Honokiol Loaded Mixed Micelles Fororal Delivery Using Novel F127 and TPGS as Carriers
-, -, -, -, -, -g
(1. Affiliated Hospital of Integrated Traditional Chinese and Western Medicine, Nanjing University of Chinese Medicine, Nanjing, 210028, China; 2. Department of Pharmaceutical Analysis and Metabolomics, Jiangsu Province Academy of Traditional Chinese Medicine,Nanjing, 210028, China; 3. School of Pharmacy, Anhui University of Chinese Medicine,Hefei, 230031, China; 4. Hope Medicine (Nanjing) Co. , Nanjing, 211500, China)
ABSTRACT: OBJECTIVE The purpose of this research was to develop a self-assembled micelle using biocompatible copolymers Pluronic F127 (F127) and Vitamin E d-α-Tocopheryl polyethylene glycol 1000 succinate (TPGS) to enhance the oral bioavailability and anti-cancer efficiency of honokiol (HK). METHODS The optimized prescription honokiol micelle (HK-M) was prepared by an ethanol solvent evaporation method. HK-M was characterized by transmission electron microscopy(TEM) and HPLC. The dialysis bag method was used to assesse the cumulative amount of HK released from the HK-M. Caco-2 cells were applied to measure the permeability of HK-M. The bioavailability and in vivo anti-tumor effect were also evaluated. RESULTS At the ratio of 4∶1 (F127∶TPGS), the HK-M was transparent and colourless with a small size (23.28 ± 2.01)nm and a spherical shape. The apparent solubility of HK in HK-M was dramatically increased to 4.76 mg/mL, suggesting that HK-M had good stability. Furthermore, encapsulation in micelles led to a sustained release of HK. HK-M enhances HK's permeability across Caco-2 cell monolayer. Compared with free HK, there was a 1.17-fold increase in the relative oral bioavailability for HK-M. Moreover, HK-M achieved a higher inhibition rate on tumor volume (35.17%) than HK group (14.86%). CONCLUSION These findings present an oral HK micelle formulation containing F127 and TPGS, with improved solubility, increased oral bioavailability, and enhanced anti-cancer activity.
KEYWORDS: honokiol; F127; TPGS; micelles; Caco-2 cell; oral bioavailability; anti-tumor
Honokiol (HK), a natural product of biphenylene lignans, is isolated from the bark of Magnolia officinalis[1]. It has been reported that HK possess a series of positive effects, e.g. anti-cancer, anti-inflammatory, anxiolytic, anti-depressant, anti-convulsant and anti-nociceptive effects[2]. Furthermore, it is noteworthy that HK has been widely used in cancer therapies. The primary mechanism of HK is believed to have an inhibitory effect on tumor growth and may induce cancer cell apoptosis. Particularly, HK could inhibit DNA polymerases β and λ, and increase bleomycin sensitivity of human cancer cells[3]. A study from Sengupta et al. demonstrated that, HK inhibited the coactivation of Stat3 in an LKB1-dependent manner, result in the suppression of pluripotency factors expression. Further, they found that HK inhibited breast tumorigenesis in mice in an LKB1-dependent manner[4]. Moreover, HK targeted mitochondria to attenuate cancer progression and metastasis[5]. However, low solubility and bioavailability limits the application of HK[6]. Therefore, it is essential to exploit a novel formulation to ameliorate solubility, enhace oral bioavailability and incrase cancer prevention of HK.
Nano-drug carrier systems (NDCS), such as solid dispersions[7-9], liposomes[10-12], micelles[13], and nanoparticles[14-15], can be exploited to increase the bioavailability of hydrophobic drugs[16]. Among various preparation techniques, mixed micelle is regarded as one of the most potential and efficacious carrier attributed to the special core-shell structure[17-18]. The abilities of high drug loading, control drug release and increase membrane permeability make mixed micelles an excellent NDCS[19].
To ameliorate solubility, enhance oral bioavailability and incrase cancer treatment of HK,we established a novel NDCS by F127 and TPGS. TPGS, a derivative of natural vitamin E, is very stable and does not hydrolyze under normal conditions[20]. Those advantages make TPGS a valuable candidate for the formation of mixed micelles, e.g. more stable for drug delivery, and higher encapsulation efficiency[21]. In specific, TPGS can reverse multidrug resistance by inhibiting P-gp-mediated drug transport[22]. Pluronic has become a common material for preparing micelle owing to its non-toxic, non-irritating, non-immunogenic properties[23]. As the most prominent member amongst Pluronic©, Pluronic©F127 is well-known for the low toxicity and the ability to encapsulate any hydrophobic ingredients[24-25]. F127 is able to change the structure of the cell membrane, and promote the transmembrane absorption of P-gp substrate[26-27]. Preparation of mixed micelles using F127 and TPGS as carrier materials is more stable than single micelle. Meanwhile, mixed micelles can combine and manifest advantages of both materials to promote the oral absorption of drugs[27].
In the present study, a novel NDCS mixed micelle encapsulating HK (HK-M) was prepared using two surfactants (F127 and TPGS). The shape and size of HK-M were determined by transmission electron microscopy (TEM) and laser particle size analyzer.Invitrostudies were carried out to ascertain the release and the permeability, and the bioavailability of HK-M was evaluated in male Sprague-Dawley rats (SD rats) and the anti-tumor activity was estimated by male nude miceinvivo. Our study aimed to provide a foundation for the development of oral HK preparations.
1 Materials and methods
1.1 Materials
HK standards, HK (purity > 98%), and magnolol standards (purity > 98%, were used as an internal standard) were purchased from Meilun Biological Technology Co., Ltd (Dalian, China). TPGS were purchased from Sigma-Aldrich (St. Louis, MO, USA). Pluronic F127 was purchased from BASF Ltd. (Shanghai, China). Dulbecco's modified Eagle's medium (DMEM) was obtained from Thermo Fisher Scientific (Bridgewater, NJ, USA). The Caco-2 cell line and the MCF-7 cells were acquired from Nanjing KeyGEN Biotech. Co. Ltd. (Nanjing, China). Milli-Q water (Millipore, Bedford, MA, USA) and chromatographic grade methanol (Tedia Company Inc., Fairfield, CT, USA) were used for high performance liquid chromatography (HPLC) analysis. All other reagents were of analytical grade.
1.2 Animals
The procedures involving animals and their care were conducted in conformity with the ARRIVE guidelines of Laboratory Animal Care (Kilkenny, Browne, Cuthill, Emerson & Altman, 2012). Male SD rats (200 ± 20)g and male nude mice (22 ± 2)g were purchased from the SLAC Lab Animal Center of Shanghai (Shanghai, China). All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the Jiangsu Provincial Academy of Chinese Medicine(AEWC-20180711-36).
1.3 Preparation of HK-loaded mixed micelles
HK-M was prepared via ethanol solvent evaporation method[28]. In brief, HK was added to ethanol in which TPGS and F127 co-dissolve in different proportions by slow stirring to form a transparent solution. The solution remove the ethanol by vacuum drying method. The product was reconstituted in deionized water to obtain a pellucid solution. The solution was filtered with a 0.45 μm membrane to remove the non-incorporated HK.
1.4 Characterization of HK-M
1.4.1 The shape and size analysis The particle size and Zeta potential of HK-M were measured by particle size analyzer (ZEN-3600, Malvern Instruments, Worcestershire, UK). Polydispersity index (PDI) assess the particle size distribution of HK-M. The shape of HK-M was characterized by TEM (JEM-200CX, JEOL, Japan).
1.4.2 Drug loading and entrapment efficiency The concentration of HK was measured by HPLC system, the separation module: Waters 2695; the UV/Vis detector: Waters 2489; the reversed-phase C18 column:(250 mm×4.6 mm, 5 μm); the mobile phase: methanol/water (75∶25,v/v); the flow rate: 1.0 mL/min; the detection wavelength: 290 nm; the column temperature: 35 ℃; The injection volume: 10 μL[29].
The encapsulation efficiency (EE %) and drug loading (DL %) of HK-M were determined by microporous membrane filtration. In short, the HK concentration of the micelle solution before and after passing through a 0.22 μm microporous membrane was measured. EE% and DL% were calculated using the following equations[30].
EE%=weight of HK in the filtered mice llessolution / weight of HK in the unfiltered mice llessolution×100% (1)
DL%=weight of HK in the filtered mice llessolution / weight of HK in the filtered mice llessolution+weight of added excipients×100%
(2)
1.4.3 Dilution and storage stability In storage stability studies, the optimized samples HK-M (n=3) were stored at 4 ℃.The average size and EE% of the HK-M were then quantified at 1, 3, 7, 10, and 15 days to appraise the storage stability, respectively. The dilution stability of HK-M was studied by diluting in deionized water (1~250 folds) at room temperature (25 ℃). The particle size and EE% after each dilution fold was measured[31].
1.5 In vitro release studies
The dialysis bag method was conducted to estimate the release behavior of HK-M and free HKinvitro. Briefly, 1 mL suspension containing HK-M or HK (dissolved in methanol) was prepared. Then 1 mL solution was introduced into dialysis membrane bags (molecular weight cut off of 3 500 g/mol; Green Bird Inc., Hefei, China). The dialysis bags were immersed into 200 mL fresh PBS (pH 1.2 and 6.8) containing 0.5 % (v/v) Tween 80 (Sinopharm Chemical Reagent Co., Ltd) with a stirring speed of 140 r/min at 37 ℃. At predetermined time intervals (0, 0.5, 1, 2, 4, 6, 8, 10, 14, 24 h), the 1 mL of the dissolution medium were withdrawn and replaced with an equal volume of fresh release medium.The above samples were centrifuged at 12 000 r/min for 5 min at 4 ℃. The amount of HK released in the supernatant was evaluated by HPLC mentioned above.
1.6 Transport experiments
The human colon carcinoma Caco-2 cell line has been recognized by the FDA in the application of human intestinal absorption. In our study, Caco-2 cells were used to estimate the permeability of HK-M[32]. Caco-2 cells were cultured in DMEM. Caco-2 cells were seeded in a Transwell plate until the cell density reached 1×106cells/cm2. Monolayer cells were differentiated and formed around 21 days after the initial seed. The transepithelial electrical resistance (TEER) values more than 600 Ω/cm2were used for drug transmembrane.
In the apical side (AP) to basolateral side (BL) transport test, 0.5 mL HK (dissolved in DMSO, 50 μg/mL) or HK-M (the concentration of HK was 50 μg/mL) solution was added to AP as the supply pool and 1.5 mL HBSS (pH 7.4) was added to the BL as a receiving pool. In the BL to AP transport test, 1.5 mL HK or HK-M solution was added to the BL and 0.5 mL HBSS was added to the AP. Aliquots of 200 μL from the receiving pool were taken out at 120 min. The above samples were added to 200 μL of methanol, vortexed and sonicated for 20 min, centrifuged at 12 000 r/min for 5 min at 4 ℃. The concentration of HK in the supernatant was measured by HPLC. The apparent permeability coefficients (Papp) were calculated using the following equation:
(3)
dQ/dt (μmol·L-1·s-1) is the transport rate, A (cm2) is the surface area of the transport film, and C0(μmol·L-1·cm-3) is the initial concentration of HK in the donor.

(4)
the Papp(AP-BL)is the absorption permeability and Papp(BL-AP)is the secretory permeability.
1.7 Pharmacokinetic studies in vivo
For the pharmacokinetic study, SD rats were randomly divided into two groups(n=6 for each group) and fasted overnight prior to the experiment. Group Ⅰ and Ⅱ were orally administrated with free HK (80 mg/kg in 0.5% hydroxypropyl methylcellulose) and HK-M (at doses equivalent to 80 mg/kg HK), respectively.
At predetermined times (10, 20, 30 and 45 min and 1, 2, 4, 6, 8, 10 and 12 h), blood samples were collected into the heparinized tubes from the orbital vein and immediately centrifuged to obtain the supernatant. 100 μL supernatant with 10 μL internal standard solution (10 μg/mL) were vortexed for 15 s. 500 μL methanol was added then vortexed for 3 min and centrifuged at 14 000 r/min for 5 min at 4 ℃. The supernatant was transferred and evaporated to dryness under a nitrogen atmosphere. The dried residue was rinsed with 100 μL methanol and water (1∶1) and centrifuged at 14 000 r/min for 5 min. After centrifugation, 20 μL of the samples was injected into HPLC. The mobile phase was acetonitrile with 0.2% formic acid (50∶50) (v/v). Other chromatographic conditions remained the same as previously described[33].
1.8 In vivo anti-tumor activity of HK-M
1.8.1 Animal grouping and model establishment The male nude mice (22 ± 2) g were maintained under pathogen-free conditions with free access to food and water for one week. A volume of 0.1 mL MCF-7 cells were resuspended in logarithmic growth phase at a density of 1×107cells/mL, and then inoculated into the right iliac crest of male nude mice to establish models. When the tumor volume reached 60 mm3, the mice were randomly divided into three groups (n=6 for each group): control, HK, and HK-M.
1.8.2 Administration and treatment The control, the HK and the HK-M group were intragastrically administrated with 0.9% saline solution, free HK (50 mg/kg in 0.5% hydroxypropyl methylcellulose) and HK-M (at doses equivalent to 50 mg/kg of HK) from the 1st to the 12th day, respectively. Tumor size was monitored with a caliper in two dimensions (length and width) was measured at day 1, 3, 5, 7, 9, 11, and 13. Tumor volumes were calculated based on the equation and analyzed statistically[34]:
At the end of the experiment, the animals were euthanized and tumors were harvested and weighed. And then the tumor was subjected to hematoxylin and eosin (HE) staining to evaluate the pathological changes.
1.9 Statistical analysis

2 Results
2.1 Characterization of HK-M
The physicochemical characteristics parameters of HK-M were showed in Table 1. The results indicated that the ratio of F127 and TPGS at 4∶1 could increase the solubility of HK to the greatest extent,yielding the highest solubility at 4.76 mg/mL. Simultaneously, HK loading efficiency was up-regulated to 8.27% and entrapment efficiency was raised to 91.64% as well. As observed from TEM (Figure 1), the microstructure of HK-M was spherical and homogeneous, and the size distribution appeared well intensive at 23.28 nm with 0.068 PDI value (Figure 2A). The zeta potential of HK-M was -2.43 mV (Figure 2B).

Table 1 Characteristics of

Figure 1 TEM micrographs and image of HK-M

Figure 2 Particle size (A) and zeta potential (B) of HK-M
As depicted in Figure 3A, there was no significant change in the average size and EE% after 15 days' storage. Moreover, no turbidity or layer separations were observed in HK-M after 15 days' storage.

A B
The particle sizes and EE% of HK-M showed no significant change in the dilution stability study (Figure 3B), suggesting that formulation had a stabilizing effect against dilution. This phenomenon could be explained by the fact that HK was spontaneously incorporated into the micelle cores in the aqueous environment.
2.2 In vitro release
As described in the methods,invitrocumulative release profiles of HK from free HK or HK-M in simulated gastrointestinal medium (PBS, pH 1.2 and 6.8) were shown in Figure 4. A slower release rate was obtained for HK-M while the free HK was faster in both media. In the first 10 h, HK displayed a burst release at both pH 1.2 and 6.8. Free HK showed corresponding values of 68.16% and 85.40%, while 19.82% and 11.12% of HK were released from HK-M at pH of 1.2 and 6.8, respectively. Based on the cumulative release of HK-M at pH 1.2 was greater than that at pH 6.8. We suspected that the harsh environment within the stomach might break the structure of hydrophilic segments of HK-M, but does not destroy the core-shell structure of HK-M. Ultimately, the drug would be safely delivered to the small intestine and be absorbed by small intestine. These results inferred that HK-M efficiently solubilize HK and have the potential for use as oral bioavailability enhancers.

A B
2.3 Transport of HK and HK-M
In this study,parameters for transport (permeability and efflux ratio) of HK and HK-M from two directions (AP-to-BL and BL-to-AP) were investigated. As shown in Table 2, for AP-BL transport, HK-M significantly increased the permeability of HK (P<0.05). Papp[(5.72±0.39)×10-7cm/s] was higher than that of the HK group [(3.16±0.35)×10-7cm/s]. For BL-AP transport, Pappof HK-M was (4.33±0.31)×10-7cm/s, a certain degree which was higher than that of HK [(2.57±0.29)×10-7cm/s]. The efflux ratio was reduced from 0.81 to 0.76. These results indicated that HK-M increased the absorption of HK.

Table 2 Permeability and efflux ratio of HK and HK-M in Caco-2 cell
2.4 Pharmacokinetic studies
The plasma concentration-time curve and the pharmacokinetic parameters of HK and HK-M were shown in Table 3 and Figure 5. After oral administration of HK-M, the plasma concentrations of HK increased to Cmax(0.940±0.075) μg/mL at (0.708±0.102) h. While in free HK group, Tmaxwas (0.958±0.102) h and Cmaxwas (0.415±0.023) μg/mL. HK-M increased Cmaxnotably (P< 0.01), signifying that mixed micelles could extensively increase oral bioavailability of HK. Additionally, the half-life (T1/2) of HK-M decreased from (5.123±1.183) h to (3.619±0.331) h, which indicated that mixed micelle promote drug absorption into the blood. The average area under the curve from time 0 to infinity (AUC0-∞) of free HK and HK-M were (5.915±0.687) and (3.365±0.425) μg·mL-1·h-1, respectively. The bioavailability of HK-M relative to that of free HK was 175.8%. The relative bioavailability of HK-M group was markedly higher than that of free HK group (P<0.01).
He immediately summoned all the best doctors in the country, and they came with all their prescriptions14 and their medicine bottles, but next day the princess was stiff and cold in death

Table 3 Pharmacokinetic parameters of HK and
2.5 Anti-tumor effect

A B
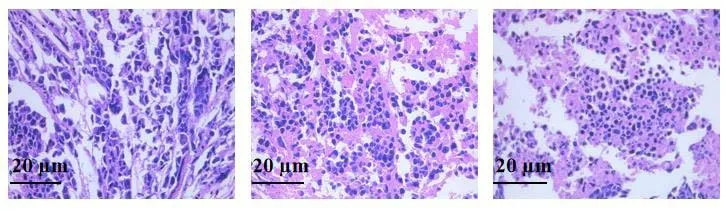
A B C
The tumor xenograft model was established to investigate theinvivoanti-tumor effect of HK-M. Both HK and HK-M exhibited therapeutic effects on tumors, accompanied with necrosis in the tumors after administration. Compared to HK therapy, HE staining of tumors after HK-M treatment showed enlarged intercellular spaces and increased tissue necrosis Figure 6. The volume-time curve and final tumor volumes during 2 weeks treatment was shown in Figure 7. Compared with control group, both free HK and HK-M displayed a noteworthy anti-tumor effects at the same doses. But the inhibition rate of HK-M was 35.17%, which was extremely higher than that of free HK (14.86%).

Figure 5 The plasma drug concentration-time curve in rats after oral administration of 80 mg/kg of HK and HK-M
3 Discussion
Among the several types of nano-sized carrier systems, e.g. nano-spheres, antibodies, HK is considered as an effective therapeutic option. However, low oral bioavailability have limited its effect. Thus, the proper delivery rote for HKinvivoremain challenging.
Amounts of new technologies have been established to modify the solubility and to improve oral absorption of poorly soluble drugs. In recent years, mixed micelles have attracted much attention for its strong solubilizing effect on insoluble drugs.Therefore, developing novel mixed micelles to improve the oral bioavailability of HK is essential. In this study, a mixed micelle of HK was formulated. Amphoteric polymer adjuvants (F127 and TPGS) self-assembled into mixed micelles wrapping the HK into the core. TEM showed that HK-M was spherical and homogeneous. HK can be efficiently encapsulated into the core of HK-M, which is an important prerequisite for increasing HK solubility.
Especially, free HK could only dissolve in some suspending agent or auxiliary solvent including Cremophor EL, ethanol, tween 80 and carboxymethylcellulose sodium[35], while the apparent solubility of HK in HK-M was dramatically increased to 4.76 mg/mL. The HK was encapsulated inside the micelles to prevent from the gastrointestinal tract environment, resulting in a sustained and controlled manner of HK releasing. Consequently, HK concentration in the gastrointestinal fluid changed smoothly. The particle size of HK-M was in the range of 10-100 nm, which facilitated the transmembrane transport of drug-loaded micelles in the gastrointestinal tract. The critical micelle concentration of micelles composed of polymers is lower than micelles composed of low molecular surfactants, so it can withstand the dilution of gastrointestinal fluids.
HK-M increased the transport ability of HK to across the Caco-2 cell monolayers. This result might be attributed to the usage of F127 and TPGS as carrier materials, which not only increase the aqueous solubility but also enhance the permeation of HK. Furthermore, HK-M was able to prolong the drug retention time in the gastrointestinal tract and increase the drug absorption due to the high adhesion to the intestinal wall. Additionally, we chose two different pH media to simulate the gastrointestinal environment, the drug remains within the micelle unless the shell structure of micelles has been destructed. The structural stability of mixed micelles prevented the drug escaping from the core of the micelles. Micelles protected the drug against damage from gastric acid and digestive enzymes, contributing to the better sustained-release property of HK.
Invivoexperiment further confirmed that HK-M could significantly increase the plasma concentration of HK, and led to the increment of anti-tumor effect of HK. Collectively, the mixed micelles system formed by F127 and TPGS could effectively enhance the anti-cancer efficacy of HK.
4 Conclusion
An oral NDCS based on F127 and TPGS mixed micelle was exploited to enhance the oral absorption of HK. HK was encapsulated into the core of HK-M, and exhibited a sustained release behavior. HK-M considerably enhanced the solubility, oral bioavailability and anti-tumor efficacy of HK.Thus, HK-M has an immense clinical application potential and prospect as a promising carrier for HK in cancer treatment.

