Configuration interaction study on low-lying states of AlCl molecule*
Xiao-Ying Ren(任笑影), Zhi-Yu Xiao(肖志宇), Yong Liu(刘勇), and Bing Yan(闫冰)
Institute of Atomic and Molecular Physics,Jilin University,Changchun 130012,China
Keywords: AlCl molecule,MRCI-F12,potential energy curves,spin–orbit coupling
1. Introduction
Some aluminum halide molecules(AlX,X =F,Cl,Br,I)have been detected in the astrophysical environment[1]and are also easy to generate chemiluminescent reactions in the gas phase through high temperature pyrolysis.[2]In recent years,monochloride has received more and more attention.[3–6]In order to further understand the physical and chemical processes involving these molecules, the characteristics of their electronic states including spectroscopic constants and transition properties are extremely important. Aluminum halides have also aroused the increasing research interest due to their potential applications in molecular laser cooling.In theory,Wells and Lane[4]have performed ab initio studies of the electronic states of AlH and AlF,showing that the A1Π–X1Σ+transition of two molecules may be strong candidates for laser cooling.For AlCl,the A1Π1–X1Σ+0+transition is also a possible candidate for laser cooling.[7]
For AlCl molecule, many experimental and theoretical studies have been performed previously.The spectrum of AlCl molecule focused mainly on the ground state X1Σ+and several low-lying states. Hedderich et al.[8]discovered the highresolution emission spectrum of AlCl and found a rotational transition occurring in a range of 5 mm–7 mm. Lanhoff et al.[9]predicted the spectroscopic constants of the ground state and excited states with energy up to 61000 cm−1by high-level ab initio calculations. In 2011, Wells and Lane[4]observed the transition of AlCl3in the discharge experiment of AlCl and conducted vibration analysis. In 1951, Sharma[7]found that the wavelength band of 415.4 nm–397 nm was similar to the Cameron band of CO,which was caused by the transition from the first excited state of AlCl to the ground state. Dearden et al.[10]used 332 nm–570 nm laser resonance enhancement photoionization spectroscopy to study the highly excited states of 24000 cm−1–60000 cm−1and obtained spectroscopic constants for seven new states of AlCl. In addition,Saksena et al.[11]observed the a3Π–X1Σ+transition of the AlCl molecule at a high resolution and determined the rotation constant of the a3Π state. Mahieu et al.[12]also studied the A1Π–X1Σ+system of AlCl and obtained the accurate rotational constants and vibrational constants of these two states,and discussed the predissociation of the A1Π state at the v′=10 vibration energy level.In 1987,Rogowsiu and Fontijn[13]determined the radiative lifetime of the AlCl A1Π state(τ=6.4 ns)by using laserinduced fluorescence technique. In theory,Brites and Hochlaf
[14]calculated the potential energy curves of electronic states for AlCl and its cationic species AlCl+and AlCl2+at the MRCI level, and derived a set of accurate spectroscopic constants. Langhoff et al.[9]computed spectroscopic constants for the lowest six singlet states and the lowest five triplet states of AlCl by the MRCI+Q method. And the lifetime of the v′=0 level of the A1Π state for AlCl is calculated to be 5.2 ns.The latest ab initio computation[3]was reported in 2016, in which the potential energy curves,permanent dipole moments,and transition dipole moments for the X1Σ+, a3Π, and A1Π states were studied by the MRCI+Q method with the ACVQZ basis set.[15]
Although AlCl molecule has been studied for a long time,our knowledge of the excited states of the molecule,especially the complete picture of spin–orbit coupling in electronic excited states,is far from enough. In this paper,we perform the explicitly correlated multireference configuration interaction plus Davidson correction (MRCI-F12+Q) method to calculate the PECs of the 13 Λ–S states of the AlCl molecule. The scalar relativistic (SR) correction is considered in this work.The SOC interactions between the Λ–S states are evaluated by the SO matrix elements. After considering the SOC effect, 13 Λ–S states split into 24 Ω states. The spectroscopic constants of Ω states are evaluated.The permanent dipole moments (PDMs), transition dipole moments (TDMs), Franck–Condon factors (FCFs), and the radiative lifetimes of several lowest transitions are also predicted.
2. Computational methods
For AlCl molecule, we perform 71 single point energy calculations for each state with the internuclear distance in a range of R value of 0.8 ˚A–8.5 ˚A to construct the potential energy curves. The high-level ab initio calculations on the electronic structure are performed with the quantum chemistry MOLPRO 2012 program package.[16]The symmetric point group of AlCl is C∞v. The C2vpoint group is selected for the AlCl electronic structure calculation. The C2vpoint group has an irreducible representation of A1/B1/B2/A2,and the corresponding relationships with the C∞νpoint group are Σ+=A1, Π=B1+B2, Δ=A1+A2, and Σ−=A2, respectively. A total of 13 Λ–S states corresponding to the two lowest dissociation limits, neutral Al(2Pu)+Cl(2Pu)and ionic pair Al+(1S)+Cl(1S), are investigated in this work. Firstly,the restricted Hartree–Fock (HF) method is used to obtain the single-configuration wavefunction of the ground state for the AlCl molecule; then the state-averaged complete active space self-consistent field (SACASSCF)[17,18]is used. In the SA-CASSCF computations, we involve a total of 13 electronic states including three1Σ+, two1Π, one1Δ, one1Σ,two3Σ+, two1Π, one3Δ, and one3Σ states. The MRCIF12 method is utilized to estimate the dynamical correlations.At the same time, the Davidson[19]correction(+Q)balances the size-consistency error of multireference method. Moreover, the calculation is extended to including the scalar relativistic effect by means of an electronic third-order Douglas-Kroll[20–22]integral.The basis set chosen in MRCI-F12 calculation is the cc-CVQZ-F12 for Al and Cl atoms.
In the following calculations, the influence of the SOC effect on the MRCI-F12 calculation level is considered. The SOC matrix element[23,24]is dealt with by the state-interacting technique via Breit–Pauli operator.[25]The off-diagonal spin–orbit matrix elements are calculated at the MRCI-F12 level,while the diagonal spin–orbit matrix elements are substituted with the MRCI-F12+Q energy. The SOC effect makes the 13 Λ–S electronic states split into 24 Ω states.The PECs of the 13 Λ–S states and 24 Ω states draw and meet the rule of avoided crossing. On the basis of the PECs in Λ–S and Ω states, the spectroscopic constants of the bound states can be determined by solving the numerical solution of the one-dimensional nuclear Schr¨odinger equation through the Level program,[26]including the excitation energy Te, the equilibrium internuclear distance Re, the vibration constant ωeand ωexe, and the harmonic vibration constant Be. The dissociation energy Deis obtained by subtracting the energy at Refrom the energy at a large interval. The transition properties and the spin–orbit matrix elements of several electronic states of AlCl molecule are calculated. The permanent dipole moments(PDMs),the transition dipole moment(TDMs),and the Franck–Condon factors(FCFs)are also calculated at the MRCI-F12 level. According to the calculated TDMs and FCFs, we predict the radiative lifetimes of several lowest transitions.
3. Results and discussion
3.1. Potential energy curves and spectroscopic constants of 13 Λ–S states
We calculate 13 Λ–S states of AlCl molecule by the method of MRCI-F12+Q, including 7 singlet states and 6 triplet states. Figure 1 displays the potential energy curves of 13 Λ–S states of AlCl molecule. These states correspond to two dissociation limits of Al(2Pu)+Cl(2Pu) and Al+(1S)+Cl−(1S)and are listed in Table 1. As shown in Table 1, except for the 31Σ+state, which is associated with the ion-pair dissociation limit Al+(1S)+Cl−1(1S), the other Λ–S states correspond to the same neutral atomic dissociation limit Al(2Pu)+Cl(2Pu).The energy interval between the two atomic limits is computed at the internuclear distance R=6 ˚A with the inclusion of Coulomb compensation for the energy.
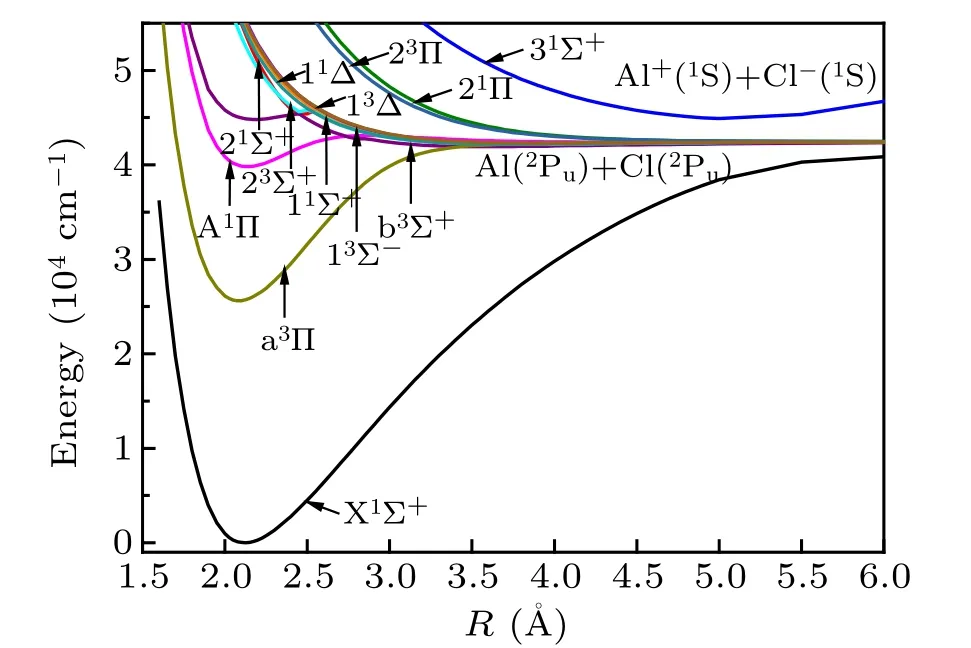
Fig.1. PECs of Λ–S states for AlCl.
From Fig.1,we can see that the two singlet states X1Σ+and A1Π and one triplet state a3Π are typical bound states,while the other states are repulsive states or quasi-bound states.It can be seen from Fig. 1 that there is an avoided crossing point between b3Σ+and 23Σ+states at internuclear distance R=2.4 ˚A, which results in the formation of a potential well in the 23Σ+state and a potential barrier in the b3Σ+state.

Table 1. Dissociation relationships of Λ–S states of AlCl.

Table 2. Computed and experimental spectroscopic constants of low-lying Λ–S states of AlCl.
The spectroscopic constants of these three states are calculated by the MRCI-F12 method, and those results obtained from previous experimental and theoretical studies are listed in Table 2 for comparison. The ground state X1Σ+of the AlCl molecule is characterized mainly by the closedshell electronic configuration 7σ28σ29σ23π4(85.043%) at Re. The deviations of Re, ωe, and Decalculated by MRCI-F12/CVQZ+Q+DK from the previous experimental value,[25,30]are 0.0046 ˚A, 6 cm−1, and 0.11 eV, respectively. Therefore, it indicates that the Davidson correction is still important for the MRCI-F12 approach to solve the sizeextensivity problem. For the first excited state a3Π,the wavefunction of a3Π is mainly described by the electronic configuration 7σ28σ29σα3π44πα(86.177%). The Davidson correction and SR correction contribute about 1162 cm−1to the value of Te,the previous calculated Revalues by various methods are similar and our calculation is about 2.09 ˚A, which is somewhat smaller than the experimental value of 2.1 ˚A. At the same time, the differences among ωevalues obtained by different methods are also small. To the best of our knowledge,no experimental value for Deof a3Π state is found. The Deof the a3Π state is evaluated to be 2.37 eV, which is in good agreement with the theoretical result of 2.25 eV.[3]For the A1Π state, the wavefunction is described mainly by the electronic configuration 7σ28σ29σα3π44πβ(78.954%). The Re, ωe, and Devalues for the A1Π state are calculated to be 2.131 ˚A,456.7 cm−1,and 0.53 eV,respectively,which are in good agreement with the previous theoretical values.[3]While all the computed values of Tedeviate from the experimental values by larger than 500 cm−1.[10]
Figure 2 shows the permanent dipole moments (PDMs)of the 13 Λ–S states of AlCl molecule. For clarity,the PDMs of singlet states and triplet states are represented in Figs.2(a)and 2(b), respectively. The PDMs of X1Σ+, a3Π, and A1Π states for AlCl molecule have been calculated previously.[3]As shown in Fig. 2, all the calculated PDMs at a large internuclear distance are almost zero a.u. (atomic unit) with the exception of the 31Σ+state. This is because the center of positive charges with the position vector λ is set to be at zero, which is corresponding to the dissociation limit of neutral atom. The variation of PDM can reflect the ionic characteristics of these states. The PDM of 31Σ+state is negative and becomes linearly-dependent R when R is larger than 6.0 ˚A.This indicates that the dissociation limit of the 31Σ+state comes from ion-pair Al+(1S)+Cl(1S). The PDMs of X1Σ+and a3Π states have negative peaks with values of −4.26 a.u.and −1.04 a.u. at R=4.4 ˚A and R=2.9 ˚A,respectively.

Fig.2. Permanent dipole moments of Λ–S states for AlCl molecule.
3.2. Spin–orbit coupling of the Λ–S states
The SOC effect has a significant effect on the AlCl molecule and cannot be ignored. The SOC effect leads the energy level to be split and the Λ–S states to be mixed with a common Ω. To clarify the strong interactions between the electronic states of AlCl,we calculate the values of SOC matrix element|Hso|.The calculated SO matrix elements are plotted in Fig.3 as a function of internuclear distance. The symbols of computed SO matrix elements are listed in Table 3.From Fig. 3, we can see that |Hso| is in a range of 0 cm−1–160 cm−1. The PECs of A1Π state and b3Σ+state have a crossing phenomenon at R=2.7 ˚A. As illustrated in Figs. 1 and 3,at the crossing point between A1Π and b3Σ+states,the Hsovalue is −138 cm−1; and near the crossing point located is the v′=9 vibrational level of A1Π high(v′=9). Therefore,the predissociation of the A1Π(v′=9)state can occur through the SO coupling with b3Σ+state.

Table 3. SO matrix elements of Λ–S state.

Fig.3. Spin–orbit matrix elements of several Λ–S states,with definition of the SOi symbols in Table 3.
3.3. PECs and spectroscopic constants of Ω states
Taking the SOC effect into account,the two dissociation limits split into five dissociation limits.The 13 Λ–S states split into 24 Ω states, including six states of Ω=0+, five states of Ω=0−, eight states of Ω=1, four states of Ω=2, and one state of Ω = 3. The detailed dissociation relationships of Ω states and the corresponding energy splitting are illustrated in Table 4. The computed energy intervals of Al(2P3/2u)–Al(2P1/2u) and Cl(2P1/2u)–Cl(2P3/2u) are 120 cm−1and 875 cm−1, which are in excellent agreement with the experimental values[27–29]of 112 cm−1and 882 cm−1,respectively.As for the ion-pair limit Al+(1S0g)+Cl−(1S0g), we estimate the 1/R Coulomb compensation from R=6 ˚A to infinite distance. The computational value of the Al+(1S0g)+Cl(1S0g)is 245 cm−1higher than experimental result of 19109 cm−1,which maybe result from the fact that it is not far enough to treat the AlCl molecule as two independent ions at R=6 ˚A.
In Fig.4,we show the calculated PECs of the 24 Ω states of AlCl. The potential energy curves are plotted following the avoided crossing rule of states with the same symmetry. Taking the SOC effect into account, there are strong interactions and a number of avoided crossings between these Ω states,especially in the ultraviolet region. There are three avoided crossing points located at R=2.2 ˚A (Ω=0+), R=2.45 ˚A(Ω=0),and R=2.4 ˚A(Ω=1),respectively,which are shown in Fig. 4. The state Ω=3 is purely generated from the 13Δ state, therefore, the shape of the PEC for Ω = 3 is similar to that of the corresponding Λ–S state. For the remaining Ωstates, the shapes of the PECs are very complex in an energy range of 37000 cm−1–47000 cm−1.Some of them(e.g.0+(6),0+(3),0−(4),0−(2),1(3),and 1(6))have two or more shallow potential wells due to the SOC effect.

Table 4. Dissociation relationship of the Ω states of AlCl.

Fig.4. PECs of a total of 24 Ω states.
Based on our calculations,the spectroscopic constants of the bound states are computed and the results are listed in Table 5. From Fig. 4, we can see that the state X0+is almost completely derived from the Λ–S state X1Σ+. Therefore, the spectroscopic constants are also close to those of the X1Σ+state. By comparison,it can be obtained that the Deof X1Σ+state is 0.505 eV higher than that of X0+state. After considering the influence of SOC,the a3Π state splits into four states(0+(2),0−(1),1(1),and 2(1)),which have deep potential wells of 16260 cm−1,15901 cm−1,15834 cm−1,and 16246 cm−1,respectively. Comparing with the a3Π state, the Revalues of these four Ω states are shortened. However, the Tevalues of the four Ω states are larger than those of the corresponding Λ–S state. As can be seen from Table 5, only four states X0+,0+(2),1(1),and 2(1)are observed experimentally. Our results are in good agreement with the available experimental and previous theoretical values.[3,7,10,12,31,33]However,there is no experimental value for the vibration constant ωexe. So far, the 0−(1)and 1(2)states have not been observed in experiment.
As shown in Fig. 5, the Λ–S components of the (1)1,(1)2, (0)1, and (2)1 states are complex. With the change of R,the Λ–S components of 1(1)state gradually become a mixture of 23Π,b3Σ+,23Σ+,11Σ−,11Δ,and a3Π states. Besides,the Λ–S component of the 1(2) state is the mixture of A1Π,23Σ+,13Δ,a3Π,21Π,and b3Σ+states in a range of R=3 ˚A–4.5 ˚A. From Fig. 5, we can see that the Λ–S components of these states have a complex mixture at the internuclear distance range from 2.5 ˚A to 4.5 ˚A,which indicates that there are complex SOC interactions between these Λ–S states. And the position where the composition suddenly changes corresponds to the avoided crossing points of the PECs(see Fig.3).
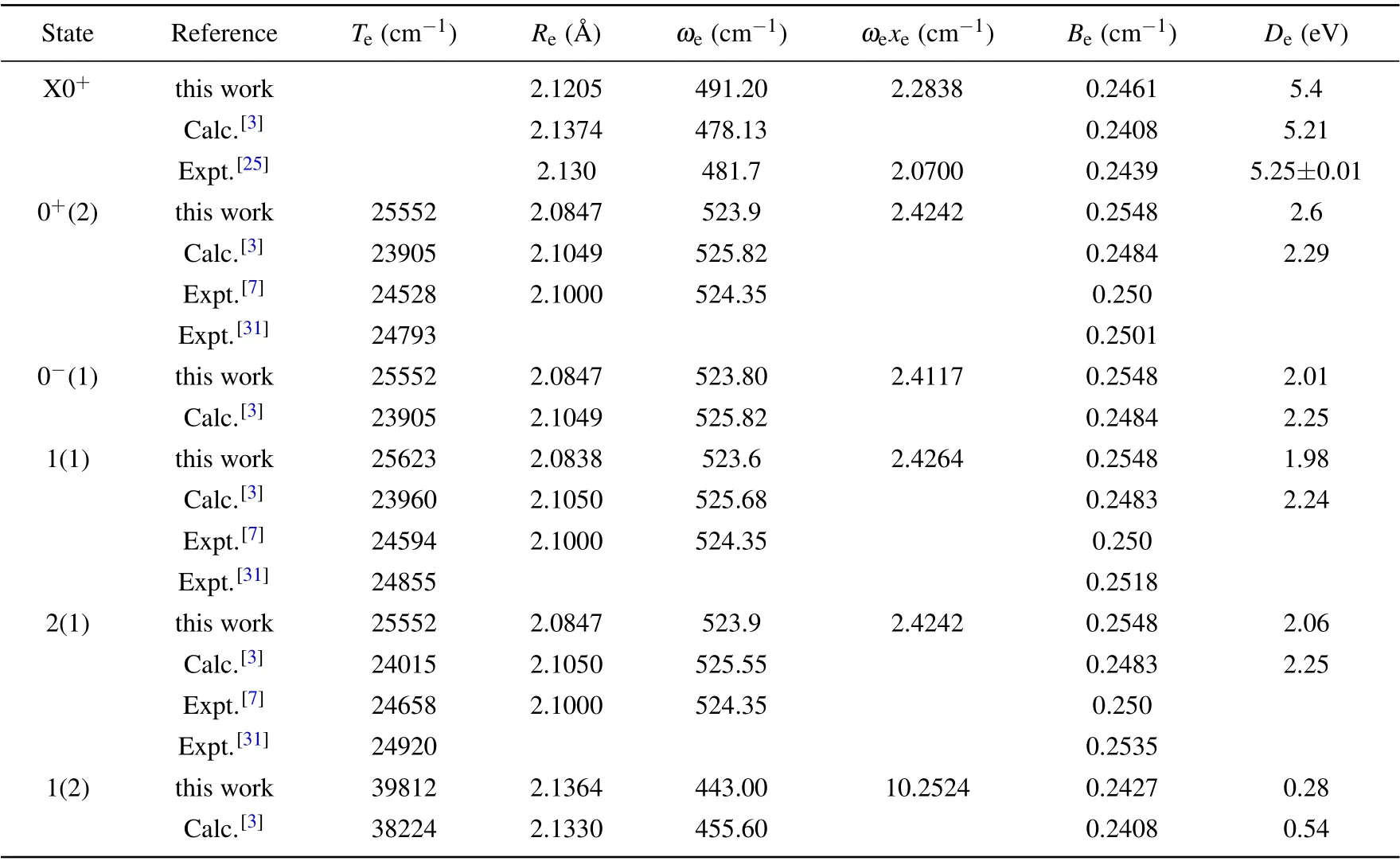
Table 5. Computed spectroscopic constants of Ω states of AlCl.

Fig.5. Curves of Λ–S component of X0+,0−(1),0+(2),1(1),(1)2,and 1(2)states.
3.4. TDMs,FCFs,and raiative lifetimes of AlCl molecule
The transition dipole moments containing 1(1)–X0+,1(2)–X0+, and 0+(2)–X0+are calculated each as a function of R in a range from 1.9 ˚A to 8 ˚A. The corresponding TDM curves are shown in Fig.6.

Fig.6. Transition dipole moments of AlCl.
It is obvious from the figure that the absolute TDM value of the 1(2)–X0+transition is much larger than the absolute TDM values of 1(1)–X0+and 0+(2)–X0+in the Franck–Condon region. The main reason is that the 1(2)–X0+transition comes mainly from the spin-allowed transition A1Π–X1Σ+, while the transitions 0+(2)–X0+and 1(1)–X0+come from the spin-forbidden transition a3Π–X1Σ+. It can also be seen that the TDM value of 1(2)–X0+decreases rapidly with the increase of R, which is due to the fact that the Λ–S components of 1(2) state changes from A1Π state to other triple states near R=3.2 ˚A.
The FCFs of the 0+(2)–X0+, 1(1)–X0+, and 1(2)–X0+transitions are calculated with the help of the LEVEL program,[34]and the results are displayed in Table 6. With the calculations of TDMs and FCFs,we calculate the radiative lifetime τ of the vibration level v′at a specified state,which is calculated from the following formula:

where TDM (in atomic unit) is the average electronic transition dipole moment, qν′ν′′is the Frank–Condon factor of the two vibration levels, ΔEν′ν′′ is the energy difference in units of cm−1.
The results of the radiative lifetime and the reference experimental values are listed in Table 7. The radiative lifetime of 1(2)–X0+is much longer than that of 0+(2)–X0+and 1(1)–X0+. The radiative lifetime for 1(2)–X0+is in unit of ns,and the other two are in unit of ms. Among them, the radiative lifetime of 0+(2)–X0+calculated by us has a certain gap with the theoretical value.[3]The reason is that the lifetime of spinforbidden 0+(2)(a3Π0+)–X1Σ+0+arises mainly from the spin–orbit couplings. In Ref.[3],only three Λ–S states,X1Σ+,a3Π,and X1Π are included in the SOC computations. While ourcomputations indicate that the 21Σ+state is mixed with the 0+(2)(a3Π0+) state, since the 21Σ+–X1Σ+is a spin-allowed transition, the mixing with 21Σ+state in the 0+(2)(a3Π0+)state will shorten the lifetime of 0+(2)(a3Π0+)–X1Σ+0+. The absence of high-lying 21Σ+state is the main source of difference in lifetime. The radiative lifetime τ of 1(1)–X0+is very close to a theoretical value.[3]The radiative lifetime τ of 1(2)–X0+is 5.48 ns,which is in agreement with experimental value of 6.4±2.5 ns.[35]

Table 6. Franck–Condon factors of selected transitions for AlCl.

Table 7. Spontaneous radiative lifetimes of AlCl molecule,with SOC coupling taken into consideration.
4. Conclusions
In this work,the low-lying states of the AlCl molecule are calculated by the high-level ab-initio MRCI-F12+Q method.We make different corrections to obtain the accurate spectroscopic constants of the three lowest electronic states of AlCl. The PECs of 13 Λ–S states are calculated, which are related to the two dissociation limits Al(2Pu)+Cl(2Pu) and Al+(1S)+Cl−(1S).On the basis of PECs,the calculated spectroscopic constants are in good agreement with the experimental values. The calculated PECs show that the A1Π and a3Π states intersect with some other excited states and the SOC coupling effect cannot be ignored. After considering the SOC effect,13 Λ–S states split into 24 Ω states,and the two dissociation limits split into five dissociation limits. With the help of calculated SO matrix elements,the predissociation mechanism of the A1Π state is analyzed. The radiative lifetime τ of 1(2)–X0+is 5.48 ns,which is in agreement with experimental value of 6.4±2.5 ns. Our work shows that the AlCl molecule has fairly accurate and detailed electronic structure and spectral information,which plays an important role in studying the electronic structure of molecule containing heavy atoms.
- Chinese Physics B的其它文章
- Corrosion behavior of high-level waste container materials Ti and Ti–Pd alloy under long-term gamma irradiation in Beishan groundwater*
- Degradation of β-Ga2O3 Schottky barrier diode under swift heavy ion irradiation*
- Influence of temperature and alloying elements on the threshold displacement energies in concentrated Ni–Fe–Cr alloys*
- Cathodic shift of onset potential on TiO2 nanorod arrays with significantly enhanced visible light photoactivity via nitrogen/cobalt co-implantation*
- Review on ionization and quenching mechanisms of Trichel pulse*
- Thermally induced band hybridization in bilayer-bilayer MoS2/WS2 heterostructure∗

