NBN-doped nanographene embedded with five-and seven-membered rings on Au(111)surface∗
Huan Yang(杨欢), Yun Cao(曹云), Yixuan Gao(高艺璇), Yubin Fu(付钰彬),Li Huang(黄立),†, Junzhi Liu(刘俊治),3, Xinliang Feng(冯新亮),‡,Shixuan Du(杜世萱),4,§, and Hong-Jun Gao(高鸿钧),4
1Institute of Physics and University of Chinese Academy of Sciences,Beijing 100190,China
2Center for Advancing Electronics Dresden(cfaed)&Faculty of Chemistry and Food Chemistry,Technische Universit¨at Dresden,01062 Dresden,Germany
3Department of Chemistry and State Key Laboratory of Synthetic Chemistry,The University of Hong Kong,Hong Kong,China
4CAS Center for Excellence in Topological Quantum Computation,University of Chinese Academy of Sciences,Beijing 100190,China
Keywords: on-surface synthesis,nanographene,nonhexagonal rings,scanning tunneling microscopy,density functional theory
1. Introduction
Two-dimensional materials, such as graphene and transition metal dichalcogenides, have been investigated extensively due to their exotic properties and promising potential applications.[1–13]Nanographenes (NGs), which are predesigned polycyclic aromatic hydrocarbons (PAHs) with atomically precise structures, can serve as model carbon nanostructures to study their unique physical and chemical properties,as well as novel nanoscale semiconductors on their own.[14–19]For graphene-based nanoscale materials, the subtle adjustments of structures could contribute to significant changes of their physical properties.[20–22]Flexible tailoring band gap of NGs can be realized by modulating the size,dopants,and edge structures.[23–27]Particularly,it is worth to note that the incorporation of non-hexagonal rings into honeycomb lattice enables one to tailor the electronic structures and magnetic properties of graphene nanostructures.[21,28–30]Nonetheless, non-hexagonal ring-containing graphene nanostructures remain rare experimentally due to the lack of feasible synthetic approaches.[30]On-surface synthesis approach based on surface-assisted catalytic reaction on noble metallic substrates has been utilized to achieve a variety of atomically well-defined graphene nanostructures in the past decade.[20,31,32]Armchair- and zigzag-edged graphene nanoribbons and NGs with various shapes (such as triangulenes, antiaromatic NG, and nonplanar porous NG)have been achieved on surfaces using rationally designed precursors,[20,31–38]which suggest that NGs fused with nonhexagonal rings have high chance to be realized through onsurface approach.
Herein, we present the synthesis of a NBN-doped nanographene embedded with five-and seven-membered rings(NBN-575-NG) on Au(111) and its electronic properties.The targeted NG was realized from an NBN-hepa precursor preinstalled with a heptagonal ring and NBN unit (4b,15bdiaza-4b1-borabenzo[fg]dibenzo[4,5:6,7]cyclohepta[1,2,3-op]tetracene).[39]Scanning tunneling microscopy(STM)measurements verified the planar NG structures after surfaceassisted cyclodehydrogenation, while non-contact atomic force microscopy (nc-AFM) images confirmed the existence of five-and seven-membered rings. Scanning tunneling spectroscopy (STS) spectra revealed that the lowest unoccupied molecular orbital (LUMO) state of the NBN-575-NG locates at 2.6 V, which is supported by the density functional theory(DFT)calculations on a freestanding NBN-575-NG,showing a bandgap of 2.38 eV.
2. Methods
2.1. Sample preparation,STM/STS measurement
The experiments were performed using a CreaTec ultrahigh vacuum (UHV) low-temperature STM/nc-AFM system with a base pressure of 2×10−10mbar. The Au(111)surface was cleaned by repeated cycles of Ar+ion sputtering and subsequent annealing at 700 K for 20 min. The NBN-hepa precursors were sublimated at 430 K onto the Au(111)substrate,which was held at room temperature during molecular deposition. When the sample was annealed at 640 K and 700 K for 40 min, the surface-assisted cyclodehydrogenation occurred,resulting in formation of intermediate dimers and NBN-575-NGs,respectively. All STM and nc-AFM measurements were performed at 5 K with a qPlus sensor. STM topography images were acquired in constant current mode and the voltages represented the bias on the sample with respect to the tip. Nc-AFM images were obtained in the frequency modulation mode using a CO-functionalized tip.[40,41]The dI/dV spectra were carried out using the lock-in technique(Vrms=20 mV).
2.2. First-principles calculations
Density functional theory (DFT) calculations were performed by using the generalized gradient approximation(GGA) in the form of Perdew–Burke–Ernzerhof (PBE) generalized gradient approximation.[42]The Vienna ab initio simulation package was used to do the structural relaxations and electronic-structure calculations.[43,44]The energy cutoff of the plane-wave basis sets was 500 eV. The K-point sampling was done only at the Γ point.The structures were relaxed until the residual forces were smaller than 0.01 eV/˚A.
3. Results and discussion
Figure 1(a) illustrates the synthetic process of the NBN-575-NG on Au(111)from an NBN-hepa molecule which has a heptagonal ring and preinstalled NBN unit(4b,15b-diaza-4b1-borabenzo[fg]dibenzo[4,5:6,7]cyclohepta[1,2,3-op]tetracene).The detailed synthesis method for the precursor could refer to our recent work.[39]The existence of the heptagonal ring leads to a saddle-shaped geometry of the precursor due to the strong steric hindrance between two groups of hydrogen pairs on adjacent benzene rings, as illustrated by the green shades in Fig. 1(a). After annealing the precursors on Au(111) at 640 K,only one pair of adjacent benzene rings(green shades)undergoes cyclodehydrogenation in most precursors,resulting in the formation of an intermediate compound with one fivemembered ring. Further annealing at 700 K leads to the full cyclodehydrogenation of the other pair of benzene rings and enables the achievement of planar NBN-575-NGs.
Figure 1(b) shows the large-area STM image of precursors deposited on the Au(111) surface, which exhibits many small clusters(as indicated by the red circle in Fig.1(b))that occupy the fcc and elbow areas on Au(111)reconstruction surface at low coverage. The representative topography of the clusters is shown in the zoomed-in STM image in Fig. 1(c).Bright protrusions are observed, which result from the nonplanar saddle-shaped geometry of the precursors. The apparent height and the lateral size of the cluster are 330 pm and 3.1 nm respectively as indicated by the line profile in Fig.1(d).Considering the lateral size of a single precursor(1.05 nm)and the dispersed electronic states of the molecules, we infer that one cluster contains four self-assembled precursors.
After annealing the sample at 640 K for 40 min, two new structures are obtained as revealed in Fig. 1(e). The zoomed-in STM image in Fig. 1(f) displays the formation of full-cyclodehydrogenated flower-shaped NGs(as indicated by blue circles) and dimers with two bright protrusions (as indicated by rectangles) on the surface, suggesting that the yield is not good at this temperature. The lateral size of the dimers is 3.3 nm, while the height for the protrusions of the dimers is 260 pm, and for the planar regions is 160 pm, as shown in the profile in Fig. 1(g). The topography of the dimers suggests that they are intermediate products, in which cyclodehydrogenation only occurred at one side of a molecule, and the protrusions should be attributed to the tilted uncyclized benzene rings on each molecule. At this stage, chiral intermediate dimers are also observed (highlighted by red and yellow rectangles), which are constructed by two prochiral intermediate monomers with the same chirality. Both lefthanded and right-handed intermediates can be found on the surface, indicating that the cyclodehydrogenation at 640 K could react on either side of the precursor. Notably, there are already full-cyclodehydrogenated NGs at this annealing temperature, but more than 70% of the precursors are halfcyclodehydrogenated dimers at this step.
Further annealing the sample at 700 K for 40 min resulted in completely full-cyclodehydrogenated planar flowershaped NGs on the surface(Fig.1(h)). The disappearance of the dimers suggests that the molecules prefer to being monodispersed when they are fully cyclodehydrogenated. Figure 1(i)shows the topography of the NGs, which have a typical lateral size of 2.4 nm (Fig. 1(j)). The height of the NGs is 160 pm, similar to the height of the planar regions of the intermediate dimers. The whole surface is covered by perfect flower-shaped NGs with a yield of about 95%.

Fig.1.Chemical structures and STM images of the precursors,the intermediate products and NBN-575-NGs on Au(111).(a)Synthetic strategy of the NBN-575-NG.(b)STM image of the precursors adsorbed on Au(111)substrate(V =−200 mV,I=10 pA).The representative cluster is indicated by a red circle.(c)Zoomed-in STM image of the precursors(V =−40 mV,I=10 pA).(d)Line profile along the red dashed line in(c). The apparent height and the lateral size of a representative cluster are 330 pm and 3.1 nm, respectively. (e) STM image of the intermediate products after annealing the sample at 640 K for 40 min(V =−1 V,I=10 pA).(f)Zoomed-in STM image of the intermediate products(V =−200 mV,I=20 pA).(g)Line profile along the purple dashed line in(f). The apparent height and the lateral size of an intermediate dimer are 260 pm and 3.3 nm,respectively. (h)STM image of the NBN-575-NGs after annealing the sample at 700 K for 40 min. (V =−200 mV,I=10 pA).(i)Zoomed-in STM image of the NBN-575-NGs(V =−200 mV,I=20 pA).(j)Line profile along the green dashed line in(i). The apparent height and the lateral size of an NBN-575-NG are 160 pm and 2.4 nm,respectively.
Bond-resolved nc-AFM measurements were carried out with a CO-functionalized tip to reveal more details of the NGs.[40,41]Figures 2(a) and 2(b) show the STM image and the corresponding nc-AFM image of an intermediate dimer. In Fig. 2(b), only two bright protrusions can be observed, which arise from the tilted benzene rings of the halfcyclodehydrogenated intermediates as marked by the red cycles in Figs.2(a)and 2(b).There is subtle difference on the positions of the bright protrusions in Figs. 2(a) and 2(b), which results from different working conditions between STM and nc-AFM imaging. For the fully-cyclodegydrogenated planar NG (Fig. 2(c)), nc-AFM image in Fig. 2(d) clearly resolves the five- and seven-membered rings and the bond configurations that are consistent with the expect structure. These results confirm the successful synthesis of NBN-575-NGs.
We carried out STS measurements to investigate the electronic structures of the NBN-575-NGs. Figure 3(a) shows the characteristic differential conductance (dI/dV) spectrum(red)acquired at the center of an NBN-575-NG as marked by a red spot in the inset STM image of Fig.3(a). Compared with the black dI/dV curve on Au(111) substrate with the same tip,the dI/dV spectrum on NBN-575-NG has a distinct peak at 2.6 eV, which corresponds to the LUMO state. The spectra do not exhibit any evident signals for the highest occupied molecular orbital (HOMO) state, which may be attributed to the overlap between HOMO state of NGs and the surface state of the Au(111) substrate. DFT calculated density of states(DOS)of a freestanding NBN-575-NG reveal that the LUMO and HOMO states locate at 2.14 eV and −0.24 eV, respectively,corresponding to an energy gap of 2.38 eV(the top inset in Fig.3(a)).The calculated HOMO and the LUMO orbitals of a freestanding NBN-575-NG are displayed in Fig. 3(b). The HOMO orbital shows small contribution from B and the adjacent C atoms, while strong contributions on LUMO orbital from both B and N atoms are presented. These results demonstrate that NG keeps excellent unlocalized electronic states contributed by π-electrons after the introduction of nonhexagonal rings and NBN dopants.
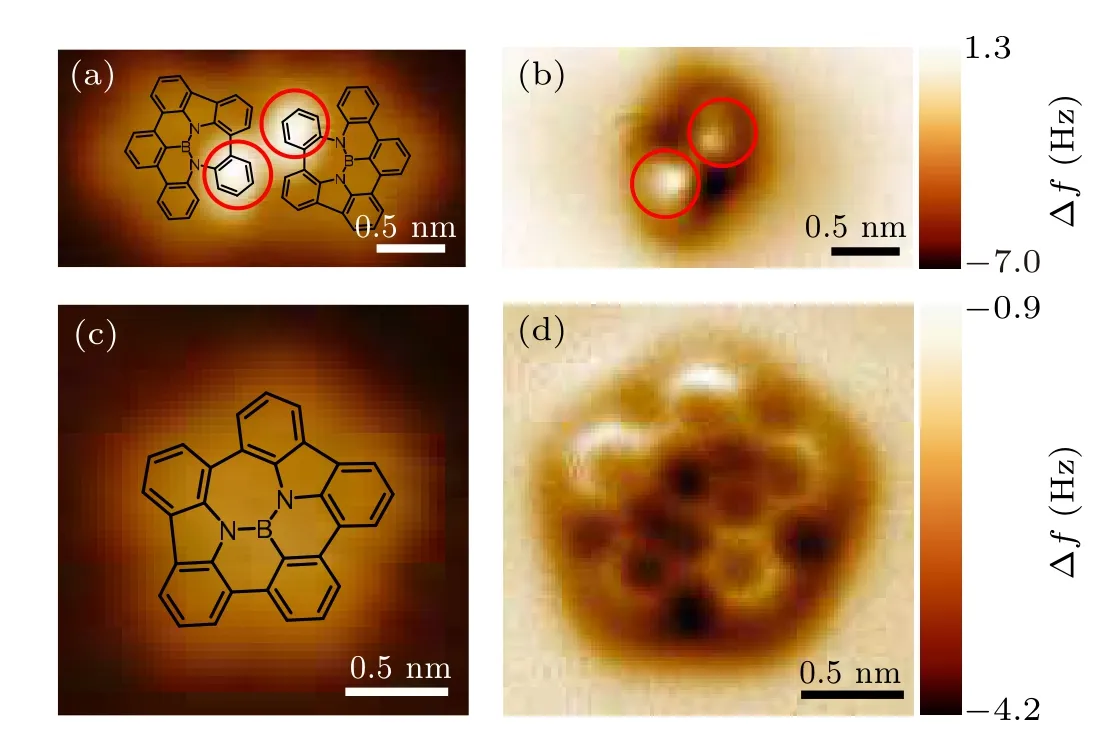
Fig. 2. STM and nc-AFM images of an intermediate dimer and NG on Au(111). (a)STM image of the intermediate dimer with superposed molecular structures(V =−200 mV,I=10 pA).(b)Constant-height nc-AFM image of(a). The red circles in(a)and(b)highlight the out-of-plane benzene rings.(c) STM image of the NBN-575-NG with superposed molecular structures(V =−200 mV,I=10 pA).(d)Constant-height nc-AFM image of(c).

Fig.3. Electronic structures of the NBN-575-NG. (a) Differential conductance (dI/dV) spectra taken on the center of the NBN-575-NG (red) and on bare Au(111)(black). The LUMO state of the NBN-575-NG locates at 2.6 eV.(V =−1 V,I=10 pA;modulation voltage Vrms=20 mV).The top inset shows the DFT calculated bandgap of 2.38 eV on a freestanding NBN-575-NG. (b) Calculated molecular orbitals of the NBN-575-NG at HOMO and LUMO energies. The isosurface is set to be 0.001 e/Bohr3.
4. Conclusions
We have demonstrated the synthesis of a novel NBN-575-NG on Au(111) from an NBN-hepa precursor. Stepwise annealing the precursors on Au(111)results in the formation of intermediate products and targeted NGs. STM images demonstrate the uniform structures and high yield(95%)of the NBN-575-NGs. Nc-AFM image confirms the existence of five-and seven-membered rings.STS spectra reveal the LUMO state locates at 2.6 eV.DFT calculations show the freestanding NBN-575-NG possesses an energy gap of 2.38 eV. This bottomup synthesis of the NBN-575-NGs with semiconductor electronic structure can promote future applications of carbonbased nanostructures in nanoelectronics.
- Chinese Physics B的其它文章
- Corrosion behavior of high-level waste container materials Ti and Ti–Pd alloy under long-term gamma irradiation in Beishan groundwater*
- Degradation of β-Ga2O3 Schottky barrier diode under swift heavy ion irradiation*
- Influence of temperature and alloying elements on the threshold displacement energies in concentrated Ni–Fe–Cr alloys*
- Cathodic shift of onset potential on TiO2 nanorod arrays with significantly enhanced visible light photoactivity via nitrogen/cobalt co-implantation*
- Review on ionization and quenching mechanisms of Trichel pulse*
- Thermally induced band hybridization in bilayer-bilayer MoS2/WS2 heterostructure∗

