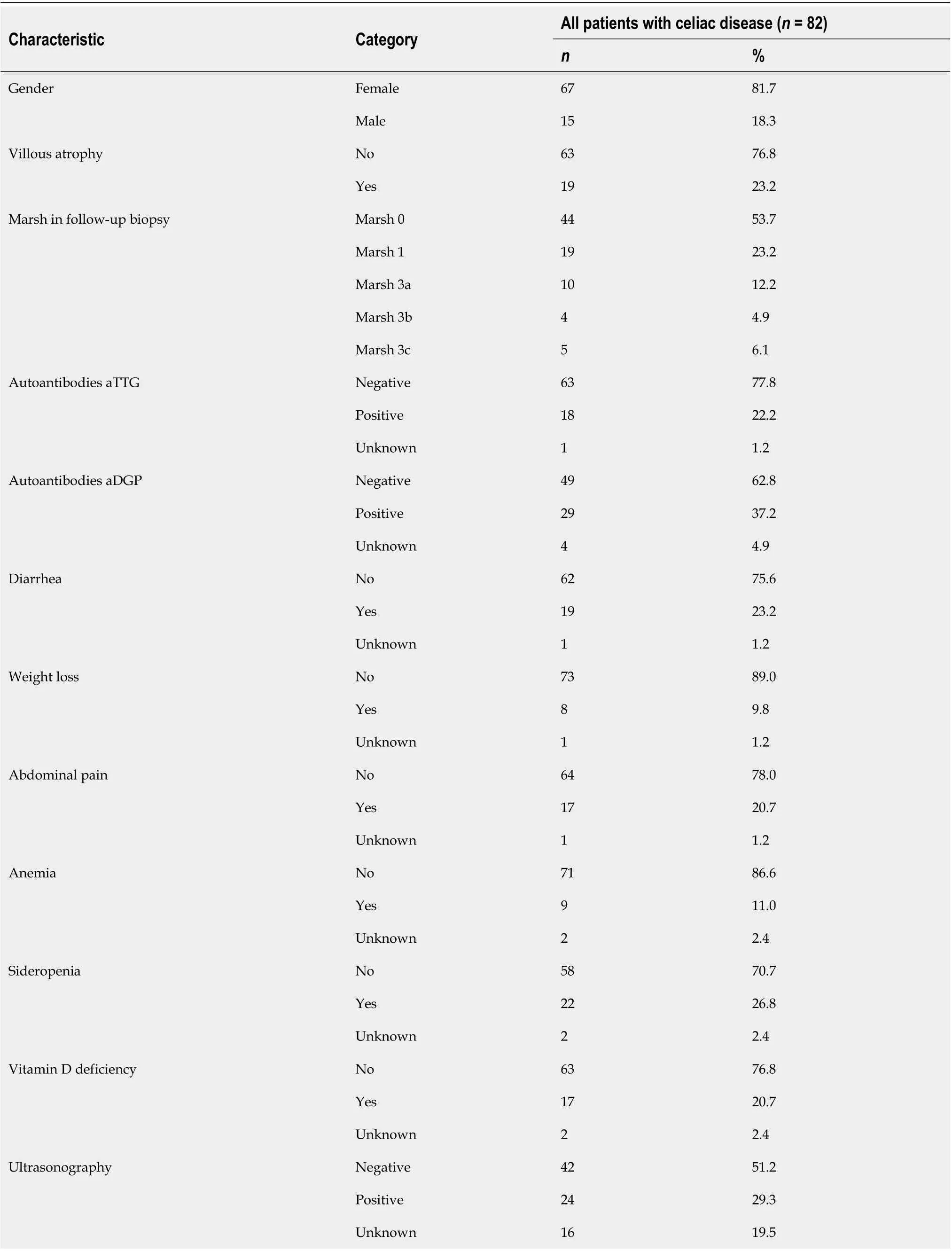
Table 1 Summary of patient characteristics at the time of biopsy on gluten-free diet
Data of 19 patients (23.2%) with persistent VA (10× Marsh 3a, 4× Marsh 3b, 5× Marsh 3c) were compared with data of 63 patients (76.8%) with either Marsh 0 (44×) or Marsh 1 (19×) classification on the follow-up duodenal biopsy. These two groups did not differ with respect to age at diagnosis, sex, or length of GFD at follow-up biopsy (details in Table 2).
In patients with persistent VA aTTG IgA was positive in nine cases; IgG was positive in one case (nine cases in any aTTG); aDGP IgA was positive in 13 cases; and aDGP IgG was positive in 11 cases (14 cases in any aDGP). In this study group, abdominal ultrasonography was available in 17 cases, and signs of active CD were found in 11 of these. Eight patients had diarrhea, four had weight loss, three had abdominal pain, on had anemia, four had sideropenia, and eight had vitamin D deficiency (Table 3). Only diarrhea and vitamin D deficiency were significantly more common in patients with persistent VA than in patients with mucosal recovery.
The sensitivity, specificity, and positive and negative predictive value of aTTG IgA positivity for prediction of VA were 50%, 96.8%, 81.8%, and 87.1%, respectively. The sensitivity, specificity, and positive and negative predictive value of aDGP IgA positivity for prediction of VA were 72.2%, 81.7%, 54.2%, and 90.7%, respectively (Table 4). In analysis of antibody titers, we calculated the cutoff values with highest total sensitivity and specificity. The calculated cutoff values were 13.4 U/mL and 6.7 U/mL for aTTG IgA and IgG, respectively, and 22.6 U/mL and 28.8 U/mL for aDGP IgA and IgG, respectively. For these cutoff values, we reached sensitivity and specificity of 66.7% (95%CI: 41.0-86.7) and 93.7% (95%CI: 84.5-98.2) for aTTG IgA and 72.2% (95%CI: 46.5-90.3) and 90.0% (95%CI: 79.5-96.2) for aDGP IgA, respectively (details in Table 5). Recalculation of the optimal cutoff values showed the best negative predictive value for aDGP IgA 91.5% (95%CI: 83.6-95.8).
The sensitivity, specificity, and the positive and negative predictive value of ultrasonography for prediction of persistent VA were 64.7%, 73.5%, 45.8%, and 85.7%, respectively. The positive predictive value of diarrhea, abdominal pain, sideropenia, or anemia for VA was low (Table 6). The combination of recalculated cutoff values for aTTG IgA and aDGP IgA with small bowel ultrasonography increased the specificity and positive predictive value for VA prediction. Ultrasonography combined with aTTG IgA reached 98% (95%CI: 89.2-99.9) specificity and 88.9% (95%CI: 51.9-98.3) positive predictive value, with aDGP IgA 97.8% (95%CI: 88.5-99.9) specificity and 90.0% (95%CI: 55.2-98.5) positive predictive value. Negative predictive value was slightly decreased, 84.2% (95%CI: 77.3-89.3) and 84.9% (95%CI: 77.2-90.3) for small bowel ultrasonography combined with aTTG IgA and aDGP IgA respectively (Table 5).
DISCUSSION
In our study, we searched for non-invasive markers of persistent VA in patients with CD who claimed to adhere to GFD. Persistent VA is one of the risk factors for lymphoproliferative malignancy[12]and possibly higher mortality rates[13], irrespective of the cause of VA. Identification of patients at higher risk of persistent VA could lead to more personalized approaches and closer follow-ups, including repeated evaluation of adherence to GFD, and thorough searches for nutritional deficiencies and complications of CD. Potential benefits of a repeated biopsy are broadly discussed[2]. Any non-invasive method that can facilitate creating indications for repeated biopsy or facilitate discharge of patients that tested negative from specific gastroenterological care would be helpful. Serology and ultrasonography are considered non-sensitive markers of persistent VA. In our study, we demonstrated 50% sensitivity and 85.7% specificity for aTTG and 77.8% sensitivity and 75% specificity for aDGP. There are conflicting results regarding this topic in the literature. A recent metanalysis demonstrated a sensitivity of 50% for aTTG IgA and a sensitivity of only 45% (95%CI: 34-57) for anti-endomysium antibodies[5]. Nevertheless, it is essential to stress that these tests be designed for detection of new cases of CD, for which purpose their cutoff values were determined. Even after determining the new cutoff values, the sensitivity of autoantibodies for prediction of VA improved slightly to 66.7% for aTTG IgA and 72.2% for aDGP IgA; however, we were able to reach high specificity and negative predictive values of 93.7% and 90.8%, respectively, for aTTG IgA, and 90% and 91.5%, respectively, for aDGP IgA. The recalculated cutoff value for TTG IgA in our study is about one-third lower than the standard diagnostic cutoff value. Not only the negative result of test but also the numeric value of antibodies might be important for test accuracy and clinical consequences. Patients with lower levels do not need to undergo follow-up duodenal biopsy to evaluate persistent VA. A similar study was performed for aTTG IgA in a larger group by Fanget al[18], who found significant differences in mucosal healing between undetectable and detectable aTTG IgA; however, owing tolimitations of the serology kit,they were unable to determine an ideal cutoff limit. Intermittent gluten exposure may explain the false negative serology tests even in the presence of incomplete mucosal recovery and non-optimal sensitivity of aTTG IgA.

Table 2 Comparison of characteristics according to persistence of villous atrophy
The use of aDGP appears to be a better method than the use of aTTG IgA for detection of persistent VA, particularly in the use of both IgA and IgG antibodies. A combination of aDGP tests led to a sensitivity of 77.8% and a negative predictive value of 91.8%. One study found 87% sensitivity and 89% specificity of aDGP IgG for prediction of nonresponsive CD[6], while another study found only 48% sensitivity but 91% specificity of aDGP IgA for prediction of persistent VA[7]. The wide use of aDGP in future studies may contribute to a more precise role of it in detection of VA despite GFD. Although one study referred to a poor outcome of aDGP IgA in detection of absence of mucosal healing in children[19], other studies obtained different, more positive results[20,21]. A commercially available point-of-care test for both DGP antibodies was referred to as an alternative to classical serology testing with better sensitivity for CD follow-up in one prospective study[22].
The strengths of our study include a complex analysis of many non-invasive parameters for detection of persistent VA in patients with CD on a long-term GFD combining serology tests, clinical parameters, and bowel ultrasound. Many radiologic studies regarding CD are limited to some specific findings on cross-sectional imaging[23]. The role of bowel ultrasound is firmly established in the diagnosis and management of Crohn’s disease[24]. Experience with bowel ultrasound in CD is rather limited; however, its use is expanding and signs corresponding with malabsorption and active CD are well defined[15,16,25]. This examination is routinely used during follow-up of CD patients in our hospital. With easier access to ultrasound examination and increasing experience in many institutions in recent years, it may be challenging to use it for the follow-up of patients with CD in the future. Particularly in patients with positive aTTG IgA or aDGP IgA, ultrasound abnormalities should indicate the need for endoscopic biopsy of duodenal mucosa despite the absence of clinical symptoms.
The limitations of our study are its retrospective design and a relatively small number of patients in the study group. Because the study group was small, we could not subdivide the patients with simple VA and patients with refractory CD. This could have an impact on the results because most patients have negative CD-specific antibodies at the time of refractory CD diagnosis. However positive CD-specific serology can be present in 19%-30% of patients with refractory CD and does not exclude the diagnosis[26]. Another limitation is an inability to evaluate adherence to a GFD using any objective method. It is well known that negative serology is not a reliable marker of adherence to a GFD[27]. Consultation with a skilled dietitian is regarded as the gold standard for monitoring adherence to a GFD[28]. The positivity of gliadin-33-mer or gluten immunogenic peptides in stool are good markers of ongoing gluten consumption[29,30]; however, it is not widely available and is able to evaluate only consumption of gluten in the last few days prior to the examination. Any objective method for long-term GFD evaluation could theoretically improve the result of all studies on this topic and patient management[31].
Relatively higher prevalence of any symptom despite adherence to GFD may be caused by some overlap of CD and functional disorders in patients consenting withinvasive examination. Nevertheless, either symptom related to CD may stimulate the patient to undergo uncomfortable endoscopy. We cannot assess asymptomatic patients’ refusals of follow-up endoscopy owing to their well-being. This situation in a retrospective study represents real-life medicine.

Table 3 Comparison of autoantibodies´ positivity, ultrasonography, laboratory and clinical markers in patients with and without villous atrophy, in groups with available parameters
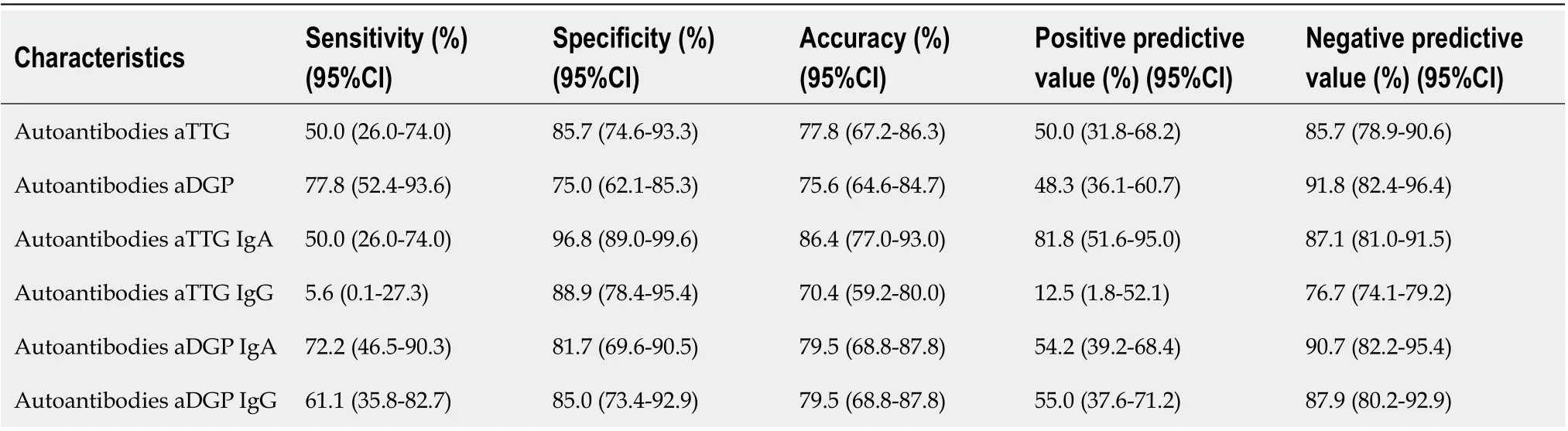
Table 4 Sensitivity, specificity, positive and negative predictive value of anti-tissue transglutaminase antibodies and anti-deamidated gliadin peptide antibodies autoantibodies (with standard cutoff values according to laboratory references 18 U/mL for anti-tissue transglutaminase antibodies and 20 U/mL for anti-deamidated gliadin peptide antibodies) for prediction of villous atrophy in patients with celiac disease

Table 5 Sensitivity, specificity, positive and negative predictive value of recalculated cutoff values of anti-tissue transglutaminase antibodies and anti-deamidated gliadin peptide antibodies autoantibodies titers and their combination with small bowel ultrasonography for prediction of villous atrophy in patients with celiac disease
Most patients in our study were known to have avoided gluten consumption for more than 2 years; therefore, inter-individual differential mucosal recovery likely plays no role in our results. In patients with VA, some symptoms and signs of malabsorption are more common; nevertheless, the predictive role of diarrhea and vitamin D deficiency for the diagnosis of atrophy was poor. In symptomatic patients on GFD, a re-biopsy should be considered[11].
In our study, we did not show that serologic tests of aTTG and aDGP with standard diagnostic cutoff values were optimal markers of persistent VA. Nevertheless, calculation of the best cutoff values of aTTG and aDGP IgA for prediction of VA improved the sensitivity, specificity, and negative predictive value. The combinationof serology and expert bowel ultrasound examination may achieve better accuracy for the detection of atrophy. Signs of persistent VA should be considered after 1-2 years of GFD. Asymptomatic patients with lower levels of both aDGP IgA and IgG do not need to undergo follow-up duodenal biopsy to determine the presence of persistent VA.
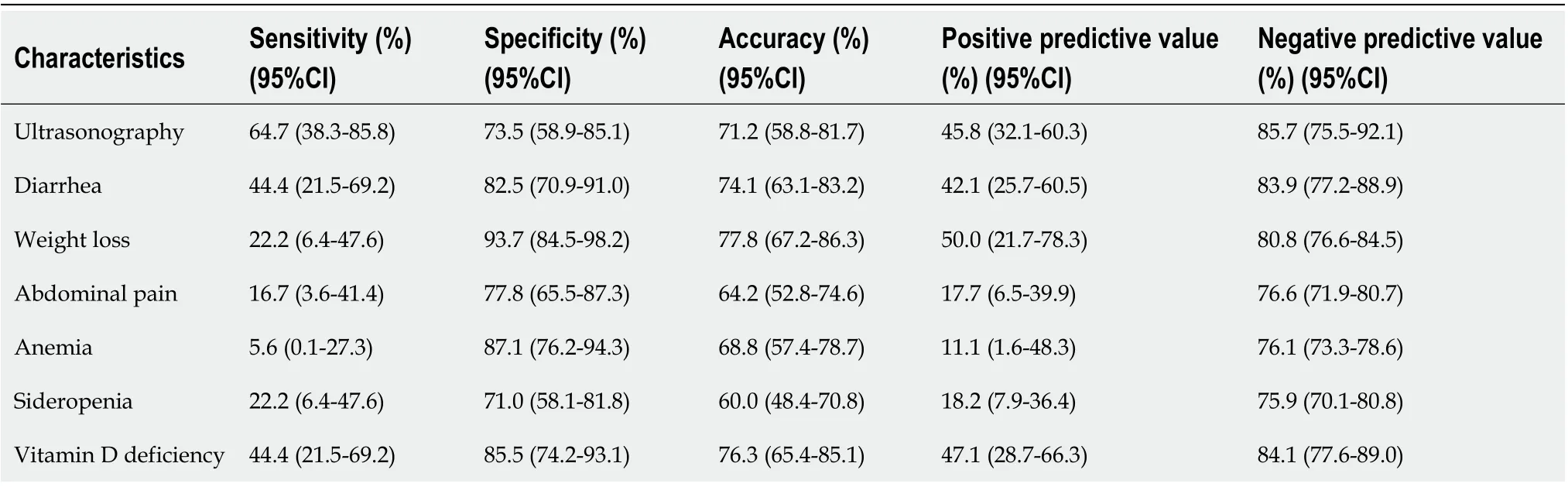
Table 6 Sensitivity, specificity, positive and negative predictive value of bowel ultrasonography, clinical and laboratory markers for prediction of villous atrophy in patients with celiac disease
ARTICLE HIGHLIGHTS

 World Journal of Gastroenterology2020年26期
World Journal of Gastroenterology2020年26期
- World Journal of Gastroenterology的其它文章
- Two-day enema antibiotic therapy for parasite eradication and resolution of symptoms
- Expression of Notch pathway components (Numb, Itch, and Siah-1) in colorectal tumors: A clinicopathological study
- Combining protein arginine methyltransferase inhibitor and anti-programmed death-ligand-1 inhibits pancreatic cancer progression
- Adipose-derived mesenchymal stem cells alleviate TNBS-induced colitis in rats by influencing intestinal epithelial cell regeneration, Wnt signaling, and T cell immunity
- Helicobacter pylori-induced inflammation masks the underlying presence of low-grade dysplasia on gastric lesions
- Intratumoral heterogeneity of hepatocellular carcinoma: From single-cell to population-based studies
