Hepatic microenvironment underlies fibrosis in chronic hepatitis B patients
Qun-Yan Yao, Ya-Dong Feng, Pei Han, Feng Yang, Guang-Qi Song
Abstract
Key words: Microenvironment; Liver fibrosis; Chronic hepatitis B; Human stellate cell; Interleukin-1β
INTRODUCTION
Chronic hepatitis B virus (HBV) infection is a leading cause of liver morbidity and mortality worldwide, primarily due to the complications of end-stage liver disease such as decompensated cirrhosis and hepatocellular carcinoma[1,2]. Continuous viral infection-associated inflammation and direct liver damage caused by viral components are accompanied by the transformation of hepatic stellate cells (HSCs) into activated myofibroblasts, fibroblast propagation, and deposition of extracellular matrix, resulting in fibrosis and in the most severe form, liver cirrhosis[3,4]. Staging hepatic fibrosis in chronic liver disease is a key indicator in judging the condition, deciding on the treatment strategy and evaluating the treatment effect. With the development of non-invasive approaches, especially imaging technology, the accuracy of the assessment of liver fibrosis has been improved[5-10]. Assessing fibrosis stage plays an important role in disease management and prognostication for individual HBV patients considering that the fibrosis degree and the time to progression to cirrhosis are heterogenous[10,11]. There is an absence of effective clinical treatments for liver fibrosis progression; thus, establishing a suitablein vitromicroenvironment in order to design novel therapeutics and identify molecular biomarkers to stratify patients is urgently required.
Due to the lack of HBV receptors in mice, thein vivostudy of HBV-induced liver fibrosis is time consuming and costly. For anin vitrostudy, neither cell lines nor 3D liver organoids can correctly simulate the patient's viral hepatitis internal environment. Recent studies have shown that various factors such as metabolic factors, growth factors, inflammatory factors, and microRNA all have an impact on the progression of fibrosis[10,12-17]. Therefore, determining the roles that the hepatic environment plays in patients will greatly facilitate the establishment of a suitablein vitroenvironment, which will represent specific pathogenic factors in hepatitis patients. Such microenvironment factors are important for developingin vitrofibrosis assays which can be used for identifying novel drug targets and drug screening. The hepatic microenvironment consists of hepatocytes, liver sinusoidal endothelial cells, resident Kupffer cells, HSCs, as well as infiltrating immune cells and a variety of secreted factors including growth factors, cytokines and chemokines, and extracellular matrix. Among the various non-parenchymal cells, HSCs play central roles in liver fibrosis following their activation into profibrotic myofibroblast-like cells in diseases such as chronic alcohol intake, hepatitis B and C, fatty liver disease, obesity and diabetes[18,19]. We set out to screen a subset of preselected microenvironment factors in chronic HBV patients with different degrees of fibrosis, and used HSCs to study how these factors interact to modulate fibrogenesis and progression of fibrosis in chronic hepatitis B patients.
MATERIALS AND METHODS
Patient samples
Human liver specimens were collected from patients who had undergone liver biopsy due to chronic hepatitis B from January 2016 to June 2017 at the Department of Gastroenterology, Zhongshan Hospital, Fudan University (Shanghai, China). The tissues were fixed in formalin and embedded in paraffin for hematoxylin-eosin and silver impregnation staining as described previously[10]. The scores for inflammation (recorded as G0-4) and fibrosis (recorded as S0-4) were examined in a blinded fashion by an experienced gastrointestinal pathologist. The clinicopathologic characteristics of all samples are shown in Table 1. This study was approved by the Clinical Research Ethics Committee of Zhongshan Hospital Fudan University, and written informed consent was obtained from each patient.
RNA extraction
Total RNA was extracted from liver tissue embedded in paraffin using the RNeasy FFPE Kit (Qiagen, Germany) according to the manufacturer’s protocol. Briefly after paraffin was removed from the samples, they were treated with lysis buffer containing proteinase K for 15 min. After lysis, the samples were incubated at 80ºC for another 15 min to reverse formalin crosslinking. Genomic DNA was then removed using DNase and DNase Booster Buffer. Finally, concentrated RNA was purified using RNeasy MinElute spin columns.
LX-2 cell culture and treatment
LX-2 cells (Merck, Germany) were cultured following the manufacturer’s instructions. Briefly, the cells were thawed in high glucose DMEM supplements with 10% HI-FBS (Gibco, heat inactivated, Australia). Treatment was conducted in the same medium. The cells were treated with cytokines or growth factors for 16 h or 48 h and then collected for subsequent analysis. Human recombinant cytokines or growth factors used in this study were from R&D System as follows: Transforming growth factor (TGF)-β1, epidermal growth factor (EGF), fibroblast growth factor (FGF) (1:1 mixture of FGF1 and FGF2), platelet-derived growth factor (PDGF), interleukin (IL)-1β and tumor necrosis factor (TNF)-α. The final concentration used for all growth factors and cytokines was 10 ng/mL.
Reverse transcription–quantitative PCR analysis
Reverse transcription–quantitative PCR (RT–qPCR) analyses were performed using PrimeScriptTMRT Master Mix (TaKaRa) and THUNDERBIRDTM SYBR qPCR Mix (TOYOBO) kits following the manufacturers’ instructions. Well-known fibrosis marker genes, including fibroblast activation protein (FAP), αlpha-smooth muscle actin(α-SMA), tissue inhibitor of metalloproteinase 1 (TIMP1), collagen type 1 alpha (COL1A) and toll-like receptor (TLR) family genes were chosen to assess the degree of fibrosis.EGF, TGF-β1, PDGF, connective tissue growth factor (CTGF) andFGFfamily genes were chosen to assess the expression of growth factors.IL-1β,IL-6, TNF-α, andNod-like receptor protein 3 (NLRP3) were chosen to assess the expression of inflammatory factors. The primer sequences are listed in Supplementary Table 1. RTqPCR reactions were performed with the ABI thermal cycler, and the primer sets were determined to be quantitative. Threshold cycles and melting curve measurements were performed using software.Pvalues were calculated by the Student-ttest.
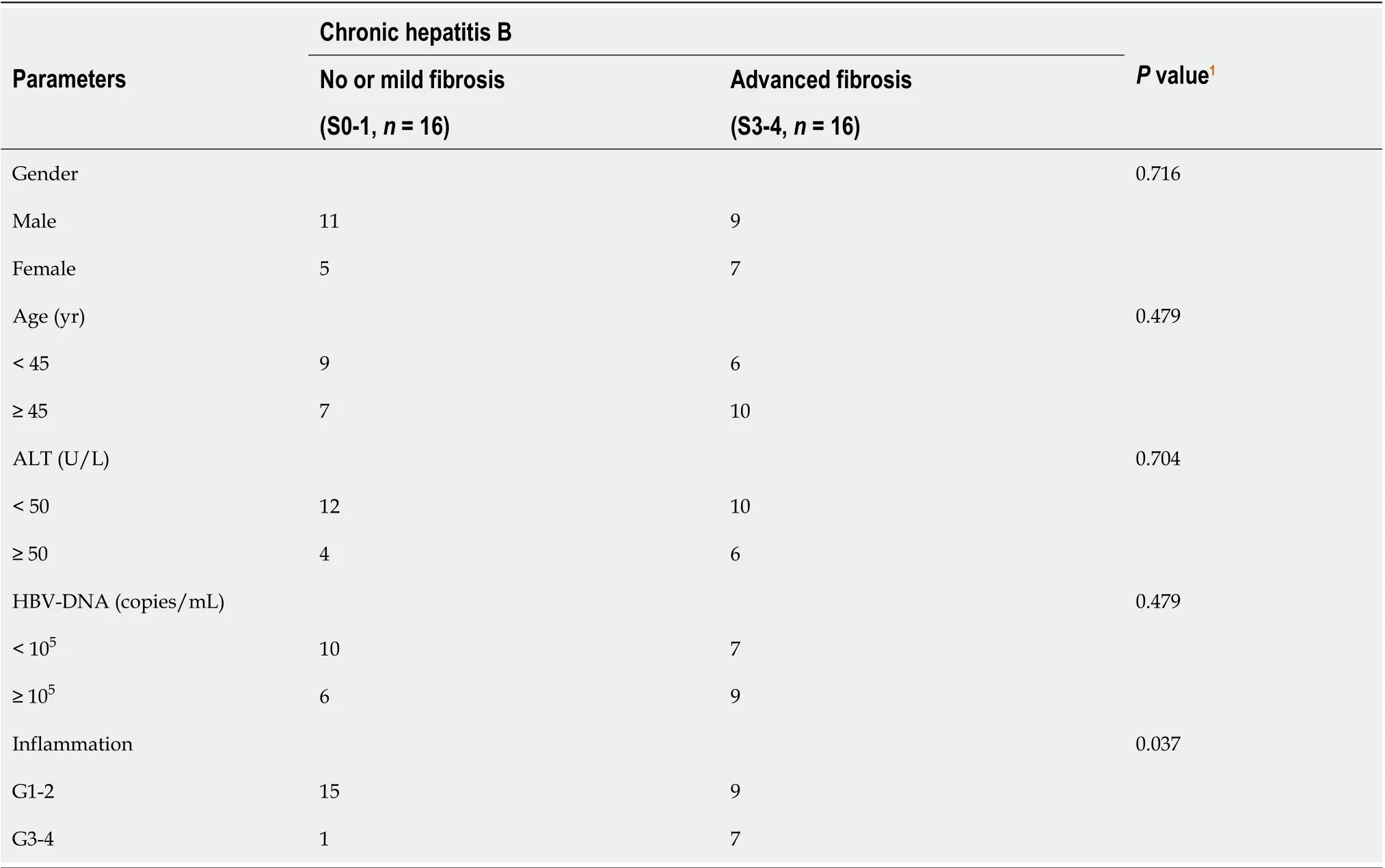
Table 1 Clinicopathological features of the chronic hepatitis B patients included in this study
Statistical analysis
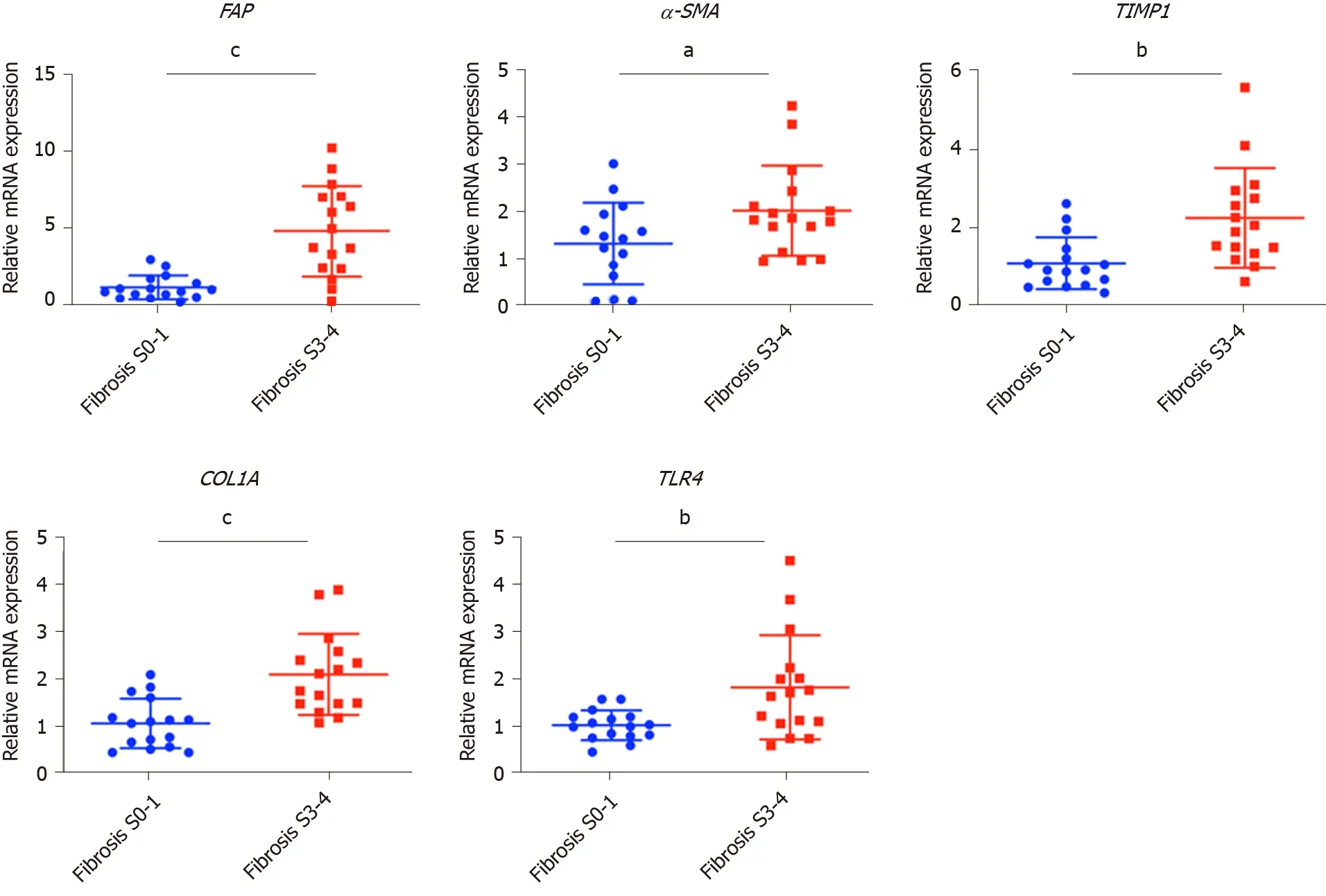
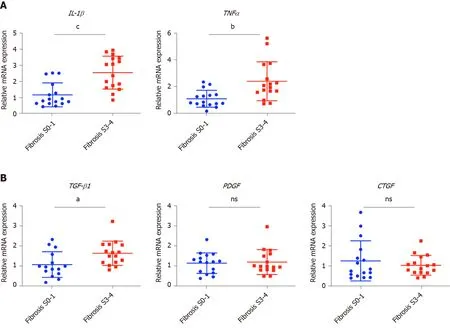
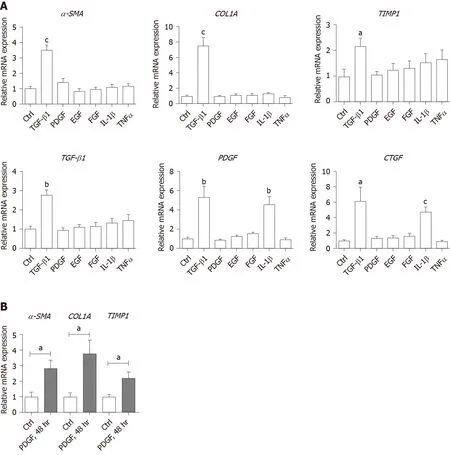
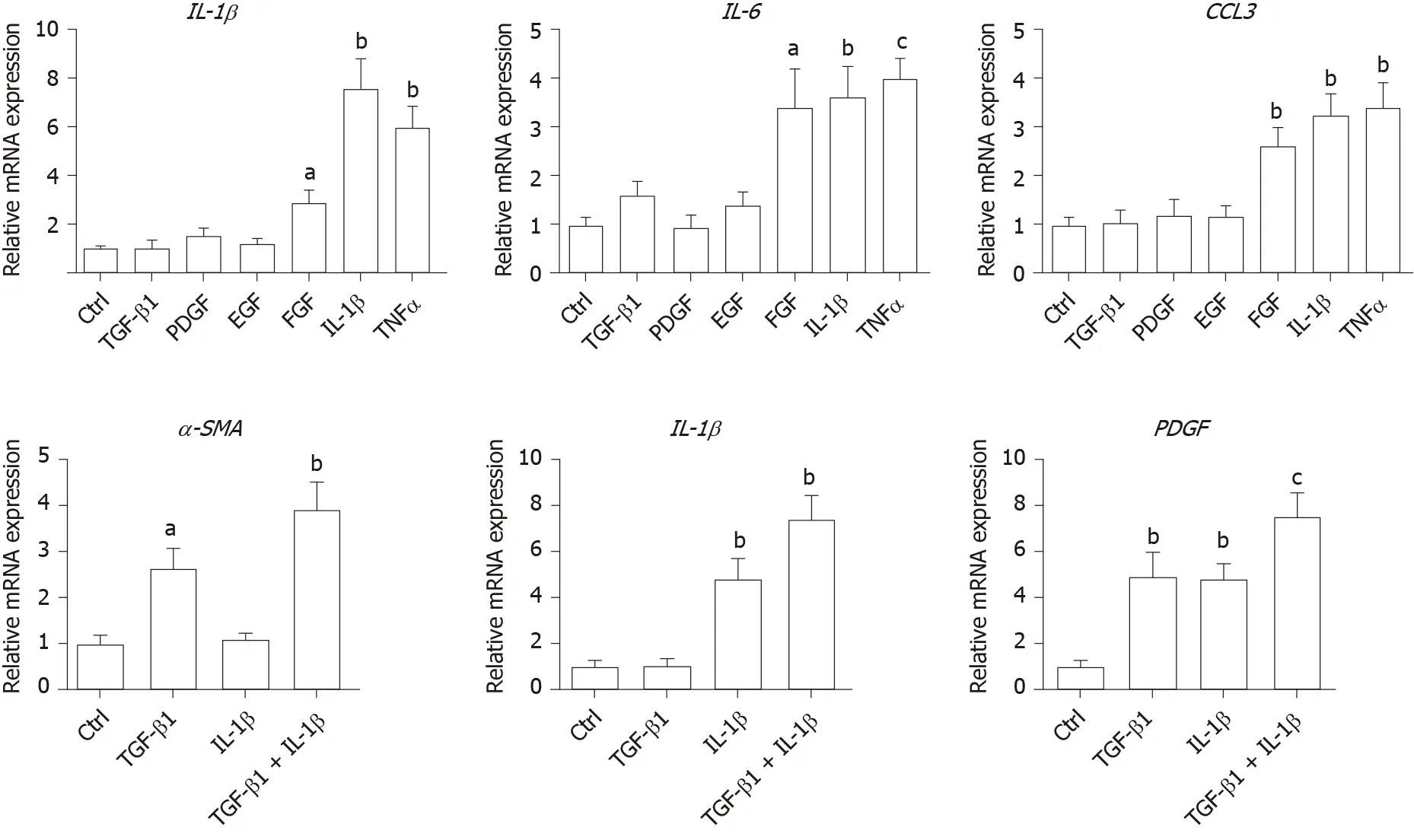
Values presented are expressed as the means ± SE. For all thein vitrostudies, statistical comparisons were performed using the non-parametric Mann–Whitney–Wilcoxon two-tail test. To compare the human clinical characteristics (Table 1), the Chi-square test was performed.P< 0.05 was considered significant.a: 0.01 To examine the hepatic microenvironment underlying fibrosis, a group of 32 chronic HBV patients who had not received antiviral treatment were selected. Liver biopsy was conducted in these patients to determine if they met the criteria for antiviral treatment. Fibrosis stage was assessed by experienced pathologists using silver impregnation staining, and the fibrotic tissue area greatly increased as fibrosis progressed[10,20,21](Supplementary Figure 1). To pinpoint which hepatic microenvironment factors are critical for fibrosis in chronic HBV patients, the patients were divided into two groups according to fibrosis stage: no or mild fibrosis (S0-1), and advanced fibrosis (S3-4). The patients in both groups showed comparable demographics, including gender and age (P= 0.465 andP= 0.288, respectively, Table 1). Liver function in the two groups of patients was also comparable indicated by alanine aminotransferase (ALT) level (P= 0.446, Table 1). Interestingly, inflammation level in the two groups of patients showed a strong trend towards a positive correlation (χ2= 4.167,P= 0.041)(Supplementary Figure 1 and Table 1), indicating a more pro-inflammatory microenvironment in patients with advanced fibrosis although their viral parameters and other clinical characteristics were comparable. Fibrosis in chronic HBV patients involved various extracellular factors, including growth factors, cytokines and other factors, altogether laying the foundation of the pathological microenvironment. To identify the most critical components, quantitative gene expression of these factors in the HBV patients’ liver samples was carried out (Table 1). As expected, the gene expression ofFAP, a-SMA, TIMP1andCOL1A1, markers of fibrosis and extracellular matrix deposition[22,23], were up-regulated in patients with advanced fibrosis (Figure 1).TLR4was significantly up-regulated and further confirmed that several pathways related to fibrosis, were more active in advanced fibrosis patients (Figure 1)[24], while otherTLRsshowed comparable levels in the two groups of patients (Supplementary Figure 2). 统计分析研究中相关数据。对呈均数标准差“±s”表现的计量数据,检验采取独立配对t,当数据检验显示P<0.05时,说明数据比较存在统计学意义。 To confirm the clinicopathological results, we quantitated the expression levels of inflammatory factors, includingIL-1β, IL6, NLRP3andTNF-α, which contribute to fibrosis directly or indirectly[25-31]. Of the cytokines profiled,IL-1βandTNF-α, but notIL6andNLRP3were up-regulated in patients with advanced fibrosis, correlating with the more severe inflammation found in these patients (Figure 2A, Table 1 and Supplementary Figure 3A). This indicated that IL-1β and TNF-α may progress fibrosis in HBV patients. With regard to the growth factors, we quantitated the gene expression ofTGF-β1, EGF, FGF1, FGF2, FGF7, PDGFandCTGF. OnlyTGF-β1showed increased expression in patients with advanced fibrosis (Figure 2B), suggesting its critical function in activated myofibroblasts[32]. TheFGFsshowed comparable levels in the two groups of patients indicating that they were not critical factors in the progression of fibrosis (Supplementary Figure 3B). Interestingly,PDGFwhich can cause liver fibrosis independent of theTGF-βpathway, andCTGFaTGF-βdownstream modulator which can amplify profibrogenic action, were not increased in patients with advanced fibrosis (Figure 2B)[33,34]. Altogether,TGF-β1, IL-1βandTNF-αwere identified as critical components of the hepatic microenvironment and may underlie the advancement of fibrosis in HBV patients. In order to verify whether growth factors and inflammatory factors can be used to simulate the hepatic microenvironment of HBV hepatitis patientsin vitro, LX-2 an immortalized human HSCs cell line was used to study how various microenvironment factors impact fibrogenesis and propagate the pathologic process. Four growth factors and two inflammatory factors, including TGF-β1, PDGF, EGF, FGF, IL-1β, and TNF-α, were selected for thein vitrostudy. Of these factors, consistent with the wellestablished function of TGF-β1 in activating HSCs, TGF-β1 increased the expression ofα-SMA, COL1A1, andTIMP116 h post-treatment (Figure 3A). TGF-β1 also induced the expression of growth factors including itself,PDGFandCTGF. IL-1β and TNF-α, both up-regulated in HBV patients with advanced fibrosis had no impact on activating HSCs (Figure 3A). Interestingly, IL-1β but not TNF-α treatment led to up-regulation ofPDGFandCTGF, suggesting its potential impact on modulating fibrosis (Figure 3A). It is noteworthy that at 48 h, but not 16 h post-treatment, PDGF also led to moderate induction ofα-SMA, COL1A1, andTIMP1(Figure 3B), suggesting that IL-1β can potentially activate LX-2 cells by upregulating these factors although in a delayed manner. As inflammation is a key player during fibrosis in HBV patients, we determined the gene expression of a few cytokine factors following treatment with the aforementioned factors. Although most growth factors generally had a minimum impact, FGF as well as IL-1β and TNF-α, increased gene expression of the cytokine factors (Figure 4A). These data suggest that not only HSCs can respond to cytokines by inducing PDGF and CTGF to activate HSCs, but they can also propagate the inflammatory process. Such crosstalk between the profibrotic and proinflammatory pathways plays important roles in regulating fibrosis. We then treated LX-2 cells with IL-1β combined with TGF-β1, and found that this treatment inducedα-SMA, IL-1βas well asPDGF, suggesting both fibroblast activation and the proinflammatory response (Figure 4B). Although PDGF induction occurs in a synergistic manner, no statistical significance was noted between either monotreatment or combination treatment (Figure 4B). Taken together, our data suggest that as a central player, HSCs mediate fibrosis by responding to various microenvironment factors and exert pleiotropic functions including not only activating hepatic fibrosis but also propagating the proinflammatory response. Figure 1 Profiling of fibrosis marker genes in liver samples from patients with hepatitis B virus infection. qPCR of fibrosis marker genes in hepatitis B virus patients with advanced fibrosis (S3-4, n = 16) compared to patients with less fibrosis (S0-1, n = 16). Values presented are expressed as the means ± SE; Statistical comparisons were made using the Mann–Whitney–Wilcoxon test; P value < 0.05 was considered significant; aP < 0.05; bP < 0.01; cP < 0.001. FAP: Fibroblast activation protein; α-SMA: Alpha-smooth muscle actin; TIMP1: Tissue inhibitor of metalloproteinase 1; COL1A1: Collagen type 1 alpha 1 chain; TLR4: Toll-like receptor 4. Our findings indicate that the inflammatory factor IL-1β is a central player in fibrogenesis and progression of fibrosis in chronic hepatitis B patients. IL-1β may activate HSCsviaPDGF, and synergize with TGF-β1 in the progression of fibrosis. To establish a suitablein vitromicroenvironment for HBV-induced liver fibrosis, not only TGF-β1 but also IL-1β should be considered as a necessary environmental factor. In our previous study, we demonstratedin vivothat the hepatic microenvironment strongly supported hepatic trans-programming, suggesting the importance of the microenvironment in determining cell fate[35]. For chronic HBV patients, the hepatic microenvironment consists of a variety of resident and infiltrating host immune cells, secreted factors and extracellular matrix proteins. How the microenvironment in HBV patients contributes to liver fibrosis is not well characterized. Currently, an effective clinical therapy to suppress the progression of liver fibrosis is still unavailable. 3D organoid technology, which will provide an effective tool for fundamental mechanism research and drug screening[36], still lacks a suitable culture system to simulate the patient's viral hepatitis internal environment. Hence, the development of novel drugs to treat fibrosis urgently requires accuratein vitromodels. Identifying critical microenvironment factors and determining how these factors interact with each other to modulate fibrosis is important in order to design novel therapeutics and identify molecular biomarkers to stratify patients. Figure 2 Profiling of inflammatory factors and growth factors in liver samples from patients with hepatitis B virus infection. A: qPCR of inflammatory factors; and B: Growth factors in hepatitis B virus patients with advanced fibrosis (S3-4, n = 16) compared to patients with less fibrosis (S0-1, n=16). Values presented are expressed as the means ± SE; P value < 0.05 was considered significant; aP < 0.05; bP < 0.01; cP < 0.001; ns: Non-significant. IL-1β: Interleukin 1β; TNF-α: Tumor necrosis factor alpha; TGF-β1: Transforming growth factor β1; PDGF: Platelet-derived growth factor; CTGF: Connective tissue growth factor. Among the secreted factors we profiled,TGF-β1, IL-1βandTNF-αwere increased in patients with advanced fibrosis. TGF-β1 is a well-known fibrosis activating factor, and our results confirmed this. Interestingly, compared with previous studies[12,14], we found that IL-1β, but not IL-6, NLRP3, or TNF-α is a central player in fibrogenesis and the progression of fibrosis in chronic HBV patients. Moreover, our results showed that the expression of PDGF, a known HSCs activation factor[15,16], did not increase with progression of fibrosis. When human stellate cells (LX-2) were treated with cytokinesin vitro, we found that either IL-1β or TGF-β1 induced the expression ofPDGF. These results indicated that IL-1β may play a critical role in the progression of fibrosis in chronic HBV patients. IL-1β, a dominant IL-1 secreted isoform produced by various cells including fibroblasts and myeloid-originated immune cells play key roles in both acute and chronic inflammation[37,38]. Circulating IL-1β is elevated in patients with chronic liver diseases, including alcoholic liver disease, chronic hepatitis B and C, and primary biliary cirrhosis[39-42]. Our study further demonstrated that in HBV patients with advanced fibrosis, elevated intrahepatic IL-1β expression may mediate inflammation and tissue damage, and propagate the profibrotic cascade. In fact, consistent with our data, in another independent study, HSCs were shown to respond to IL-1β by inducingIL-1β,IL1-Ra, andMMP-9[43]. However, it is unclear whether IL-1β is a driver or a consequence of fibrosis, and whether elevated IL-1β may have protective effects in liver regeneration given the fact that abolishing IL-1Ra encoding the antagonist of the IL-1 receptor delays regeneration after partial hepatectomy[44]. In order to further reveal whether this phenomenon is caused by HBV infection, it is necessary to further analyze samples from non-HBV patients and chronic HBV patients receiving antiviral treatments in future studies. Figure 3 Activation of hepatic stellate cells with growth factors and inflammatory factors. A: qPCR of fibroblast activation genes in LX-2 cells treated with various growth factors or cytokines for 16 h; B: qPCR of fibroblast activation genes in LX-2 cells treated with platelet-derived growth factor for 48 h. Values presented are expressed as the means ± SE; P value < 0.05 was considered significant; aP < 0.05; bP < 0.01; cP < 0.001. α-SMA: Alpha-smooth muscle actin; COL1A1: Collagen type 1 alpha 1 chain; TIMP1: Tissue inhibitor of metalloproteinase 1; TGF-β1: Transforming growth factor β1; PDGF: Platelet-derived growth factor; CTGF: Connective tissue growth factor. In the current study, HBV patients with advanced fibrosis also showed a high degree of inflammation despite viral parameters among these patients being comparable (Table 1). Therefore, the microenvironment in hepatitis patients provides the possibility for cross-talk between profibrotic and proinflammatory signals[45]. Interestingly, the proinflammatory response can be elicited in the “fibroblast” cell line LX-2 following treatment with IL-1β and TNF-α, providing a positive feedback loop to exacerbate inflammation. Finally, by combining IL-1β and TGF-β1, we observed not only fibroblast activation but also the inflammatory response, which may serve as a model to determine further aspects of the pathogenesis of liver fibrosis in HBV patients. In summary, our findings suggest that IL-1β is a central player in the hepatic microenvironment of viral hepatitis patients and plays a critical role in modulating fibrosis and the cross-talk between profibrotic and proinflammatory stimuli, and may converge on stellate cells to propagate these key pathologic events. Figure 4 Hepatic stellate cells may serve as a proinflammatory source. A: qPCR inflammation signature genes in LX-2 cells treated with various growth factors or cytokines; B: qPCR of alpha-smooth muscle actin, interleukin 1β and platelet-derived growth factor gene levels in LX-2 cells treated with transforming growth factor β1 combined with interleukin 1β. Values presented are expressed as the means ± SE; P value < 0.05 was considered significant; aP < 0.05; bP < 0.01; cP < 0.001. IL-1β: Interleukin 1β; IL6: Interleukin 6; CCL3: C-C motif chemokine ligand 3; α-SMA: Alpha-smooth muscle actin; PDGF: Platelet-derived growth factor. Chronic hepatitis B virus (HBV) infection is a leading cause of liver morbidity and mortality worldwide. Liver fibrosis resulting from viral infection-associated inflammation and direct liver damage plays an important role in disease management and prognostication. The mechanisms underlying the contribution of the liver microenvironment to fibrosis in HBV patients are not fully understood. There is an absence of effective clinical treatments for liver fibrosis progression; therefore, establishing a suitablein vitromicroenvironment is urgently required in order to design novel therapeutics and identify molecular biomarkers to stratify patients. In this study, we set out to screen a subset of preselected microenvironment factors, including growth and inflammatory factors, in chronic HBV patients with different degrees of fibrosis. In addition, hepatic stellate cells (HSCs) were used to study how these factors interact to modulate fibrogenesis and progression of fibrosis in chronic hepatitis B patients. We examined the gene expression of key microenvironment factors using liver samples from patients with more advanced fibrosis compared to those with less severe fibrosis. We also carried out anin vitrostudy using the human stellate cell line LX-2. Different recombinant cytokines and growth factors or their combination were used to study how these factors interacted with LX-2 cells and to pinpoint the cross-talk between the aforementioned factors and screen the most important factors. Of the secreted factors examined, transforming growth factor (TGF)-β1, interleukin-1β and tumor necrosis factor (TNF)-α were increased in patients with advanced fibrosis. We found that besides TGF-β1, IL-1β can also induce a profibrotic cascade by stimulating the expression of connective tissue growth factor and platelet-derived growth factor (PDGF) in LX-2 cells. Furthermore, the proinflammatory response can be elicited in LX-2 cells during treatment with IL-1β and TNF-α, suggesting that stellate cells can respond to proinflammatory stimuli. When IL-1β and TGF-β1 were combined, we observed not only fibroblast activation as shown by α-SMA and PDGF induction, but also the inflammatory response as shown by increased expression of IL-1β. Collectively, our data from HBV patients andin vitrostudies demonstrate that the hepatic microenvironment plays an important role in mediating the crosstalk between profibrotic and proinflammatory responses and modulating fibrosis in chronic HBV patients. Our findings indicate that the inflammatory factor IL-1β is a central player in fibrogenesis and progression of fibrosis in chronic hepatitis B patients. IL-1β may activate HSCsviaPDGF, and synergize with TGF-β1 in fibrosis progression. To establish a suitablein vitromicroenvironment for HBV-induced liver fibrosis, not only TGF-β1 but also IL-1β should be considered as a necessary environmental factor. Our study demonstrated that the hepatic microenvironment involves crosstalk between profibrotic and proinflammatory factors in hepatitis patients and underlies fibrosis. The treatment of stellate cells with IL-1β combined with TGF-β1 may serve as anin vitromodel for fibrotic HBV infected patients and can reflect the liver microenvironment. We acknowledge Yan-Ting Zou and Shu-Yu Li for their excellent laboratory assistance.RESULTS
HBV patients with advanced fibrosis show more inflammation
Inflammatory factors and TGF-β are up-regulated in patients with chronic HBV infection and advanced fibrosis
IL-1β can potentially elicit the profibrotic cascade in HSCs
HSCs can serve as a pro-inflammatory response
DISCUSSION
ARTICLE HIGHLIGHTS
Research background
Research motivation
Research objectives
Research methods
Research results
Research conclusions
Research perspectives
ACKNOWLEDGEMENTS
 World Journal of Gastroenterology2020年27期
World Journal of Gastroenterology2020年27期
- World Journal of Gastroenterology的其它文章
- Histopathological landscape of rare oesophageal neoplasms
- Details determining the success in establishing a mouse orthotopic liver transplantation model
- Modified percutaneous transhepatic papillary balloon dilation for patients with refractory hepatolithiasis
- Serum ceruloplasmin can predict liver fibrosis in hepatitis B virusinfected patients
- Acceptance on colorectal cancer screening upper age limit in South Korea
- Transarterial chemoembolization with hepatic arterial infusion chemotherapy plus S-1 for hepatocellular carcinoma
