Effects of Fuke Qianjin Formula on hormones and their receptors and metabonomics study in uterine fibroids model rats
LI Ymei, TANG Jie, LUO Hongshn, XIA Bohou, LIN Limei, LIAO Dunfng*
a.College of Pharmacy, Hunan University of Chinese Medicine, Changsha, Hunan 410208, China
b.Key Laboratory for Quality Evaluation of Bulk Herbs of Hunan Province, Changsha, Hunan 410208, China
Keywords Fuke Qianjin Formula (妇科千金方, FKQJF)Uterine leiomyoma Grease Gas chromatography-mass spectrometry (GCMS)Metabolomics Estrogen Progesterone Estrogen receptor Progesterone receptor
ABSTRACT Objective To investigate the effects of different fractions from Fuke Qianjin Formula(妇科千金方,FKQJF)on uterine leiomyoma (UL) to determine the best fraction.Methods FKQJF was extracted and isolated to obtain polysaccharides (FKP), flavonoids (FKF), and grease (FKG).140 female SPF SD rats were divided into 14 groups [model (MOD), normal control (NC), Gouliuqing (GLQ), Mifepristone (MFST), FKQJF,low, medium, and high dose of polysaccharides (l-FKP, m-FKP,and h-FKP), low, medium, and high dose of flavonoids (l-FKF,m-FKF, and h-FKF), low, medium, and high dose of grease(l-FKG, m-FKG, and h-FKG)], and uterine fibroids model rats were treated with drugs for four weeks.Serum levels of estrogen and progesterone were measured using enzyme-linked immunoassay assay (ELISA) kits.The expression of estrogen receptor(ER-α, ER-β) and progesterone receptor (PR) in the uterus was observed using immunohistochemistry (IHC).Serum metabolite profiles and FKG were analyzed using gas chromatography-mass spectrometry (GC-MS).Results FKQJF, h-FKF, m-FKG, and h-FKG significantly downregulated the estrogen level in the uterine fibroid model rats(P < 0.01).FKQJF, h-FKF, and h-FKG significantly reduced the level of progesterone in the uterine fibroid model rats (P < 0.01).The levels of ER-α, ER-β, and PR in uterine fibroid model rats were significantly decreased by FKQJF and h-FKG (P < 0.01).The levels of ER-α, ER-β, and PR in the fibroid model rats were decreased by m-FKG (P < 0.05).Additionally, serum metabolism results revealed that h-FKG and FKQJF could regulate related endogenous metabolites and make the pathological indices of uterine fibroids in rats close to the normal group.Forty-six components were identified in the oil, accounting for 91.97% of the total oil components.Conclusion FKQJF and h-FKG showed a significant anti-myoma activity and significantly improved the pathological state of the uterus in rats with hysteromyoma.The mechanism of action may be related to the regulation of estrogen progesterone and its receptor in uterine fibroid model animals.These findings proved the effect of FKQJF on uterine leiomyoma and provided an experimental basis for its clinical research and application.
1 Introduction
Uterine leiomyomas (ULs) are the most common benign tumors in the female reproductive system and are monoclonal tumors that arise from the excessive proliferation of uterine smooth muscle cells[1].It has an estimated incidence of up to 75% in women of reproductive age; however, only 20% – 30% of these cases are symptomatic[2].Symptoms associated with these benign tumors include irregular vaginal bleeding, menorrhagia, abdominal mass, dysmenorrhea,abnormal leucorrhea, anemia, and other clinical symptoms[3].The specific pathogenesis of UL remains unclear.At present, it is known that high levels of estrogen and progesterone can promote the formation and development of uterine fibroids[4,5].The main treatments for UL are surgical operation and hormone therapy; however, surgical treatment and hormone therapy can easily cause female endocrine disorders, accelerated aging, and increase the incidence of breast diseases and endometrial cancer[6,7].Therefore, it is of great clinical significance to identify safe and effective drugs for treating uterine fibroids.
Traditional Chinese medicine (TCM) is commonly used as an alternative to surgical treatment and hormone therapy.Fuke Qianjin Formula (妇科千金方,FKQJF) is a TCM protection variety in China, which has been selected and integrated by a series of folk prescriptions.It has been included in theChinese Pharmacopoeia2020 edition.FKQJF consists of Qianjinba (Moghania macrophylla), Jinyinggen (Rosa Laevigata Radix),Chuanxinlian(Andrographis Herba),Danmianzhen(Zanthoxylum dissitumHemsl.), Gonglaomu (Mahoniae Caulis), Jixueteng(Spatihlobi Caulis), Danggui (Angelicae Sinensis Radix), and Dangshen (Codonopsis Radix).FKQJF functions to clear heat and remove dampness, benefit Qi, and remove blood stasis and is mainly used for treating abnormal leukorrhea and abdominal pain caused by heat stagnation.It is one of the main TCMs used to treat chronic pelvic inflammatory disease,endometritis, and chronic cervicitis[8].
Modern pharmacological studies have shown that many components of FKQJF have many antitumor activities[9-14].This study explored the pharmacological effect of FKQJF on uterine fibroids and determined its effective fractions in UL model rats, the results of which would provide an experimental basis for further research on FKQJF.
2 Materials and methods
2.1 Drugs
Extract of FKQJF(20170228)was provided by Zhuzhou Qianjin Pharmaceutical Co., Ltd..
2.2 Reagents
Progesterone injections (161001, Zhejiang Xianju Pharmaceutical Co., Ltd., Taizhou, China), estradiol benzoate injections (20161201, Sichuan Jinke Pharmaceutical Co., Ltd., Chengdu, China), Gongliuqing Capsules (161201, Sichuan Shenghe Pharmaceutical Co.,Ltd.,Chengdu,China),chloral hydrate(20161023, Sinopharm Chemical Reagent Co., Ltd.,Shanghai, China), and mifepristone (12203764378,Zhejiang Xianju Pharmaceutical Co., Ltd., Taizhou,China) were purchased.Estrogen receptor alpha (bs-0725R), estrogen receptor beta (bs-0116R), and progesterone receptor (bsm-33169M) were purchased from Bioss Biotechnology Co., Ltd.(Wuhan, China).Progesterone kits (AK0017OCT30014/96T) and estradiol kits (AK0017OCT27013/96T) were purchased from Elabscience Biotechnology Co., Ltd.(Wuhan,China).Methoxyamine(BCBP2843V),pyridine(STBF7305V), and BSTFA (BCBJ4275V) were purchased from Sigma (USA).Malic acid (20170127,Shanghai Source Leaf Organisms Technology Co.,Ltd., Shanghai, China), high-purity helium (Guangdong Huate Gas Co., Ltd., Foshan, China), ethyl acetate (20160227, Tianjin Fushi Fine Chemical Co., Ltd.,Tianjin, China), sodium chloride (161203, Xiqiao Chemical Co., Ltd., Shanghai, China ), n-hexane(20170509, Guangdong Guanghua Technology Co.,Ltd., Shantou, China), anhydrous sodium sulfate(20170418, Jiangsu Qiangsheng Functional Chemical Co., Ltd., Suzhou, China), BF3-methanol (B822337,Shanghai Maclean Biochemical Technology Co., Ltd.,Shanghai,China),and sulfuric acid(20120922,Tianjin Chemical Reagent Research Institute, Tianjin,China) were purchased.
2.3 Preparation of different fractions of FKQJF
Polysaccharides (FKP): 1 000 g FKQJF was defatted with ethanol and then extracted by refluxing with distilled water and removing the precipitate.The solutions were precipitated by adding ethanol, resulting in an alcohol content of 80% in the final solution and kept at 4 °C overnight.Thereafter, the samples were centrifuged at 4 000 rpm for 5 min (Beckman Coulter,USA), precipitates were collected (the supernatant was used to extract the flavonoids), and then a 1∶4 n-butanol/chloroform mixed solution was added to remove the protein.Finally, they were dried under a vacuum freeze-dryer (EYELA, Japan) for two days to obtain crude polysaccharides[15,16].
Flavonoids (FKF): the collected supernatant was extracted with petroleum ether and chloroform repeatedly, extracted with water saturated with n-butanol,and finally evaporated to obtain flavonoids[17].
Grease (FKG): a total of 1 000 g FKQJF was loaded into a supercritical CO2fluid extraction vessel (Hangzhou Huali Pump Co., Ltd., China).The parameters employed were as follows: time, 240 min; temperature of the extraction vessel, 40 °C; pressure, 25 MPa;temperature of separation vessel I, 35 °C; pressure of separation vessel I, 15 MPa; temperature of separation vessel II, 30 °C; and pressure of separation vessel II, 8 MPa[18,19].
2.4 Animal experiments
Seven-week-old female Specific Pathogen Free (SPF)grade Sprague-Dawley (SD) rats (180 – 220 g) were purchased from Hunan Slack Jingda Experimental Animal Co., Ltd.[Laboratory Animal Quality Certificate No.SCXK (Xiang) 2016-0002].The experimental unit used a license number SYXK (Xiang) 2013-0005.Rats were housed in an air-conditioned room at 21 –24 °C with 12 h of light.Rats had free access to food pellets and water throughout the study period.All animal experiments were conducted in accordance with the guidelines established by the Animal Experimental Ethics Committee of the Hunan University of Chinese Medicine and were approved by this committee (LLBH-201808050003).
A total of 140 rats were randomly divided into 14 groups (n=10 per group): the normal control (NC)group, model (MOD) group, Gongliuqing (GLQ)group, Mifepristone (MFST) group, FKQJF group,groups of low, medium, and high doses of polysaccharides (l-FKP, m-FKP, and h-FKP), groups of low,medium, and high doses of flavonoids (l-FKF, m-FKF, and h-FKF), and groups of low, medium, and high doses of grease (l-FKG, m-FKG, and h-FKG).Rats in the NC group were injected with normal saline (0.5 mL/d) through the lower limb lateral muscle every day for 8 w, whereas the other groups were administered intramuscular injections of progesterone(1 mg/kg) on Monday, Wednesday, and Friday, and injected with estradiol benzoate (2 mg/kg) on Tuesday, Thursday, and Saturday for 8 w[20].
Rats were orally administered from the 9th week for 4 w, and the dose of each group was as follows:GLQ, 0.3 g/kg; MFST, 5.4 mg/kg; FKQJF, 2.4 g/kg;l-FKP,0.38g/kg;m-FKP,0.76g/kg;h-FKP,1.52 g/kg; l-FKF, 0.22 g/kg; m-FKF, 0.44 g/kg; h-FKF,0.88 g/kg; l-FKG, 0.038 g/kg; m-FKG, 0.076 g/kg;h-FKG, 0.152 g/kg; and NC, 1 mL normal saline per rat.
During the experiment, eight rats died during the modeling process, eliminating outliers; therefore, the final number of rats in each group was eight.
At the end of treatment, rats were anesthetized with pentobarbital sodium (80 mg/kg), and blood was collected from the abdomen, centrifuged at 2 000 rpm for 10 min at 4 °C, and then stored at-80 °C.The uterus was removed, weighed, fixed in 4% neutral paraformaldehyde for 48 h, and sliced using an RM2016 pathology slicer (Leica, Germany).
2.5 Enzyme-linked immunosorbent assay (ELISA)detection of serum levels of estradiol (E2) and progesterone
To study the effects of FKQJF, FKP, FKF, and FKG on E2and progesterone, the concentrations of E2and progesterone in serum samples were determined using ELISA kits according to the manufacturer’s instructions using a Multiskan MK3 multifunction microplate reader (Thermo Company, USA).
2.6 Hematoxylin and eosin (HE) staining for observation of the pathological change in UL tissue
Uterine slices were stained with HE, followed by dewaxing, dyeing, dehydration, redyeing, dehydration, transparency, and sealing.After the gum was dried, rat uterus sections were observed and analyzed under the Nikon Eclipse optical microscope(Nikon, Japan).
2.7 Immunohistochemistry (IHC) staining for detection the expressions of ER-α, ER-β, and progesterone receptor (PR) in UL tissue
Uterine slices were routinely deparaffinized in xylene and passed through graded alcohols.Thereafter, they were incubated with 3% H2O2at room temperature for 10 min to eliminate endogenous peroxidase activity.Tissue slices were microwaved twice for 10 min in 10 mmol/L sodium citrate buffer to induce antigen retrieval, and 5% goat serum was used to block nonspecific epitopes.Slices were then incubated with the appropriate primary antibody (1∶200) overnight at 4 °C, washed thrice in 0.01 mol/L phosphate-buffered saline (PBS) for 5 min each time, combined with one drop of biotin-labeled secondary antibody,incubated at 37 °C for 30 min, and washed with PBS thrice for 5 min each time.Detection was achieved using 3,3'-diaminobenzidine substrate and counterstained with hematoxylin.One immunohistochemical slice was taken from each rat and magnified 20 times under a microscope, and five fields of view were randomly selected.Using Image-Pro-plus software, the integrated optical density (IOD) values of ER-α, ER-β,and PR were calculated, and the average IOD values were taken as the protein expression levels of ER-α,ER-β, and PR of the sections.
2.8 Determination of metabolites in rat serum by gas chromatography-mass spectrometry (GC-MS)
2.8.1 Serum samples pretreatmentThe serum samples were thawed at 4 °C for 1 h, and 50 μL of L-malic acid (1 mg/mL) as an internal standard was added to 100 μL serum sample and mixed using a vortex mixer.Thereafter, 450 μL of methanol was added to precipitate proteins.The samples were placed for 8 min and then centrifuged at 13 000 rpm for 15 min at 4 °C.The supernatant was transferred into a 2 mL GC-MS glass vial and evaporated to dryness under a gentle stream of nitrogen gas.Furthermore, 50 μL of methoxyamine-pyridine (20 mg/mL) was added into each residue and incubated for 1 h at 70 °C.Thereafter, 100 μL of N, O-Bistrifluoroacetamide (BSTFA)derivatization reagent was added to the aforementioned mixture, mixed for 30 s, and incubated for 1 h at 70 °C.Finally, the samples were centrifuged at 13 000 rpm for 15 min, and 100 μL of the supernatant was transferred to a 250 μL GC vial for GC-MS detection[21].
Quality control (QC) samples were prepared by mixing an equal volume of experimental samples for methodological investigation, including precision,repeatability, and stability.The processing of QC samples was the same as that used for the preparation of the samples mentioned above.Six QC samples were included in the analytical batch for GC-MS monitoring.
2.8.2 GC-MS procedureThe derivatized samples were separated on a nonpolar HP-5 column (30 m ×0.25 mm ×0.25 μm, Agilent, USA) of a GC-MS spectrometer (GC-MS-QP 2010, Shimadzu, Japan)using the following conditions: sampling volume,0.8 μL; carrier gas, helium (99.999%); flow rate,1.0 mL/min; and split ratio, 20∶1.The initial oven temperature was maintained at 60 °C for 5 min, and then increased to 130 °C at a rate of 20 °C/min, 190 °C at a rate of 8 °C/min, and 270 °C at a rate of 8 °C/min for 5 min.The inlet temperature was set to 270 °C.The temperature of the ion source was 200 °C, the solvent delay time was 6.5 min, and the scanning range of mass spectrometry was 50 – 800 m/z.During the formal analysis, the sampling sequence of all samples was randomly arranged to avoid the run order effect and systematic variation attributed to instrument-based analyses.One QC sample was analyzed after every eight sample injections.
Test results were imported into the soft independent modeling of class analogy (SIMCA 14.1) for partial least squares discriminant analysis (PLS-DA) and orthogonal projections to latent structures discriminant analysis (OPLS-DA) analyses.
Significant discriminant metabolites were selected based on the remarkable differences [variable importance for the projection (VIP) >1.2 andP< 0.05].
2.8.3 FKG component analysis(1) Pretreatment conditions of FKG: 10 mg FKG was accurately weighed into a 10 mL volumetric flask, diluted with ethyl acetate, and filtered through a 0.22 μm microporous membrane[22].(2) Gas Chromatographic (GC)conditions: column, DB-5 column (30 m ×0.25 mm ×0.25 μm); Agilent, USA; carrier gas, helium; injection volume, 1.0 μL; column flow, 1.0 mL/min; and inlet temperature, 270 °C.The programmed temperature conditions were as follows: the initial temperature was 70 °C, which was increased to 270 °C at 4 °C/min for 15 min.(3) Mass spectrometry (MS) conditions:ion source, EI; ion source temperature, 250 °C; ionization energy, 70 eV; interface temperature, 280 °C;scanning range, 35 – 800 m/z.
2.9 Statistical analysis
Data analysis was performed using SPSS 20.0 statistical software.The data are expressed as mean ±standard deviation (±s).One-way analysis of variance was used for comparisons between the groups.Statistical significance was set atP< 0.05.
3 Results
3.1 Effective fraction yields
The extraction yields of FKP, FKF, and FKG in FKQJF were 32.31%, 18.39%, and 3.03%, respectively.
3.2 Uterine coefficient (UC)
UC was significantly different between the MOD and NC groups (P< 0.01).In addition, UC was significantly different between MOD group and other groups of treated with MFST, FKQJF, l-FKF, m-FKF, h-FKF,l-FKG, m-FKG, h-FKG (P< 0.05), indicating that these drugs can inhibit the growth of UL.They all exhibited similar extents of inhibited UL growth (Figure 1).
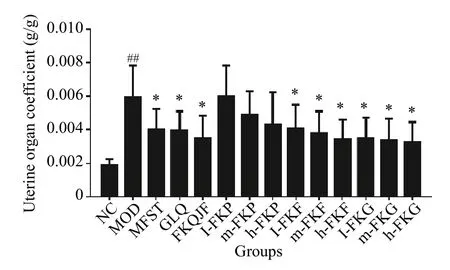
Figure 1 Determination results of the uterine coefficient in each group rats (±s, n =8)
3.3 Changes of the general uterus morphology
Uteruses of the NC group were uniform in texture,their color was bright red, and the bilateral uteruses were symmetrical and Y-shaped.No cysts, nodules,or swelling in the uteruses of the NC group rats were found.Compared with those of the NC group, evident swelling and masses were observed in uteruses of the MOD group, which were congested, with the phenomenon of hemorrhage, uterine folds and retention, and a large amount of thick secretions, and the volume and weight increased.After drug intervention, the uterine volume was smaller than that in the MOD group, and the cysts decreased or disappeared.In addition, the pathological morphology of the uteruses was effectively relieved in the MFST, GLQ,h-FKP, m-FKG, and h-FKG groups (Figure 2).
3.4 Pathologic changes in uterus tissue
HE staining of uterine tissues demonstrated that a thin uterine smooth muscle layer (SML) in NC group rats, slender smooth muscle cells (SMCs), and the inner and outer longitudinal muscles were arranged in orderly rows.The uterine SML of the MOD group rats was thicker than that of the NC group, and muscle cells were arranged randomly.Some uterine smooth muscle cells were elongated and deformed, and a large number of inflammatory cells appeared.This was accompanied by a large number of secretions in the cavity, and some uterine tissues lost the structure of the outer lining, muscle layer, and intima of normal uterine tissue.After drug intervention, the SML of the FKP group uteruses had changed slightly, and the SML in the GLQ, FKQJF, h-FKF, and h-FKG groups were very thin, SMCs were slender, the inner muscle and outer longitudinal muscle were arranged in orderly rows, and the nucleus of the fusiform or rod-shaped nucleolus was not clear (Figure 3).
3.5 Detection of E2 and progesterone in the serum of rats
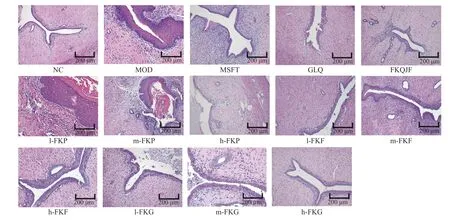
Figure 2 HE staining of the uterus in each group rats
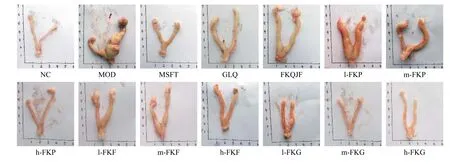
Figure 3 The uterus morphology in each group rats
Serum levels of E2and progesterone were compared among different groups (Table 1).E2and progesterone levels in the MOD group were significantly higher than those in the NC group (P< 0.01).After administration, the E2and progesterone levels in the GLQ, FKQJF, h-FKF, and h-FKG groups decreased significantly compared with those in the MOD group(P< 0.01).The E2levels in the MFST and m-FKG groups were lower than those in the MOD group (P< 0.01).ThePlevels in the MFST group were lower than those in the MOD group (P< 0.05).
3.6 Detection of ER-α, ER-β, and PR in uterus of SD rats
The expressions of ER-α, ER-β, and PR in the MOD group were significantly higher than those in the NC group (P< 0.01).After drug administration, the expressions of ER-α, ER-β, and PR in the MFST, GLQ,FKQJF, m-FKG, and h-FKG groups were significantly reduced compared to those in the MOD group (P<0.01).Among them, the FKQJF and h-FKG groups had better effects than the other groups.It was shown that drugs can play a therapeutic role by reducing the expressions of estrogen and progesterone receptors(Figure 4).
3.7 Comparison of metabolic differences between FKG and FKQJF groups
A clear boundary was observed between NC and MOD groups, indicating that endogenous metabolites in the serum of MOD rats had changed.After the intervention with FKQJF and FKG, the metabolic profile of the serum in the h-FKG and FKQJF groups was similar to that of the NC rats (Figure 5).QC samples had good repeatability, and this method was relatively stable.Therefore, the QC sample data are not included in this figure.
The data were analyzed using PLS-DA and OPLSDA methods.PLS-DA achieved maximum separation between samples by establishing a mathematical model between categories, and the interpretation ability parameter (R2Y) and prediction ability parameter (Q2) of the established model were 0.938 and 0.897, respectively, indicating that the model had good discrimination and prediction degrees.
Comparisons of the NC group with the MOD group, and the MOD group with the h-FKG group were separately subjected to OPLS-DA.The evaluation indexes of the NC group and the MOD group were R2Y =0.999 and Q2=0.953, whereas the evaluation indexes of the MOD and h-FKG groups were R2Y =0.997 and Q2=0.953, indicating that the model quality was good.
By comparison, we found that the amount of lysine, mannose, creatinine, and galactose declined in the MOD group, whereas they were upregulated byh-FKG and were close to the NC group.Additionally,the amount of valine, ribitol, and propanoic acid increased in the MOD group, whereas they were downregulated by h-FKG and were close to the NC group.
Table 1 Serum estrogen and progesterone levels in each group of rats ( ±s, n =8)
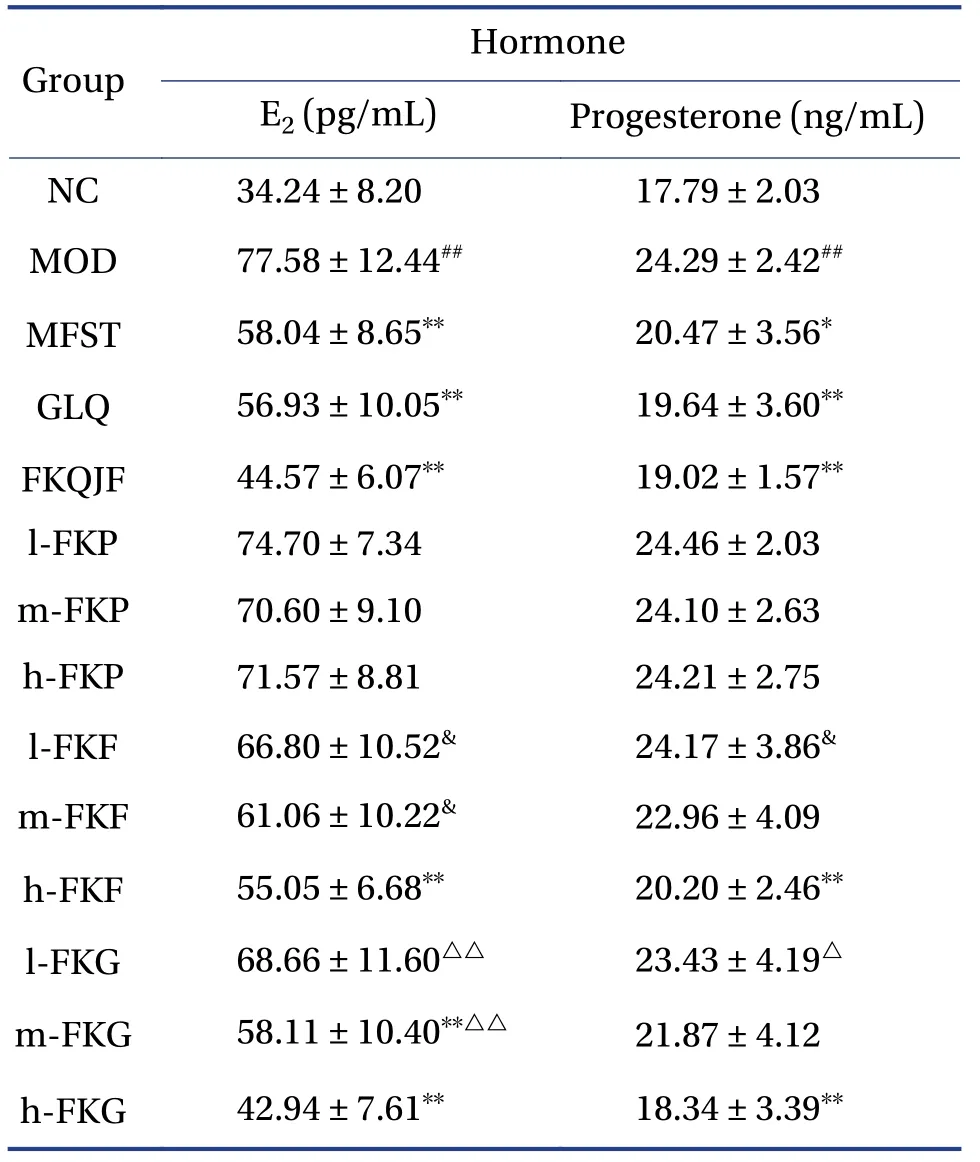
Table 1 Serum estrogen and progesterone levels in each group of rats ( ±s, n =8)
##P < 0.01, compared with the normal group; *P < 0.05 and**P < 0.01, compared with the MOD group; &P < 0.05,compared with the h-FKF group; △P < 0.05 and △△P < 0.01,compared with the h-FKG group.
Hormone Group E2 (pg/mL)Progesterone (ng/mL)NC 34.24 ± 8.20 17.79 ± 2.03 MOD 77.58 ± 12.44## 24.29 ± 2.42##MFST 58.04 ± 8.65** 20.47 ± 3.56*GLQ 56.93 ± 10.05** 19.64 ± 3.60**FKQJF 44.57 ± 6.07** 19.02 ± 1.57**l-FKP 74.70 ± 7.34 24.46 ± 2.03 m-FKP 70.60 ± 9.10 24.10 ± 2.63 h-FKP 71.57 ± 8.81 24.21 ± 2.75 l-FKF 66.80 ± 10.52& 24.17 ± 3.86&m-FKF 61.06 ± 10.22& 22.96 ± 4.09 h-FKF 55.05 ± 6.68** 20.20 ± 2.46**l-FKG 68.66 ± 11.60△△ 23.43 ± 4.19△m-FKG 58.11 ± 10.40**△△ 21.87 ± 4.12 h-FKG 42.94 ± 7.61** 18.34 ± 3.39**
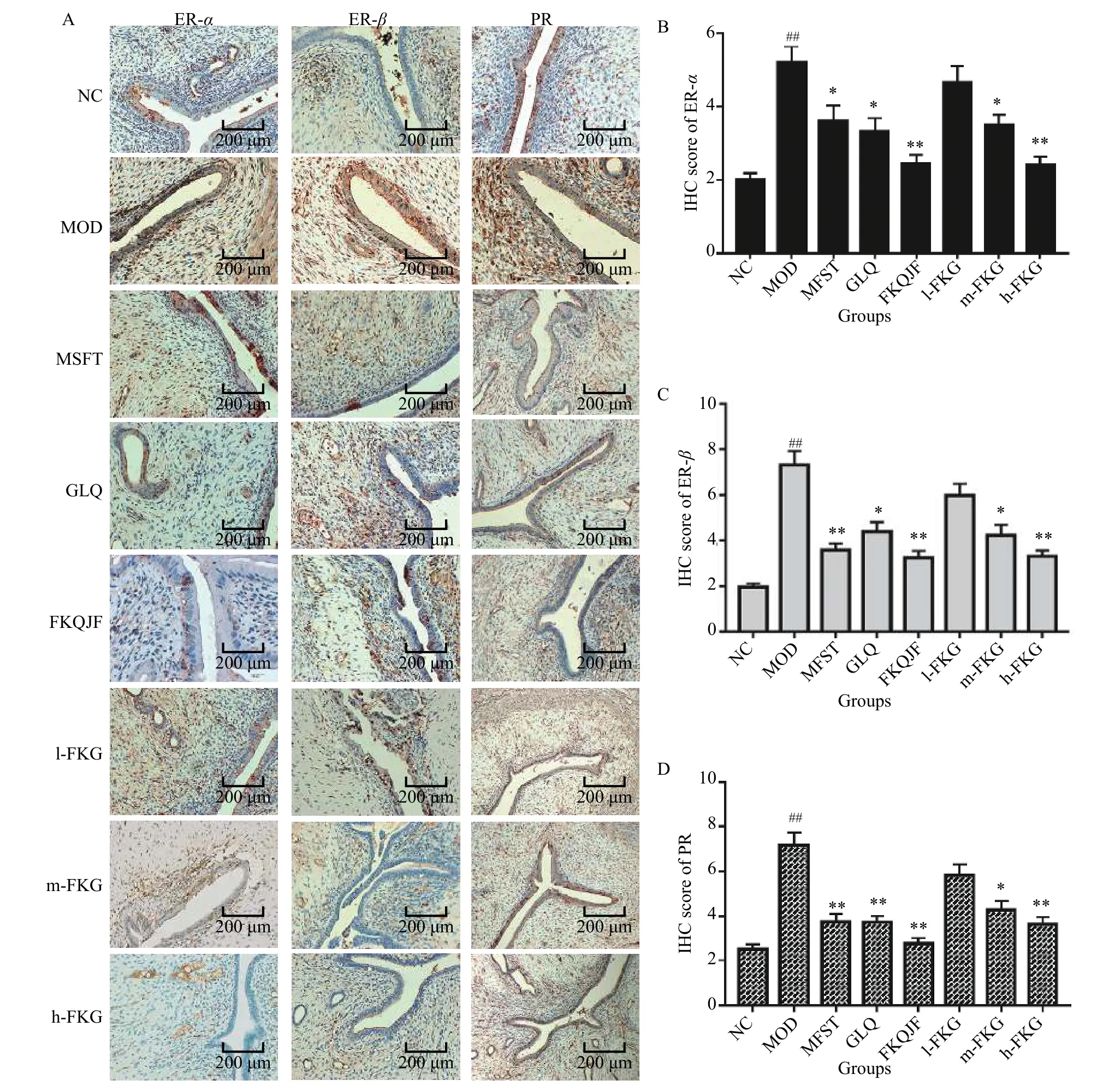
Figure 4 Expressions of ER-α, ER-β, and PR in the uterus of SD rats
3.8 Analysis of FKG components
The GC-MS total ion chromatogram of the FKG extract is shown in Figure 6, and the FKG components were matched with those in the database (Table 2).The results showed that the main components of FKG were palmitic acid (12.32%), oleic acid (12.05%),linoleic acid (10.79%), hexacosanoic acid (7.76%),undecylenic acid(7.10%),stearic acid(5.26%),eucalyptus acid (4.78%), wood tar oleic acid (4.23%),18-methyl hexadecanoic acid (3.68%), valeric acid(2.22%),pentacosanoic acid(2.02%),sitosterol(1.78%),stigmasterol(1.57%),queen bee acid(1.39%), heptadecanoic acid (1.31%), and linolenic acid (1.03%).
4 Discussion

Figure 5 Multivariate statistical analysis of metabolomics of rats serum
UL are benign tumors that evolve from uterine smooth muscle hyperplasia and are one of the most common gynecologic tumors, and their pathogenesis remain unclear.A study has shown that estrogen can promote UL growth and that progesterone also plays an important role in promoting the development of UL[23].Estrogen and progesterone regulate each other through autocrine and paracrine action, and estrogen can increase the content of progesterone receptor in fibroid cells, progesterone.As a result, it can further promote and maintain the changes in estrogen, and the two affect each other to promote the growth of fibroids[24].The low postmenopausal estrogen and progesterone status can cause fibroids to shrink or even disappear, and the occurrence and development of UL are closely associated with high concentrations of estrogen and progesterone.The biological effects of hormones can be achieved by binding to their receptors.Therefore, regulating the levels of estrogen and progesterone and their receptors can effectively control UL[25-28].

Figure 6 GC-MS total ion chromatogram of FKG

Table 2 Chemical constituents of FKG
In this study, the UL model was successfully established by the cross-intervention of estrogen and progesterone.Estrogen and progesterone induced cell proliferation and differentiation by activating corresponding estrogen and progesterone receptors,which promote uterine fibroid cell mitosis and the development of uterine fibroids.
h-FKG could effectively lower levels of estrogen and progesterone and its receptors in UL model rats,indicating that anti-UL effects of h-FKG were closely related to its down-regulation of estrogen, progesterone, and their receptors.The contents of linoleic acid, sitosterol, and stigmasterol in FKG were 10.79%,1.78%, and 1.57%, respectively.Studies have shown that conjugated linoleic acid exerts anti-estrogenic effects in human breast tissues[29],α-linolenic acid decreased ER-αexpression in murine mammary adenocarcinoma[30], and sitosterol and stigmasterol have anti-uterine fibroid effects[31].
FKG also had a regulatory effect on serum metabolism levels in uterine fibroid rats.FKG had the best effect on uterine fibroids among various fractions of FKQJF.
Lysine is a necessary amino acid, and it is able to enhance body immunity.In addition, h-FKG can effectively upregulate the level of lysine, indicating that h-FKG may regulate the immunity of rats to achieve the effect of anti-uterine fibroids.h-FKG downregulates urea in UL model rats, which also demonstrates that h-FKG regulates protein and amino acid metabolism D-mannose can inhibit the growth of tumor cells[32]; D-galactose provides energy for the body,and h-FKG can effectively up-regulate the levels of D-mannose and D-galactose, suggesting that h-FKG may be related to its regulation of energy metabolism.
In summary, this study proved that FKQJF had a certain therapeutic effect on the rat UL, and FKG was the most effective fraction of FKQJF.This study also provides an experimental basis for future research on the pathogenesis and treatment mechanism of UL and provides a new direction for the treatment of UL.However, further studies should be conducted regarding the specific components that aid in fibroid reduction.
5 Conclusion
The current study suggests that FKQJF has potent anti-myoma activity.All extracts had some degree of anti-hysteromyoma activity, among which FKG was the most effective fraction.These findings provide scientific evidence supporting the quality standardization of FKQJF and an experimental basis for clinical research and application of FKQJF.
Acknowledgements
We thank for the funding support from the Major Science and Technology Projects in Hunan Province(No.2015SK1001), Special Funds from the Central Government to Guide Local Science and Technology Development (No.2019XF5076), Natural Science Foundation of Hunan Province (No.2017JJ1023 and No.2019JJ50443), and the Research project of Hunan Education Department(No.17B198and No.19C1398).
We thank Drs.GONG Yun and ZHANG Peng of Zhuzhou Qianjin Pharmaceutical Co., Ltd.for their valuable suggestions during the entire research process.
Competing interests
The authors declare no conflict of interest.
 Digital Chinese Medicine2021年4期
Digital Chinese Medicine2021年4期
- Digital Chinese Medicine的其它文章
- P-glycoprotein mediated interactions between Chinese materia medica and pharmaceutical drugs
- Systematic review of robust experimental models of rheumatoid arthritis for basic research
- Traditional Chinese medicine compounds for the treatment of functional dyspepsia: an updated meta-analysis of randomized,double-blind, placebo-controlled trials
- Rational drug design, synthesis, and biological evaluation of novel N-(2-arylaminophenyl)-2,3-diphenylquinoxaline-6-sulfonamides as potential antimalarial, antifungal, and antibacterial agents
- Study on differential gene expression profile of serum exosomes in patients with acute cerebral infarction
- Protective effects of Fufang Ejiao Jiang against aplastic anemia assessed by network pharmacology and metabolomics strategy
