磷化钴封装在磷富集的三维多孔碳及其双功能氧电催化性能研究
肖瑶,裴煜,胡一帆,马汝广,王德义,王家成,*
1 西华大学理学院,成都 610039
2 中国科学院上海硅酸盐研究所,高性能陶瓷与超微结构国家重点实验室,上海 200050
1 Introduction
Rechargeable ZABs have attracted much attention due to their portability and high power density for resolving the energy shortage and environment pollution issues1,2. However, the application of ZAB is greatly limited duo to the sluggish kinetics at the air electrode, which highly depends on the expensive Ptgroup catalysts3,4. To address these issues, many researches have spent energy to study low-cost and high-efficiency catalysts to replace noble metal Pt/RuO25–7. Usually, a large number of active sites and high activity of active species are indispensable for efficient catalysts. For the former aspect, by adjusting the geometry of the catalyst, such as, engineering catalyst morphology with hierarchical pores or reducing the particle size,it can increase the electrochemically active sites8–12. For the latter aspect, by regulate the electronic structure of the catalytic active center, it has an appropriate adsorption energy for the intermediate during the reaction13–16.
Transition-metal phosphides (TMPs) are ideal candidates because of their good conductivity and high activity. Especially,Co2P-based catalysts are widely studied based on excellent OER17–19. However, there are few reports of Co2P-based materials as efficient ORR catalysts. For the cathode electrocatalyst of ZAB, it is indispensable to have bifunctional catalytic activity. So, there is a huge challenge to elaborately design Co2P-based catalyst with outstanding ORR performance for ZABs. Recently, some studies reported that the doping of heteroatoms with different electronegativities is an available method to enhance the activity of carbon materials by tuning electronic properties and conductivity20–23. For example, Daiet al. prepared vertically-arranged nitrogen-containing carbon nanotubes (VA-NCNTs) that exhibited highly efficient ORR activity. Because the introduction of more electronegative nitrogen atoms into the carbon plane of conjugated nanotubes will cause the adjacent carbon atoms to carry relatively high positive charges24. Moreover, many researchers suggest that the chemical environment of TMPs such as CoP25,26, Co2P27,28and NiCo2P229located plays a crucial role for stable ORR and OER.Chenet al. reported that nitrogen and phosphorus dualcoordinated iron to boost ORR performance30. To this end, it is effective to synthesize highly efficient ORR catalysts based on Co2P and P-containing carbon with their catalytic performance better than that of noble metals.
What’s more, catalytic reaction is a complex process, which not only depends on the activity of the catalyst, but also relates to number of active sites31. Recently, tremendous efforts have been devoted to fabricate catalytic materials with various structures, for instance, core-shell, array and hierarchical porous structure, to improve the electroactivity32–35. Due to the hierarchical porous structure, it not only facilitates mass and electron transport, but also exposes more active sites. For example, Guoet al. preparing 3D metal sulfide (MxSy)nanomaterials based on the 3D hierarchical porous structure exhibits satisfactory performance36.
Here, we provide a method to fabricate bifunctional oxygen electrocatalyst of Co2P-based materials by geometrical optimization and electronic adjustment. Co2P nanoparticles wrapped in P-doped porous carbon aerogels show excellent ORR and OER activity. The results indicate that the assynthesized catalyst deliver outstanding ORR activity with halfwave potential (E1/2) of 0.84 V more than other Co2P-based catalysts and comparable to commercial Pt/C. The increase in catalytic activity is mainly due to the regular hierarchical pores,high specific surface area and synergy between Co2P and Pdoped carbon matrix, which significantly optimize the electronic structure of Co2+in Co2P and thus weaken the binding force between the adsorbed OH* and the surface Co atoms in the determination step. When used as a cathode catalyst, Co2P-PCA-800-based ZAB exhibits a high open circuit voltage (1.44 V) and high power density. In addition, ZAB also exhibits higher specific energy density and better stability than noble metal bases. Finally, we believe that this method is universal and can be used to prepare other similar catalysts to solve the energy crisis.
2 Experimental
2.1 Chemicals
Cobalt nitrate hexahydrate (AR), 70% (w, mass fraction)Phytic acid solution, k-Carrageenan and 5% (w) Nafion solution were come from Aldrich (China). The 20% (w) Pt/C and 99.9%(w) RuO2were purchased from Johnson Matthey (UK). All the reagents were utilized without further purification.
2.2 Preparation of Co2P-PCA hybrid
The 1.2% (w) k-carrageenan aqueous solution was prepared at 80 °C using magnetic stirring. The Co(NO3)2solution was dropped in carrageenan solution and stirred 60 min. Then phytic acid solution was slowly added and stirred for another 60 min.Finally, it was then cooled at room temperature to obtain carrageenan-PA-M hydrogel, washed and frozen to obtain carrageenan-PA-M aerogels. The carrageenan-PA-M aerogels were pyrolyzed at 800 °C for 3 h in Ar to obtain Co2P-PCA-800.Furthermore, Co-doped carbon aerogels (Co-CA) and P-doped carbon aerogels (PCA) were also made using the same method without adding PA or Co(NO3)2. Pure Co2P nanoparticle was prepared for comparison according to the literature37.
2.3 Electrocatalytic activity evaluation
All electrochemical measurements were conducted in a threeelectrode configuration with CHI 760E electrochemical workstation at room temperature. The saturated Hg/HgCl2electrode (SCE) and graphite rod were used as the reference and counter electrodes, respectively. A glassy carbon electrode with catalyst (0.5 mg·cm−2) was used as working electrode. To prepare the working electrode, disperse 5 mg catalyst in a solution containing 500 μL of deionized water, 500 μL of ethanol, and 20 μL of 5% (w) Nafion solution for sonication for 30 min. And the catalyst ink (20 μL) was pipetted onto a polished glassy carbon electrode. For comparison, 20% (w) Pt/C ink with the same load was prepared. The electrochemical measurement was conducted in O2-saturated 0.1 mol·L−1KOH for ORR, 1 mol·L−1KOH for OER. The potential, measured against SCE converted to potential versus RHE according toERHE= 0.2415 +ESCE+ pH × 0.059. Linear sweep voltammetry (LSV)measurements were executed with a scan rate of 10 mV·s−1. The numbers of electrons transferred (n) during ORR was calculated by the following Koutecky-Levich equation at various electrode potentials based on the different rotating speeds. At the same time, the number of electrons transferred (n) and the hydrogen peroxide production (%H2O2) rate were calculated by rotating ring-disk electrode (RRDE) test.
2.4 Assembly of a zinc-air battery (ZAB)
In order to test ZABs, the prepared catalyst ink was uniformly coated on carbon paper as the cathode. A polished Zn plate was used as the anode and 6 mol·L−1KOH solution containing 0.2 mol·L−1Zn(OAc)2was used as the electrolyte. The mass loading on carbon paper was 0.62 mg·cm−2. For comparison, a mixture of 20% Pt/C and RuO2(mass ratio of 1 : 1) with the same loading was coated onto carbon paper as the cathode. The electrochemical performances of ZABs, such as cycling ability tests and specific capacities texts were recorded by a Land CT2001A system.
3 Results and discussion
The experiment process is schematically described in Fig. S1.The k-carrageenan macromolecules are random coil-like structures in aqueous solution at 80 °C. Co(NO3)2and phytic acid solution are slowly added because metal cations can induce the conversion of random coil carrageenan chains into a doublehelix structure and become carrageenan-M hydrogels36. The obtained hydrogels converted to a Co2P@P-doped carbon aerogelviafreeze drying and pyrolysis at 800 °C (Co2P-PCA-800). In this step, carrageenan as a carbon source and template and unstable small molecules decompose to form a 3D porous architecture. The X-ray diffraction (XRD) results show that the Co2P nanoparticles and Co2P-PCA-800 have main characteristic at around 40.7°, 41.0°, 43.3°, 52° and 54.1°, which could be correspond to the (121), (220), (211), (130), and (002) crystal planes of Co2P, respectively (JCPDS No. 32-0306)38(Fig. 1a).Field emission scanning electron microscopy (FESEM) shows that Co2P-PCA-800 exhibits a 3D self-supporting honeycomb porous structure. The interconnected macropores and mesopores can be observed in the network (Fig. 1b and Fig. S2). Here, we adjust the geometry morphology of carrageenan aerogel by doping Co(NO3)2and phytic acid to obtain regular hierarchical porous structure (Fig. S3). And compared to Co-CA and PCA,the Co2P-PCA-800 has a regular honeycomb porous structure while Co-CA and PCA are broken trivial network structures. The regular interconnected porous structure can be beneficial to mass transfer and ensure uniform distribution of ion current. However,when the structure collapses, the incomplete porous structure not only increases the mass transfer distance, but the lower resistance is the main channel for electrolyte ions. Therefore,most active sites are abandoned during the catalytic process39,40.And the transmission electron microscopy (TEM) shows that the Co2P nanocrystals with a size of 70–100 nm are embedded in the carbon (Fig. 1c). As shown in Fig. 1d, the lattice fringe of 0.226 nm corresponds to the (121) crystal planes of Co2P. The presence of the Co2P crystals is further confirmed by selected area diffraction (ASED) (Fig. 1e). And the elemental mapping images show a relatively uniform dispersion of Co, P and C elements and Co2P nanoparticles are encapsulated in a P-rich carbon matrix (Fig.1f).

Fig. 1 a) XRD images and Co2P; b, c) SEM and TEM images of Co2P-PCA-800; d) HRTEM image of Co2P-PCA-800; e) SAED pattern of Co2P-PCA-800; f) HADDF and EDS elemental mappings of Co2P-PCA-800.
Further investigated the composition and valence of the sample. XPS indicates the surface composition of C, Co and P in the Co2P-PCA-800. And the element contents are estimated as the percentage of 96.95%, 0.22% and 2.84%, which verifies the doping of P and Co within the carbon framework (Fig. S4). C 1sspectrum with binding energies of 284.7, 286.6, 293.2 and 296.0 eV can be ascribed to the C―C, C―O, C=O and O=C―O―P type bonds of Co2P-PCA-800 in Fig. 2a. And compared to the C 1sof Co-CA, it has a strong electron-withdrawing peak of O=C―O―P. Therefore, the doping of P can adjust the charge distribution of local Co2+. This can also be found in C 1sof PCA(Fig. S4b). This result is also consistent with that from Co 2ppeak. In the Co 2pspectrum, there are two orbital double peaks of Co2+, accompanied by two satellite peaks. And the peaks at 781.2 and 796.7 eV correspond to the Co 2p3/2and Co 2p1/2orbitals, respectively. At the same time, compared with metallic cobalt (778.2 eV), the cobalt 2p3/2peak (Fig. 2b) shifted to a more positive value at 781.2 eV, indicating that Co2+in the Co2PPCA-800 catalyst has a partial positive charge20. In the P 2pspectrum (Fig. 2c), the binding energies of 132.8, 132.9, 133.8 eV and 135.2 eV can be corresponded to the P―C, C3―PO3,C―PO3and C―O―PO3type bonds respectively41. And the P―Co peaks at 129.6 and 130.8 eV can also be found. The charge transfer between Co2P and P-doped carbon can change the electronic structure of each other, thereby having better performance13. The above results indicate that we successfully prepared Co2P and P-doped carbon hybrid materials, which are potential as active bifunctional electrocatalyst.
Raman spectroscopy for further analysis (Fig. 2d). There are two peaks around 1300 and 1600 cm−1, corresponding to the D and G peaks, which are characteristic peaks of defective carbon and graphene carbon layer, respectively. As observed, the ID/IGratios of Co2P-PCA-800 is 0.98 indicating that a higher defective degree present in Co2P-PCA-800 than the control samples. The presence of defects will change the charge distribution of adjacent carbons, which may be beneficial to the electrochemical reaction42–44. In order to determine the specific surface area and porosity of the prepared material, the N2adsorption-desorption test was performed. The N2adsorption-desorption isotherms of all the samples show typical type IV adsorption isotherm (Fig.2e) indicating the presence of mesopores. Combining with SEM,we know that the carbon materials are a hierarchical porous structure containing mesopores and macropores. The BET specific surface area of Co2P-PCA-800 is 266.98 m2·g−1higher than other comparative samples in Table S1. And the samples possess mesoporous structure in Fig. 2f. As we all know,pyrolysis temperature is also critical to the electrocatalytic performance of carbon materials, so we also prepare samples at different temperature (Fig. S5). The Co2P-PCA-800 also has a higher defective degree and larger specific surface area. With high specific surface area, the electrocatalyst can be expected to have excellent performance in the ORR/OER electrochemical test.

Fig. 2 XPS spectrum of the synthesized Co2P-PCA-800 composite: a) C 1s; b) Co 2p and c) P 2p; d) Raman spectrum of Co-CA, PCA and Co2P-PCA-800; e, f) nitrogen adsorption-desorption isotherms and pore-size distribution of Co-CA, PCA and Co2P-PCA-800.
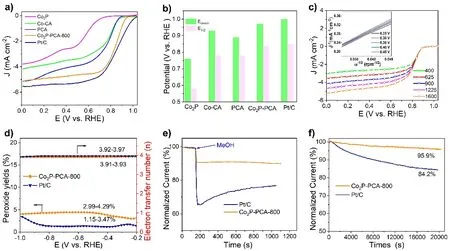
Fig. 3 a) ORR polarization curves; b) Comparison of the onset (Eonset) and half-wave (E1/2) potentials of different catalysts; c) LSV curves of the Co2P-PCA-800 at a different rotation rate (inset: K-L plots based on the ORR curves of Co2P-PCA-800 at different potentials (vs. RHE);d) Number of electrons transferred and peroxide yields of Co2P-PCA-800 and 20% Pt/C; e) Tolerance toward methanol text of Co2P-PCA-800 and 20% Pt/C; f) Current–time (i–t) chronoamperometric responses for the ORR of the Co2P-PCA-800 and 20% Pt/C.
The ORR activities of the Co2P-PCA-800 electrocatalysts were first measured by three-electrode system. The linear sweep voltammogram curves show the Co2P-PCA-800 with higher onset potential (Eonset) of 0.97 V (vs. RHE) and half-wave potential (E1/2) of 0.84 V (vs. RHE) outperforming Co-CA (0.92 V, 0.78 V), PCA (0.89 V, 0.77 V) and Co2P (0.75 V, 0.67 V) (Fig.3a). And theE1/2of Co2P-PCA-800 is comparable with Pt/C. We compare theEonsetandE1/2of the catalysts and Co2P-PCA-800 possess more positive half-wave potential (Fig. 3b). Co2P-PCA-800 shows better ORR catalytic activity indicating that the formation of the hybrid structure of Co2P and P-doped porous carbon has suitable adsorption energy. Also, we compare the ORR catalytic activity of the samples at different pyrolysis temperatures and others Co2P-electrocatalysts reported (Fig. S6 and Table S2). Compared to others, Co2P-PCA-800 also has satisfactory ORR catalytic activity, which mainly comes from the regular hierarchical porous structure and local charge change by P-doping. The LSV at different rotation rates are tested, and the results fitted by the Koutecky-Levich (K-L) plot (Fig. 3c).As the rotation speed increases, the current density increases accordingly, and the electron transfer number (n) is 3.9,suggesting that the ORR process is a primary four-electron reaction. In the meantime, the electron transfer number and hydrogen peroxide (H2O2%) are evaluated by rotating ring-disk electrode measurements. Similarly, the RRDE test shows that the Co2P-PCA-800 catalyst has a good selectivity, showing a higher electron transfer number (n) ~3.9 and a lower hydrogen peroxide yield 5% (Fig. 3d). The methanol tolerance and stability of Co2PPCA-800 are tested by chronoamperometric measurement. As shown in Fig. 3e, Co2P-PCA-800 exhibits excellent tolerance toward the methanol crossover. On the contrary, the chronoamperometric current of Pt/C catalyst dropped sharply,while the Co2P-PCA-800 was basically unchanged after methanol injection. Moreover, the chronoamperometric responses of Co2P-PCA-800 and Pt/C catalysts are measured at 0.4 V and shows that Co2P-PCA-800 has better stability than Pt/C (Fig. 3f). The results indicated that Co2P nanoparticles dispersed in P-doped carbon matrix can effectively improve the catalytic efficiency.
What’s more, Co2P-PCA-800 also has satisfactory OER performance. The potentials corresponding to the current density of 10 mA·cm−2(E10) for Co2P-PCA-800, Co-CA, and PCA are 1.70, 1.72 and 1.73 V, respectively, suggesting the enhanced and excellent OER performance for Co2P-PCA-800 (Fig. S7a). Fit the LSV polarization curve and calculate the Tafel slope to evaluate the dynamics of the catalyst. The Tafel slope of Co2PPCA-800 is 81.1 mV·dec−1(Fig. S7b), which is lower than those of Co-CA (94.4 mV · dec−1) and PCA (113.1 mV·dec−1),suggesting a more favorable kinetics of Co2P-PCA-800, while the improvement of the kinetics could be owing to the more active sites and better conductivity. To further illustrate, we conducted an electrochemical impedance test (Fig. S7c). The smallest diameter of the semicircle for Co2P-PCA-800 in the Nyquist plot indicates the high conductivity of Co2P-PCA-800.The OER performance also depends on electrochemically active surface area (ECSA). To compare ECSA, we measure the electrochemical double-layer capacitances of samplesviaa simple efficient cyclic voltammetry (CV) (Fig. S8). Co2P-PCA-800 exhibits aCdlof 12.9 mF·cm−2, which is larger than that of Co-CA (6.0 mF·cm−2) and PCA (0.5 mF·cm−2) (Fig. S7d). This high ECSA of Co2P-PCA-800 is ascribed to the larger specific surface. The outstanding electrocatalytic performance of Co2PPCA-800 can be ascribed to following reasons: (i) The improved conductive property of hybrids. Although cobalt or cobalt oxide nanoparticles have lower conductivity and are easy to aggregate,P-doped carbon is not only beneficial for electrical conductivity and electrochemical performance, but also maintain the structural stability. (ii) The high surface area and hierarchical porous structure. The porous structure and high surface area can provide high density of active sites and accelerates the mass transport. (iii) The synergy Co2P and P-doped carbon. As is known to all, the difference in electronegativity between carbon atoms and heteroatoms, the doping heteroatoms generally redistributes charge density and spin density of carbon atoms,thereby effectively regulating the work function and having a more appropriate adsorption energy.27. Here, based on the synergistic effect of Co2P and P-doped carbon, Co2P-PCA-800 shows outstanding ORR catalytic performance mainly due to proper desorption. And, the ORR reaction on the Co surface of Co2P may be as follows:
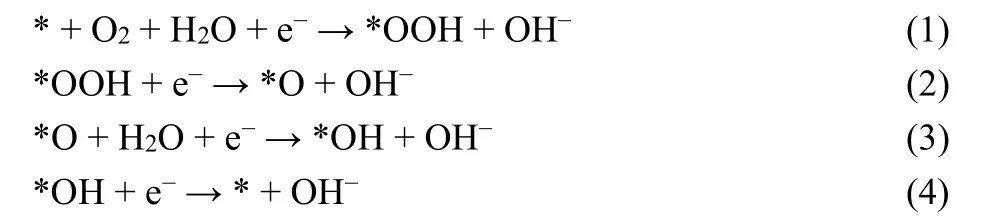
where * denotes active site on the surface10,45. Based on Geyer,the rate-determining step in the overall ORR process is the desorption of OH * on the Co surface of Co2P nanoparticles. P doping produced a strong electron-withdrawing group, which can induce Co electron transfer, and eventually lead to weakening of the binding force between the intermediate OH*and the surface Co atom25. And the real active sites for OER may be the cobalt hydroxide. The previous research has proved that transition metal phosphides could undergo surface reconstruction to form hydroxides in the OER process46,47.

Fig. 4 a) Schematic illustration of the ZAB; b) Photograph of ZAB open circuit voltage measurement; c) Discharge polarization curves and power density of Co2P-PCA-800 and Pt/C||RuO2; d) Galvanostatic discharge curves of Co2P-PCA-800 and Pt/C||RuO2 tested at 10 mA·cm−2; e, f) Cycle test at 10 mA·cm−2.
The high catalytic activities of Co2P-PCA-800 complex prompt us to prepare a ZAB by using the Co2P-PCA-800 catalyst as air cathode and zinc foil as the anode (Fig. 4a). For comparison, we assembled the cathode catalyst as precious metal-based ZAB. The open-circuit potential of Co2P-PCA-800 based ZAB is ~1.44 V (Fig. 4b). As shown in Fig. S9, there is a smaller voltage gap, which indicates excellent rechargeable capability of Co2P-PCA-800 based battery. The discharge polarization curve and power density display that Co2P-PCA-800 exhibits similar performance compared to Pt/C||RuO2. The discharge polarization and corresponding current density of 133 mA·cm−2at 0.4 V, and the peak power density of 58 mW·cm−2at 90 mA·cm−2for Co2P-PCA-800 are compared to those of the Pt/C||RuO2. But the Co2P-PCA-800-based battery perform a higher capacity than Pt/C||RuO2, such as, 741 mAh·g−1for Co2PPCA-800vs.620 mAh·g−1for Pt/C||RuO2at 10 mA·cm−2(Fig.4d). Finally, Pt/C||RuO2-based battery showed poor stability for 18 h, while the battery with Co2P-PCA-800 shows a good stability for 32 h in Fig. 4e. And the charge-discharge voltage gap of the two batteries has slightly increased at 35 cycles.However, the voltage gap rapidly increases for Pt/C||RuO2about(1.55 V), while Co2P-PCA-800 remain stable after 35 cycles(Fig. 4f). The above results demonstrate the outstanding activity and stability of Co2P-PCA-800 as cost-effective electrocatalysts for rechargeable ZABs.
4 Conclusions
In summary, we report an effective approach to fabrication Co2P-PCA-800 catalyst with high-performance catalytic activity. The as-prepared Co2P-PCA-800 has a 3D honeycomb hierarchical porous structure. Due to the high specific surface area, regular hierarchical pore structure, and P-doping adjusts the electronic structure of Co2+in Co2P, the Co2P-PCA-800 exhibited excellent ORR/OER electrocatalytic activity. When used as a ZAB cathode, Co2P-PCA-800 exhibit excellent charge and discharge performance closed to Pt/C||RuO2.
Supporting Information:available free of chargeviathe internet at http://www.whxb.pku.edu.cn.
———理学院

