狼疮性肾炎合并抗磷脂抗体阳性患者的临床特点分析
余圣俨 沈鑫 李圆圆 郑芳芳 王梅英





【摘要】 目的:通過对比分析抗磷脂抗体(aPLs)阳性和aPLs阴性狼疮性肾炎(LN)患者的临床表现、实验室检查和相关共存疾病,以期探讨aPLs对LN患者肾脏病变的影响。方法:回顾性分析2008年1月-2020年3月北京大学深圳医院风湿免疫科或肾内科住院的195例LN患者的临床资料,根据aPLs检测结果,将LN患者分为aPLs阴性组(n=132)和aPLs阳性组(n=63)。比较两组的一般资料(性别、年龄、从出现SLE首发症状到确诊LN的持续时间、高血压病史、脑血管疾病)、临床表现(发热、雷诺现象、皮疹、脱发、口腔溃疡、浆膜腔积液、癫痫发作、关节炎、肺间质病变、肺动脉高压、肠道受累、自身免疫性溶血性贫血)、实验室资料[24 h尿蛋白定量、肌酐、白蛋白、免疫球蛋白IgG、免疫球蛋白IgM、免疫球蛋白IgA、总补体溶血活性(CH50)、补体C3、补体C4、抗ds-DNA抗体滴度]及肾组织病理。结果:aPLs阴性组从出现SLE首发症状到确诊LN的持续时间与aPLs阳性组比较,差异有统计学意义(P<0.05)。aPLs阳性组自身免疫性溶血性贫血的发生率高于阴性组,差异有统计学意义(P<0.05)。aPLs阳性组24 h尿蛋白定量、肌酐、抗dsDNA抗体滴度明显高于阴性组,差异均有统计学意义(P<0.05)。38例LN患者行肾活检,其中aPLs阳性组19例,aPLs阴性组19例。aPLs阳性组慢性指数高于aPLs阴性组,差异有统计学意义(P<0.05)。结论:与aPLs阴性患者相比,aPLs阳性LN患者从出现SLE首发症状到确诊LN的持续时间短,贫血和脑血管疾病发生率显著升高,肾脏病变重且慢性指数高。对LN患者常规实施aPLs检测并对aPLs阳性患者积极治疗和密切随访观察,以期改善预后。
【关键词】 狼疮性肾炎 抗磷脂抗体 肾损伤
Analysis of Clinical Characteristics of Patients with Lupus Nephritis Complicated with Positive Antiphospholipid Antibody/YU Shengyan, SHEN Xin, LI Yuanyuan, ZHENG Fangfang, WANG Meiying. //Medical Innovation of China, 2021, 18(15): -132
[Abstract] Objective: To explore the effect of aPLs on renal lesions in LN patients, the clinical manifestations, laboratory tests and related coexisting diseases of aPLs positive and aPLs negative LN patients were compared and analyzed in this study. Method: The clinical data of 195 patients with LN treated in the Department of Rheumatology and Immunology or the Department of Nephrology, Peking University Shenzhen Hospital from January 2008 to March 2020 were analyzed retrospectively, according to the results of aPLs detection, patients with LN were divided into the aPLs negative group and the aPLs positive group. The general information (gender, age, duration of SLE, hypertension, cerebrovascular disease), clinical manifestation (fever, Renault phenomenon, rash, hair loss, mouth ulcers, serous cavity effusion, seizures, arthritis, interstitial lung disease, pulmonary hypertension, intestinal involvement, autoimmune hemolytic anemia), laboratory data [24-hour urinary protein, platelet count, creatinine, Albumin, immunoglobulin IgG, immunoglobulin IgM, immunoglobulin IgA, total complement hemolytic activity (CH50), complement C3, complement C4, anti-double-stranded DNA antibody] and renal histopathology of two groups were compared. Result: The duration from the first symptom of SLE to the diagnosis of LN in the aPLs negative group was shorter than that in the aPLs positive group, the difference was statistically significant (P<0.05).The incidence of autoimmune hemolytic anemia in the aPLs positive group was significantly higher than that in the aPLs negative group (P<0.05). The levels of 24-hour urinary protein, creatinine and anti-double-stranded DNA antibody in the aPLs positive group were significantly higher than those in the aPLs negative group (P<0.05). Renal biopsy was performed in 38 patients with LN, including 19 patients with aPLs positive and 19 patients with aPLs negative. The chronic renal index of LN patients in the aPLs positive group was higher than that in the aPLs negative group, and the difference was statistically significant (P<0.05). Conclusion: Compared with aPLs negative patients, LN patients with aPLs positive have shorter time from initial symptoms to diagnosis, significantly higher incidence of anemia and cerebrovascular diseases, severe renal lesions and higher chronic index. Routine detection for aPLs is necessary in patients with LN, and aPLs positive patients need to be treated and follow-up strictly.
[Key words] Lupus nephritis Antiphospholipid antibody Renal damage
First-author’s address: Clinical College of Peking University Shenzhen Hospital, Anhui Medical University, Shenzhen 518000, China
doi:10.3969/j.issn.1674-4985.2021.15.031
狼疮性肾炎(lupus nephritis,LN)是系统性红斑狼疮(systemic lupus erythematosus,SLE)患者最常见的脏器受累,其发生率高达30%~40%[1]。LN严重影响了SLE患者的预后。抗磷脂抗体(antiphospholipid antibodies,aPLs)作为SLE诊断标准之一已纳入国际指南。但aPLs对于SLE患者肾脏损害目前说法不一。有研究发现aPLs阳性LN患者其高血压发生率、肾间质纤维化率和血肌酐水平明显增高,是慢性肾衰竭的独立危险因素[2]。也有研究提出aPLs与LN发生与否无关[3-4]。本研究旨在通过对比分析探讨aPLs阳性和阴性LN患者的临床资料,探讨aPLs对LN患者肾脏病变的影响。现报道如下。
1 资料与方法
1.1 一般资料 选取2008年1月-2020年3月北京大学深圳医院肾内科、风湿免疫科住院的195例狼疮性肾炎的患者,根据aPLs检测结果分为aPLs阴性组(n=132)、aPLs阳性组(n=63)。其中行肾活检的有38例,aPLs阴性组(n=19)、aPLs阳性组(n=19)。LN诊断标准及分型:(1)符合1997年ACR SLE分类诊断标准;(2)24 h尿蛋白定量>0.5 g/24 h,或尿蛋白定性检测>+++,或尿常规中可见异常管型(红细胞、白细胞、颗粒或混合管型);(3)或肾活检证实。LN的病理分型根据2003年国际肾脏病协会/肾脏病理学会的分型标准。纳入标准:(1)年龄18~70岁,性别、民族不限;
(2)符合1997年ACR SLE分類诊断标准;(3)所有患者均在本院检测过抗磷脂抗体,包括抗心磷脂抗体(anticardiolipin antibody,aCL)、狼疮抗凝物(lupus anticoagulant,LA)、抗β2糖蛋白2抗体(anti-β2 glycoprotein 1 antibody,anti-β2GP1);(4)Ⅳ型及Ⅳ+Ⅴ型狼疮性肾炎需同时符合以下条件:持续性蛋白尿≥1 g/24 h(或尿蛋白/肌酐比≥1.0 mg/g)或血肌酐>115 μmol/L,且有活动性尿沉渣(>5个红细胞/高倍镜视野或大于实验室参考范围且排除泌尿性感染;或存在细胞管型);(5)Ⅲ型、Ⅲ+Ⅴ型及Ⅴ型狼疮性肾炎同时符合以下条件:持续性蛋白尿≥2 g/24 h(或尿蛋白/肌酐比≥2.0 mg/g)
或血肌酐>115 μmol/L。排除标准:(1)临床资料严重缺失;(2)aPLs阴性组患者抗磷脂抗体资料不全,即aCL、LA、anti-β2GP1这三种抗体仅检测1项或2项;(3)合并恶性肿瘤、乙肝相关性肾病。本研究已被北京大学深圳医院伦理学委员会批准,所有患者均签署知情同意书。
1.2 方法
1.2.1 临床资料收集 收集患者的一般资料(性别、年龄、从出现SLE首发症状到确诊LN的持续时间、高血压病史、脑血管疾病)、临床表现(发热、雷诺现象、皮疹、脱发、口腔溃疡、浆膜腔积液、癫痫发作、关节炎、肺间质病变、肺动脉高压、肠道受累、自身免疫性溶血性贫血)、实验室资料[24 h尿蛋白定量、肌酐、白蛋白、免疫球蛋白(IgG、IgM、IgA)、总补体溶血活性(CH50)、补体C3、补体C4、抗ds-DNA抗体滴度]及肾组织病理资料。
1.2.2 肾活检切片标本 肾活检评估:根据2003年国际肾脏病学会和肾脏病理学会(ISN/RPS)制定的狼疮性肾炎病理分型标准,有经验丰富的两名病理学家进行病理分型评估和活动性评分及慢性评分。
1.3 统计学处理 采用SPSS 25.0统计软件分析处理,正态分布计量资料用(x±s)表示,比较采用t检验;非正态分布计量资料以M(P,P)表示,采取Mann-Whitney秩和检验;计数资料以率(%)表示,比较采取χ检验、连续性矫正或Fisher确切概率法进行比较。以P<0.05为差异有统计学意义。
2 结果
2.1 两组一般资料比较 aPLs阴性组从出现SLE首发症状到确诊LN的持续时间与aPLs阳性组比较,差异有统计学意义(P<0.05)。aPLs阴性组和aPLs阳性组性别、年龄、脑血管疾病、高血压病史比较,差异均无统计学意义(P>0.05)。见表1。
2.2 两组临床表现比较 aPLs阳性组自身免疫性溶血性贫血的发生率高于阴性组,差异有统计学意义(P<0.05)。两组其他肾外表现比较,差异均无统计学意义(P>0.05)。两组静脉血栓发生率比较,差异无统计学意义(P>0.05),但aPLs阳性组血栓发生在肾静脉。见表2。
2.3 两组实验室指标比较 aPLs阳性组血清肌酐、24 h尿蛋白定量、抗dsDNA抗体滴度均明显高于aPLs阴性组,CH50、C3均显著低于aPLs阴性组,差异均有统计学意义(P<0.05),见表3。
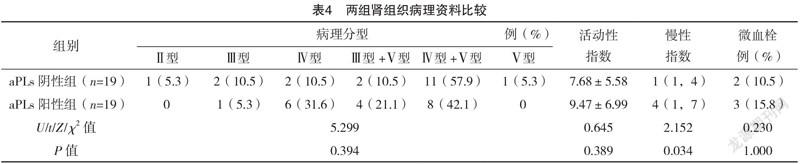
2.4 两组肾组织病理结果比较 38例LN患者行肾活检,其中aPLs阳性组19例,aPLs阴性组19例。aPLs阳性组慢性指数高于阴性组,差异有统计学意义(P<0.05)。两组病理分型比较,差异无统计学意义(P>0.05)。aPLs阳性组微血栓发生率高于aPLs阴性组,但差异无统计学意义(P>0.05)。见表4。
3 讨论
在SLE患者中,有30%~40%的患者伴有aPLs阳性[5],与本研究结果相似。本研究发现从出现SLE首发症状到确诊LN的持续时间,aPLs阳性组患者较aPLs阴性组患者时间短,提示aPLs阳性LN患者更早就诊,可能与其器官损害加重有关系。既往有研究证实aPLs可加重SLE的器官损害,且与SLE的疾病活动度相关[6-8]。此外我们的数据还提示,aPLs阳性组LN患者脑血管疾病的发生率无差异。而Sahebari等[9]发现IgM型aCL是脑血管疾病中最多见的磷脂抗体类型[9]。这种研究上的差异可能是由于本研究的样本量较少所致。
自身免疫性溶血性贫血是SLE的一个首要表现[10]。Ames等[11]的荟萃分析提示在IgG型aCL和IgM型aCL阳性的SLE患者中,自身免疫性溶血性贫血更常见,而在IgG型β2GP1阳性的患者中少见。也有研究显示贫血是LN预后不良即慢性损害的相关因素之一[12]。本研究发现,aPLs阳性组自身免疫性溶血性贫血发生率较aPLs阴性组高,推测可能是aPLs阳性LN患者肾损伤较重的因素之一。
既往研究发现合并低补体C3血症的LN患者肾脏损害更严重[12-13]。本研究结果显示aPLs阳性组补体C3较低。aPLs通过诱导补体激活途径从而导致血栓形成[14]。aPLs阳性的SLE患者补体C3、C4较低,补体激活更为明显,加重肾脏损伤[15]。另外,抗dsDNA抗体早已被纳入国际诊断标准,与SLE患者的活动性相关,尤其是在肾脏疾病之中。Gheita等[16]发现抗β2GP1阳性的SLE患者ds-DNA水平显著升高。在本研究中,aPLs阳性组患者的抗dsDNA抗体滴度较高,与其结果一致。这可能与LN的活动性有关,但是在临床上,有一些LN患者在稳定期的抗ds-DNA抗体滴度仍处于一个高水平状态,因此aPLs阳性对LN患者活动性的影响仍有待进一步研究。
虽然有些研究提示aPLs增加了SLE的器官损害风险,但是aPLs阳性对于肾脏损害的影响仍有争论。Parodis等[3]发现IgG型aPLs可导致肾脏功能损害,但aPLs与长期肾脏预后之间并没有关联。Natejumnong等[4]提出aCL陽性与LN无关,LA与LN密切相关,检测LA可早期识别肾脏不良预后的LN患者。Loizou等[17]发现aCL水平升高与LN有关。Ding等[18]则认为aCL抗体在增生型狼疮性肾炎损伤中起作用。Yap等[19]也发现aPLs增加了肾脏慢性损害的风险。本研究结果显示aPLs阳性组慢性指数明显大于aPLs阴性组,也支持aPLs加重SLE肾损害。然而有趣的是,Mehrani等[20]发现IgM抗β2GP1对LN和肾脏损害具有保护作用。之所以出现研究方面的偏差,可能与各个研究中纳入患者的种族、样本量、长期随访管理不同有关。
综上所述,与aPLs阴性患者相比,aPLs阳性LN患者从出现首发SLE症状到确诊LN时间短,自身免疫性溶血性贫血发生率显著升高,肾脏病变重且慢性指数高。然而本研究的样本数量有限,需进行多中心、大样本研究以进一步研究。另外,由于仅进行了回顾性研究,其结论的准确性和科学性尚待应用前瞻性队列进行验证。
参考文献
[1] Hanly J G,O’Keeffe A G,Li Su,et al.The frequency and outcome of lupus nephritis: results from an international inception cohort study[J].Rheumatology,2016,55(2):252-262.
[2] Moroni G,Ventura D,Riva P,et al.Antiphospholipid antibodies are associated with an increased risk for chronic renal insufficiency in patients with lupus nephritis[J].Am J Kidney Dis,2004,43(1):28-36.
[3] Parodis I,Arnaud L,Gerhardsson J,et al.Antiphospholipid Antibodies in Lupus Nephritis[J].PLoS One,2016,11(6):0158076.
[4] Natejumnong C,Ruangkanchanasetr P,Aimpun P,et al.
Significance of antiphospholipid antibodies in lupus nephritis[J].
J Med Assoc Thai,2006,89(Suppl 2):S121-128.
[5] Petri M.Epidemiology of the antiphospholipid antibody syndrome[J].J Autoimmun,2000,15(2):145-151.
[6] Ozan U,Stephane Z,Doruk E.The clinical significance of antiphospholipid antibodies in systemic lupus erythematosus[J].Eur J Rheumatol,2016,3(2):75-84.
[7] Taraborelli M,Leuenberger L,Lazzaroni M G,et al.The contribution of antiphospholipid antibodies to organ damage in systemic lupus erythematosus[J].Lupus,2016,25(12):1365-1368.
[8] Sarabi Z S,Sahebari M,Rezaie A E,et al.The Relationship Between Systemic Lupus Erythematosus Activity and Persistent Positive Antiphospholipid Antibodies[J].Current Rheumatology Reviews,2018,14(2):145-152.
[9] Sahebari M,Rastin M,Boostani R,et al.Subtypes of Antiphospholipid Antibodies in Neurologic Disorders:An Observational Study[J].Curr Rheumatol Rev,2019,15(1):59-66.
[10] Velo-García A,Castro S G,Isenberg D A.The diagnosis and management of the haematologic manifestations of lupus[J].Journal of Autoimmunity,2016,74:139-160.
[11] Ames P,Bucci M,Pastori D,et al.Antiphospholipid Antibodies and Autoimmune Haemolytic Anaemia:A Systematic Review and Meta-Analysis[J].International Journal of Molecular Sciences,2020,21(11):4120.
[12] Contreras G,Pardo V,Cely C,et al.Factors associated with poor outcomes in patients with lupus nephritis[J].Lupus,2005,14(11):890-895.
[13] Durcan L,Petri M.The Clinical and Serological Associations of Hypocomplementemia in a Longitudinal SLE Cohort[J].Seminars in Arthritis and Rheumatism,2020,50(5):1081-1086.
[14] Chaturvedi S,Braunstein E M,Yuan X,et al.Complement activity and complement regulatory gene mutations are associated with thrombosis in APS and CAPS-Science Direct[J].Thrombosis and Hemostasis,2020,135(4):239-251.
[15] Garabet L,Gilboe I M,Mowinckel M C,et al.Antiphospholipid Antibodies are Associated with Low Levels of Complement C3 and C4 in Patients with Systemic Lupus Erythematosus[J].Scandinavian Journal of Immunology,2016,84(2):95-99.
[16] Gheita T A ,Abaza N M,Hammam N,et al.Anti-dsDNA titre in female systemic lupus erythematosus patients:relation to disease manifestations, damage and antiphospholipid antibodies[J].Lupus,2018,27(7):1081-1087.
[17] Loizou S,Samarkos M,Norsworthy P J,et al.Significance of anticardiolipin and anti-β2-glycoprotein I antibodies in lupus nephritis[J].Rheumatology,2000,39(9):962-968.
[18] Ding X,Chen C,Zhang J,et al.Antiphospholipid antibodies in patients with proliferative and membranous lupus nephritis[J].Clin Rheumatol,2020,39(6):1531-1535.
[19] Yap D,Thong K,Yung S,et al.Antiphospholipid antibodies in patients with lupus nephritis:clinical correlations and associations with long-term outcomes[J].Lupus,2019,28(12):1460-1467.
[20] Mehrani T,Petri M.IgM Anti-β2 Glycoprotein I Is Protective Against Lupus Nephritis and Renal Damage in Systemic Lupus Erythematosus[J].Journal of Rheumatology,2011,38(3):450-453.
(收稿日期:2021-03-18) (本文編辑:程旭然)

