Crystal Structure, Thermal Decomposition Mechanism and Properties of Lanthanide Supramolecular Complexes Based on 2,4,6-Trimethylbenzoic Acid and 5,5'-Dimethyl-2,2'-bipyridine
Mengxue Zhou , Ning Ren , Jianjun Zhang ,*
1 Testing and Analysis Center, Hebei Normal University, Shijiazhuang 050024, China.
2 College of Chemistry & Material Science, Hebei Normal University, Shijiazhuang 050024, China.
3 College of Chemical Engineering & Material, Hebei Key Laboratory of Heterocyclic Compounds, Handan University,Handan 056005, Hebei Province, China.
Abstract:Six ternary lanthanide complexes formulated as[Ln(2,4,6-TMBA)3(5,5'-DM-2,2'-bipy)]2 (Ln = Pr 1, Nd 2, Sm 3, Eu 4, Gd 5, Dy 6; 2,4,6-TMBA = 2,4,6-trimethylbenzoate; 5,5'-DM-2,2'-bipy = 5,5'-dimethyl-2,2'-bipyridine)have been synthesized under solvothermal conditions and characterized by singlecrystal X-ray diffraction, elemental analysis, thermogravimetric analysis, etc. The results of crystal diffraction analysis show that complexes 1–6 are binuclear units, crystallizing in the triclinic Pī space group. Complexes 1–5 are isostructural, and each of the central metal ions has a coordination number of 9. The asymmetric unit of complexes 1–5 consists of one Ln3+, one 5,5'-DM-2,2'-bipy ligand, and three 2,4,6-TMBA− moieties with three coordination modes: chelation bidentate, bridging bidentate, and bridging tridentate. The coordination geometry of Ln3+ is distorted monocapped square antiprismatic. The binuclear units of complexes 1–5 form a one-dimensional (1D)supramolecular chain along the c-axis via π–π stacking interactions between the 2,4,6-trimethylbenzoic acid rings. The 1D chains are linked to form a supramolecular two-dimensional (2D)sheet in the bc plane via π–π stacking interactions between the pyridine rings. Although the molecular formulae of complex 6 and complexes 1–5 are similar, the coordination environment of the lanthanide ions is different in the two cases. The asymmetric unit of complex 6 contains a Dy3+ ion coordinated by a bidentate 5,5'-DM-2,2'-bipy and three 2,4,6-TMBA− ligands adopting bidentate and bridging bidentate coordination modes. The Dy3+ metal center has a coordination number of 8, with distorted square antiprismatic molecular geometry. The binuclear molecule of 6 is assembled into a six-nuclear unit by π–π weak staking interactions between two 5,5'-DM-2,2'-bipy ligands; then, adjacent six-nuclear units form a 1D chain via offset π–π interactions between 5,5'-DM-2,2'-bipy ligands on different adjacent units. The adjacent 1D chains are linked by C―H∙∙∙O hydrogen bonding interactions to form a 2D supramolecular structure. The thermal stability and thermal decomposition mechanism of all the complexes are investigated by the combination of thermogravimetry and infrared spectroscopy (TG/FTIR)techniques under a simulated air atmosphere in the temperature range of 298–973 K at a heating rate of 10 K∙min−1. Thermogravimetric studies show that this series of complexes have excellent thermal stability. During the thermal decomposition of the complex, the neutral ligand is lost first, followed by the acid ligand, and finally, the complex is decomposed into rare earth oxides. The three-dimensional infrared results are consistent with the thermogravimetric results. The photoluminescence spectra of complex 4 show the strong characteristic luminescence of Eu3+. The five typical emission peaks at 581, 591, 621, 651, and 701 nm correspond to the 5D0 → 7F0, 5D0 → 7F1, 5D0 → 7F2, 5D0 → 7F3, and 5D0 → 7F4 electronic transitions of Eu3+,respectively. The emission at 621 nm is due to the electric dipole transition 5D0 → 7F2, while that at 591 nm is assigned to the 5D0 → 7F1 the magnetic dipole transition. The lifetime (τ)of complex 4 is calculated as 1.15 ms based on the equation and the intrinsic quantum yield is calculated to be 45.1%. Further, the magnetic properties of complex 6 in the temperature range of 2–300 K are studied under an applied magnetic field of 1000 Oe.
Key Words:Lanthanide supramolecular complexes; Crystal structure, Thermal behavior; Luminescence spectrum; 2,4,6-Trimethylbenzoic acid
1 Introduction
Lanthanide complexes with various structures1have attracted extensive attention due to their excellent optical2–5, magnetic6–9,adsorption10and catalytic properties11,12. Due to the particularity 4felectron layer structure of lanthanides, lanthanide complexes have special luminescent properties such as high color purity, long decay times, high quantum yields, and so on13. These optical properties of lanthanide complexes have potential applications in fluorescent probes14and luminescent bioassays15. However,f–ftransition is prohibited by spin and parity16, it is necessary to select appropriate organic chromophores to enhance the luminescence of intensity, which is called “antenna effect”17. Lanthanide has strong affinity with nitrogen or oxygen atoms, so aromatic carboxylic acids ligands:3,4-diethoxybenzoic acid18, 2,4-dichlorobenzoic acid19, 1,2,3,5-benzenetetracarboxylic acid20,etc., and nitrogen-containing rigid ligands: 1,10-phenanthroline21, 2,2'-bipyridine22,2,2':6',2''-terpyridine23,etc., are usually selected as ligand to synthesize lanthanide complexes. The introduction of nitrogencontaining second ligand can not only improve the stability of the complex, but also replace the position of the solvent, reduce energy loss and enhance luminous efficiency. The second ligand can also act as an energy donor, absorbing energy and transferring to the central ion, improving the luminous intensity.Deprotonated 2,4,6-trimethylbenzoic acid anions have multiple coordination modes, such as monodentate, bidentate, bridged bidentate, bridged tridentate,etc., providing a possibility for the diversity structure of lanthanide complexes. 2,4,6-trimethylbenzoic acid as an aromatic carboxylic acid ligand combined with nitrogen-containing heterocyclic ligands 5,5'-dimethyl-2,2'-bipyridine as antenna ligands sensitizes lanthanide ions and improves luminescence efficiency. In recent years, the thermal stability, decomposition mechanism and kinetics of complexes have been significantly increased24. TG/FTIR is usually used to monitor the mass loss in the process of gas escape and thermal decomposition at different temperatures, which provides valuable information for the structure of complexes.Moreover, the potential application of trivalent lanthanide ion coordination complexes in magnetic materials has attracted great attention25. Owing to the unquenched orbital angular momentum of high spin 4funpaired electrons in trivalent lanthanide ion, these lanthanide complexes show magnetic anisotropy26. Therefore lanthanide complexes are the most promising magnetic materials.
In this paper, we utilize 2,4,6-trimethylbenzoic acid and 5,5'-dimethyl-2,2'-bipyridine to form six binuclear complexes,[Ln(2,4,6-TMBA)3(5,5'-DM-2,2'-bipy)]2(Ln = Pr 1, Nd 2, Sm 3,Eu 4, Gd 5, Dy 6). All the prepared complexes were characterizedviasingle-crystal X-ray diffraction, elemental analysisetc. The pyrolysis mechanisms of the complexes were studied by the TG-DTG-DSC/FTIR technique. Solid state fluorescence and lifetime of complex 4 were studied. In addition,the magnetic property of 6 was also investigated.
2 Experimental section
2.1 Materials and methods
Ln(NO3)3∙6H2O (Ln = Pr, Nd, Sm, Eu, Gd, Dy)(Alfa Aesar,99.9%), 2,4,6-trimethylbenzoic acid (Alfa Aesar, 99%), and 5,5'-dimethyl-2,2'-bipyridine (Alfa Aesar, 98%)were received from commercial suppliers and used as received without further purification. The Vario-EL II analyaer (Elementar, Germany)was used for elemental analyses (C, H and N)and the contents of lanthanide elements were titrated with ethylenediamine tetraacetic acid (EDTA). The TG-DTG/FTIR analyses were conducted in a simulated air atmosphere with the STA 449 F3 instrument (Netzsch, Germany)and the TENSOR 27 fourier transform infrared spectrometer (Bruker, Germany)at a heating rate of 10 K∙min−1with about 3–8 mg compounds. Excitation(λem= 621 nm)and emission spectra (λex= 330 nm)and lifetimes of solid complexes were measured on a Fluorescence Spectrometer (Edinburgh Instruments Ltd., UK). In addition,magnetic susceptibilities of the complex 6 were performed on a MPMS XL-7 magnetometer (Quantum Design, USA)from 2 K to 300 K with an applied magnetic field of 1000 Oe.
2.2 Synthesis of complexes 1–6
Complexes 1–6 have the same synthetic conditions. A mixture of 2,4,6-trimethylbenzoic acid (0.098 g, 0.60 mmol)and 5,5'-dimethyl-2,2'-bipyridine (0.037 g, 0.20 mmol)was dissolved in 6 mL ethanol solution with stirring. Adjust the pH value of the mixture to 5–7 with 1.0 mol·L−1NaOH, add the mixture drop to Ln(NO3)3∙6H2O (0.20 mmol, Ln = Pr, Nd, Sm, Eu, Gd, Dy)aqueous solution by stirring. After stirring for 40 min, the suspension was sealed in a 25 mL Teflon-lined autoclave and heated to 120 °C for 7 days and then slowly cooled to room temperature, the crystals were collected by filtration, washed with ethanol and distilled water.
[Pr(2,4,6-TMBA)3(5,5'-DM-2,2'-bipy)]2(1), Elemental Anal.Calcd. for C84H90N4O12Pr2: C, 61.96; H, 5.61; N, 3.42; Pr, 17.35,Found: C, 61.92; H, 5.57; N, 3.44; Pr, 17.30. [Nd(2,4,6-TMBA)3(5,5'-DM-2,2'-bipy)]2(2), Elemental Anal. Calcd. for C84H90N4O12Nd2: C, 62.13; H, 5.61; N,3.49; Nd, 17.66, Found:C, 62.14; H, 5.54; N, 3.42; Nd, 17.63. [Sm(2,4,6-TMBA)3(5,5'-DM-2,2'-bipy)]2(3), Elemental Anal. Calcd. for C84H90N4O12Sm2: C, 61.23; H, 5.49; N, 3.44; Sm, 18.25, Found:C, 61.21; H, 5.50; N, 3.40; Sm, 18.24. [Eu(2,4,6-TMBA)3(5,5'-DM-2,2'-bipy)]2(4), Elemental Anal. Calcd. for C84H90N4O12Eu2: C, 61.06; H, 5.52; N, 3.37; Eu, 18.45, Found:C, 61.09; H, 5.49; N, 3.39; Eu, 18.40. [Gd(2,4,6-TMBA)3(5,5'-DM-2,2'-bipy)]2(5), Elemental Anal. Calcd. for C84H90N4O12Gd2: C, 60.73; H, 5.50; N, 3.36; Gd, 18.97, Found:C, 60.70; H, 5.46; N, 3.37; Gd, 18.92. [Dy(2,4,6-TMBA)3(5,5'-DM-2,2'-bipy)]2(6), Elemental Anal. Calcd. for C84H90N4O12Dy2: C, 60.30; H, 5.44; N, 3.39; Dy, 19.48; Found:C, 60.32; H, 5.42; N, 3.35; Dy, 19.43.
2.3 Single-crystal X-ray diffraction
Crystallographic data for complexes 1–6 were collected on a Smart-1000 diffractometer (Bruker, Germany)using graphitemonochromated with Mo-Kαradiation (λ= 0.71073 Å, 1 Å = 0.1 nm, the same below)at the temperature of 298 K. Structures of complexes 1–6 were solved by means of direct methods and refined by full-matrix least-squares techniques onF2using the SHELXL-97 program. The detailed crystallographic data and structure refinements for all compounds are presented in Table 1.

Table 1 Crystal data and structure refinement for complexes 1–6 (1 Å = 0.1 nm).
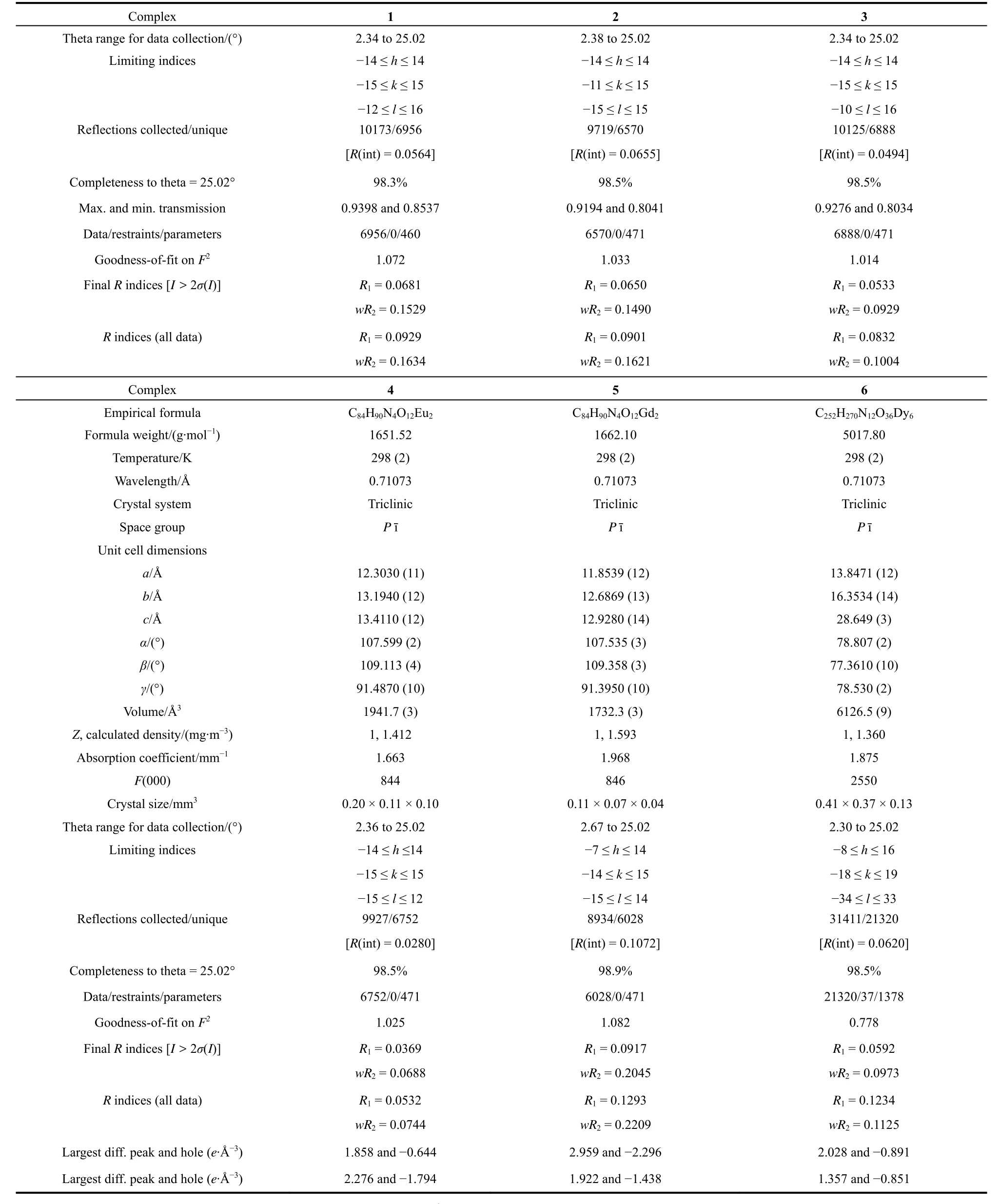
continued Table 1
3 Results and discussion
3.1 Crystal structure description of complexes 1–6
The results of crystal diffraction analysis show that although the molecular ratio of complexes 1–6 is the same, there are two different structures due to the contraction effect of lanthanide elements. Among them, complexes 1–5 are isostructural, the central ions are all nine-coordinated, while complex 6 is different with a coordination number of eight. So as a representative, the crystal structures of complexes 4 and 6 are employed to be discussed in detail.
3.1.1 Crystal structure of [Eu(2,4,6-TMBA)3(5,5'-DM-2,2'-bipy)]2
The complex 4 is a binuclear unit, crystallizing in the triclinicPī space group. As shown in Fig. 1a, the asymmetric unit of 4 consists of one Eu3+, one 5,5'-DM-2,2'-bipy ligand, three 2,4,6-TMBA−with three coordination modes: chelation bidentate,bridging bidentate and bridging tridentate (Fig. 2). The Eu3+is nine coordinated and displays a distorted monocapped square antiprismatic coordination geometry (Fig. 1b). In the polyhedron Eu1O7N2, seven oxygen (O1, O2, O3, O4, O3A, O5 and O6)atoms are derived from 2,4,6-TMBA−ligand with different coordination modes, and two nitrogen (N1, N2)atoms are derived from the bidentate 5,5'-DM-2,2'-bipy ligand. The average bond distances of Eu1-O to Eu1 is 2.451 Å, while the bond distances of Eu1-N (bound through N1, N2)is 2.653 Å.The Eu1-O and Eu1-N bond length ranges (Table 2)are similar to the bond lengths previously reported27.

Fig. 1 (a)The molecular structure of complex 4;(b)coordination polyhedron of Eu3+ center.

Fig. 2 Different coordination modes of 2,4,6-TMBA− ligand for complex 4.

Table 2 The main bond lengths for complexes 1–6.
As shown in Fig. 3a, the binuclear unit of 4 forms a onedimensional (1D)supramolecular chain along thec-axisviaπ–πstacking interactions between 2,4,6-trimethylbenzoic acid rings with the distance of 3.726 Å. The 1D chain is linked to form a supramolecular two-dimensional (2D)sheet in about thebcplanevia π–πstacking interactions between pyridine rings with the distance of 3.772 Å (Fig. 3b).

Fig. 3 (a)The supramolecular 1D chain structure of 4 along the c axis; (b)The supramolecular 2D sheet of 4 in about the bc plane.
3.1.2 Crystal structure of Dy(2,4,6-TMBA)3(5,5'-DM-2,2'-bipy)]2
The complex 6 is also a binuclear molecule, crystallizing in the triclinicPī space group. Although the molecular formula of complex 6 and 4 are similar, the coordination environment of lanthanide ions is different. As shown in Fig. 4a, the asymmetric unit of complex 6 contains a Dy3+coordinated by a bidentate 5,5'-DM-2,2'-bipy and three 2,4,6-TMBA−ligand adopting bidentate and bridging bidentate coordination modes. The Dy3+metal center has a coordination number of eight with a distorted square antiprismatic molecular geometry (Fig. 4b). Dy1-O bond distances for the bidentate 2,4,6-TMBA−ligand (O9 and O10)are 2.409 Å and 2.461 Å. Dy1-O bond distances for the bridging bidentate 2,4,6-TMBA−ligand (O1, O3, O5 and O7)are 2.394 Å, 2.274 Å, 2.374 Å and 2.326 Å, respectively. The Dy1-N1 and Dy1-N2 bond distances are 2.565 Å and 2.588 Å. The Ln-O, Ln-N distances and their average distances for complexes 1–6 are listed in Table 2. Obviously, the shrinkage effect of lanthanides is reflected in the decrease of average Ln-O bond length and average Ln-N bond length with the increase of atomic number28.

Fig. 4 (a)The molecular structure of complex 6;(b)Coordination polyhedron of Dy3+ center.
Although both complex 6 and complex 4 are binuclear molecules with similar molecular ratios, the coordination numbers of the central rare earth ions are different, furthermore,the 1D and 2D supramolecular structures are quite different. The binuclear molecule of 6 is assembled into a six-nuclear units byπ–πweak staking interactions between two 5,5'-DM-2,2'-bipy ligands with the distance of 3.752 Å, then adjacent six-nuclear unitsviaoffsetπ–πinteractions between 5,5'-DM-2,2'-bipy ligands on different adjacent units forms a 1D chain, where the distance is 3.614 Å (Fig. 5a). The adjacent 1D chains are linked by C-H···O hydrogen bonding interactions with the distance of 3.456 Å to construct two-dimensional supramolecular structure(Fig. 5b).

Fig. 5 (a)Supramolecular 1D chain in 6 along the c axis; (b)Supramolecular 2D sheet in 6 about the bc plane.
3.2 Thermal analysis
To investigate the pyrolytic dissociation mechanism and thermal stability of ternary lanthanide complexes, we measured the thermal behavior using TG-DTG-DSC under a simulated air atmosphere in the temperature range of 298–973 K with a heating rate of 10 K∙min−1. Thermal analysis curves of 1–6 are shown in Fig. S1(a–f)(in Supporting Information). The details of the pyrolysis steps (mass losses, temperature ranges, probable expelled groups and peak temperatures of DTG)are exhibited in Table 3. The complexes 1–5 have the similar pyrolysis steps, the complex 4 as a representative will be discussed in detail.
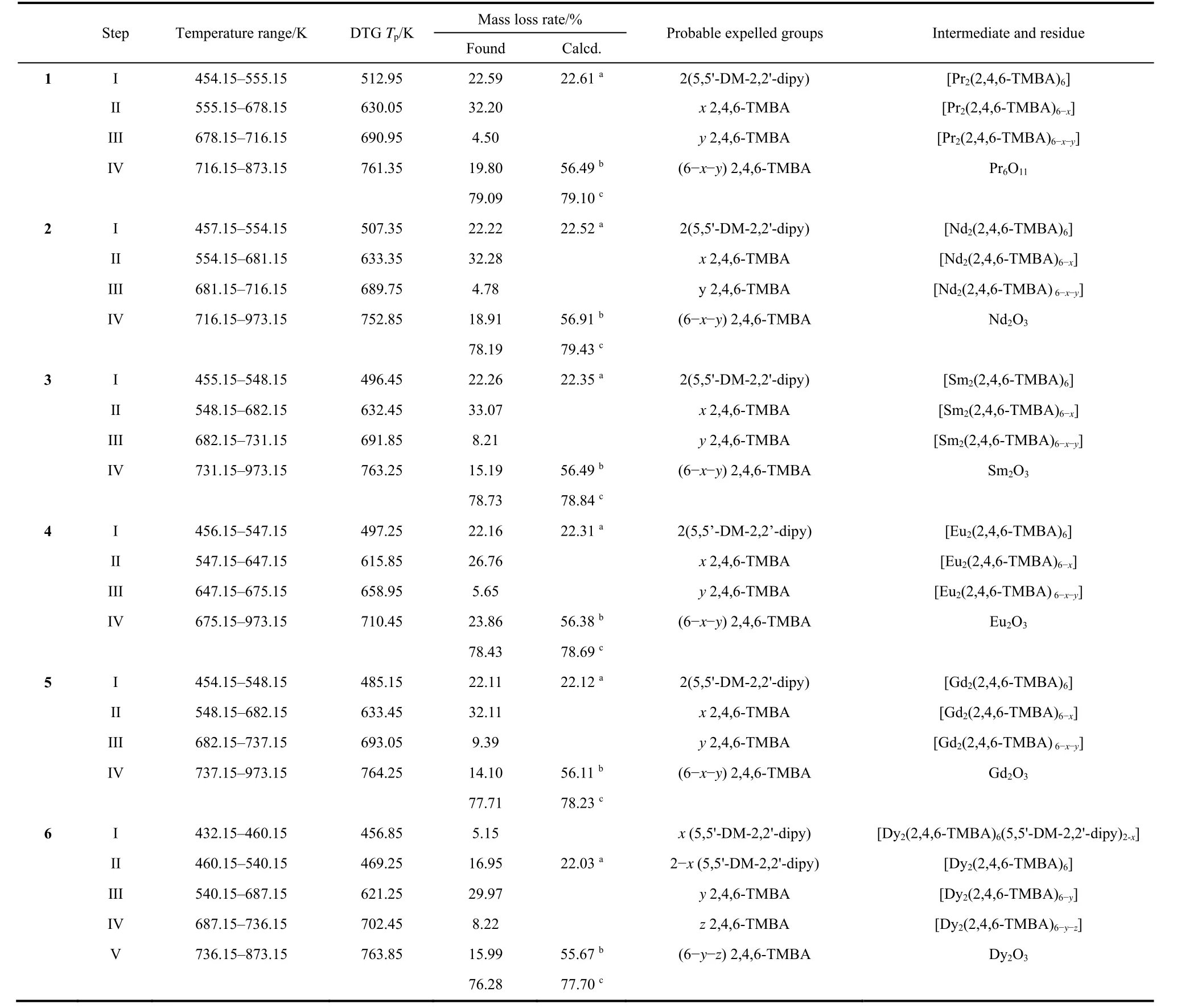
Table 3 Thermal data for the complexes 1-6 at pressure 101.3 kPa.
The thermal decomposition temperature of complex 4 is 454.15 K, which has certain thermal stability. The first mass loss between 454.15–547.15 K, is connected with symmetry peak on DTG curves centered at 497.25 K (experiment value of mass loss= 22.16%, calculated value of mass loss= 22.31%). The mass loss percent is close to the theoretical value of two 5,5'-DM-2,2'-dipy losses. Additionally, in the DSC curves of complex 4, a low endothermic peak is observed. Over 547.15K, the DTG curve of complex 4 shows that the mass loss is divided into three overlapping steps. The first one is centered at 615.85 K with mass loss of 26.76%. The second one is centered at 658.95 K with mass loss of 5.65%. The third one is centered at 710.45 K with mass loss of 23.86%. These steps are corresponding to the loss of six 2,4,6-TMBA ligands (experiment value of mass loss =56.27%, calculated value of mass loss = 56.38%). In the meantime, three exothermic peaks on the DSC curve can be found. Complex 4 was eventually completely collapsed into Eu2O3.
The decomposition temperature of complex 6 is 432.15 K. In the range of 432.15–540.15 K, the DTG curve of complex 6 shows the mass loss in the two overlapping steps. The first one is centered at 456.85 K with mass loss of 5.15%. The second one is centered at 469.25 K with mass loss of 16.95%. The DSC curve has no obvious endothermic peak in this temperature range. Between 540.15–873.15 K, the DTG curve for complex 6 shows mass losses in three overlapping steps, they are centered at 621.25, 702.45 and 763.85 K and corresponds to mass loss of 29.97%, 8.22% and 15.99%, respectively. Compared with complexes 1–5, the exothermic peak on the DSC curve of complex 6 is significantly lower at 540.15–873.15 K. Complex 6 was eventually completely collapsed into Dy2O3.
3.3 Evolved gas analysis
In order to further study the pyrolysis behavior of complexes,the TG/FTIR spectra were obtained by thermogravimetry followed by Fourier transform infrared spectroscopy system.The 3D stacked plots of the FTIR spectra are presented in Fig.6a–f and the 2D IR spectra are shown in Fig. 7a–f. We take complex 4 as an example to give a detailed explanation. It can be seen from Fig. 7d that there are four infrared absorption peaks at different temperatures (T= 500.44, 616.48, 653.26, 710.44 K),which are consistent with the strongest absorption peaks of gases escaping from four temperature ranges (456.15–547.15, 547.15–647.15, 647.15–675.15, 675.15–973.15 K). In the first stage, at 500.44 K, the characteristic signal ofνC=Cat 1469 and 1600 cm−1,νC=Nat 1558 cm−1,νC―Nat 1130 and 1218 cm−1,νC―Hat 2881–3036 cm−1,γC―Hat 826, 1029 and 1061 cm−1were observed. This indicates the loss of the neutral ligands in the first step. In the second IR spectrum at 616.48 K, the sharp absorption peak at 1797 cm−1isνC=Ofrom the carboxylic acid group, and the absorption peak in the region between 2258–2360 cm−1and 669 cm−1were attributed to the absorption peak of CO2. The absorption peaks ofνC―Hat 2889–3025 cm−1,δC―Hat 849 and 1036 cm−1,νC=Cat 1615 cm−1, and weak water peak at 3600–3800 cm−1was observed, The results were consistent with the loss of the aromatic carboxylic acid ligand in the second step of the thermogravimetric analysis. In the infrared spectrum of the third and fourth steps, only the strong absorption peak of CO2at 2258–2360 cm−1and 669 cm−1and the weak water peak at 3600–3800 cm−1were left on the infrared spectrum. This indicates that the carboxylic acid ligands have been completely cleaved.

Fig. 6 The 3D IR image of the gas escaping from the complexes 1–6 (a–f)during pyrolysis.

Fig. 7 The 2D IR spectra of the gaseous mixtures for complexes 1–6 (a–f).
For complex 6, the formation of complex can be divided into five steps according to the 3D IR spectrum of evolved gas. The five IR spectra at different temperatures (T= 452.00, 504.89,623.81, 702.35 and 764.32 K)were shown in Fig. 7f. At 452.00 and 504.89 K, the 2D infrared spectra were similar. The characteristic peak ofνC=C,νC=NandνC―Hat 1469, 1558 and 2889–3025 cm−1can be observed respectively, indicating that the bipyridine ligands was lost in the first two steps. At 623.81 and 702.35 K, the similar 2D infrared spectra were also found.The characteristic peak of CO2,νC=O andνC-H were observed at(2255–2347, 669 cm−1), 1798 and (2893–3017)cm−1respectively, indicating the loss of carboxylic acid ligands. At 764.32 K, there are only strong absorption peak of CO2and H2O in the infrared spectrum. This indicates that the carboxylic acid ligands have been completely decomposed.
3.4 Luminescence measurements
The luminescent properties of solid-state complex 4 were studied at room temperature. The excitation and emission spectra of 4 are shown in Fig. 8a. The fluorescence decay curve was shown in Fig. 8b. From the excitation spectrum, we can see a wide absorption band, that is, the absorption peak of electron transition from ligand to metal ion. The strength of the absorption band proves that carboxylic acid ligand as an antenna can effectively sensitize lanthanide ions29.
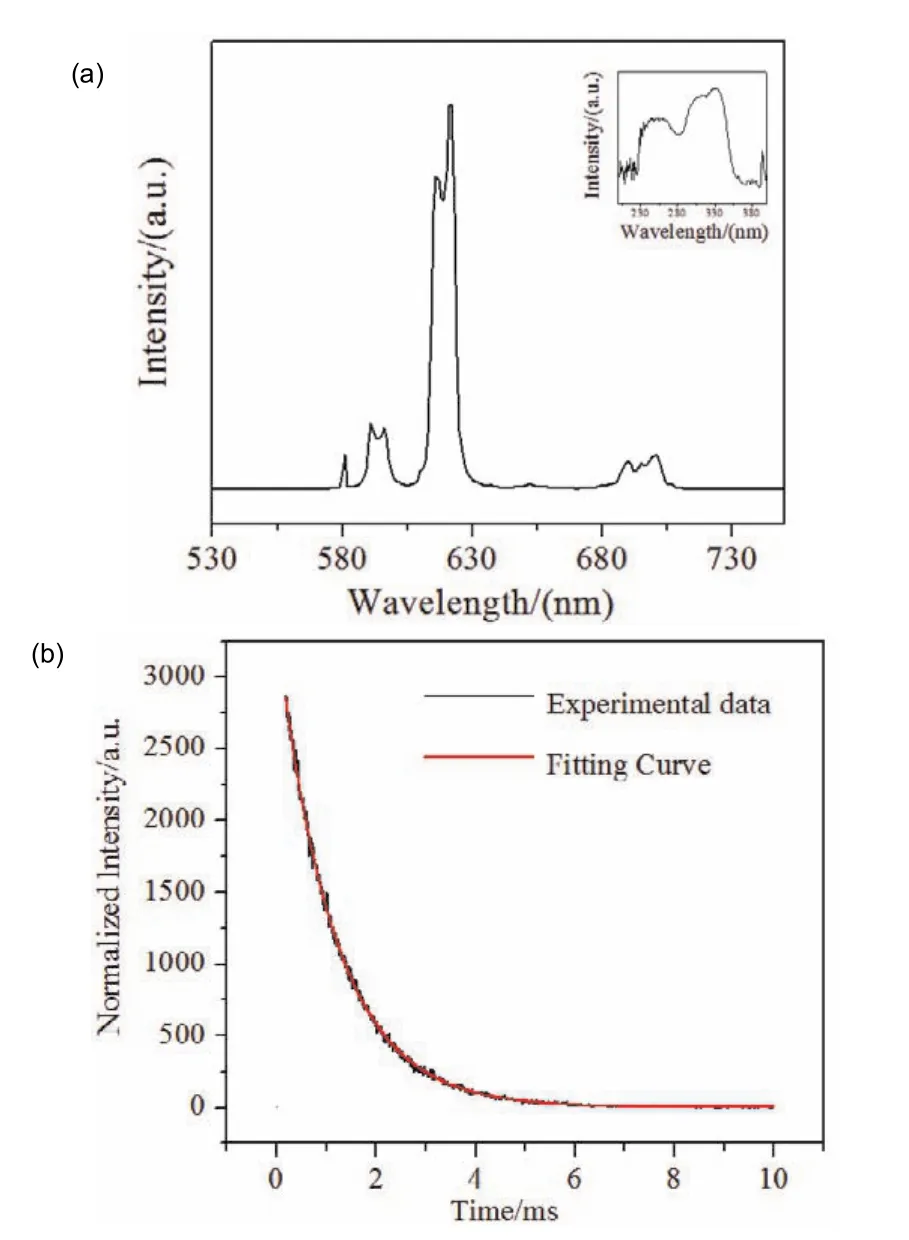
Fig. 8 (a)Emission and excitation spectrum of complex 4 in the solid state; (b)The fluorescence attenuation curves of the complex 4.
For complex 4, the emission spectrum was scanned at the excitation wavelength of 330 nm. It exhibits five emission peaks of Eu3+:5D0→7Fj(j= 0–5)(581, 591, 621, 651, 701 nm), The emission at 621 nm was the electric dipole transition5D0 →7F2,while the emission at 591 nm was magnetic dipole transition5D0→7F1. The highest peak5D0→7F2appears at 621 nm,which is the reason why the complex presents strong red light under the UV lamp. Moreover,5D0→7F2is higher than5D0→7F1indicated that the Eu3+was located in an asymmetric coordination. According to the Judd-Ofelt theory, the5D0 →7F0 transition occurs only in asymmetric environments30. While,this transition was observed at 581 nm indicating that the Eu3+is at the asymmetric center. The lifetime (τ)of complex 4 can be calculated by the following equation:

τ1andτ2are the decay times andB1andB2are the fitting constants31. Finally, the lifetime (τ)of 4 is 1.15 ms. Using the calculation formula proposed by Bunzli32et al., the intrinsic quantum yield is calculated to be 45.1%.
3.5 Magnetic properties
The magnetic properties of 6 in the temperature range of 2–300 K were studied under the applied magnetic field of 1000 Oe.TheχMTand 1/χMvsTof complex 6 are shown in Fig. 9.
For complex 6, theχMTvalue of 28.08 cm3∙K∙mol−1at room temperature is similar to the calculated value 28.34 cm3∙K∙mol−1of two isolated Dy3+(6H15/2,J= 15/2,g= 4/3). With the decrease of temperature, theχMTvalue of 6 decreased slowly in the temperature range of 300–50 K, but with the continuous decrease of temperature, theχMTvalue decreased sharply and reached the minimum value of 14.93 cm3∙K∙mol−1at 2 K, which can be attributed to the antiferromagnetic interaction between Dy3+33.

Fig. 9 Temperature dependence of the χMT, and 1/χMT susceptibility for complex 6 in the range of 2–300 K.
4 Conclusions
In conclusion, six new ternary lanthanide complexes formulated as [Ln(2,4,6-TMBA)3(5,5'-DM-2,2'-bipy)]2(Ln = Pr 1, Nd 2, Sm 3, Eu 4, Gd 5, Dy 6)have been synthesized under the solvothermal conditions. The structural analyses revealed that complexes 1–5 are isostructural and each center metal ion is nine-coordinated, while complex 6 is different with another structures and each center metal ion is eight-coordinated.Complexes 1–5 are packed into 2D sheetviatheπ–πstacking interactions while complex 6 forms a 2D sheet through theπ–πstacking interactions and C-H···O hydrogen bond interaction.Thermal analysis show that complexes 1–6 have similar thermal decomposition mechanisms and high thermal stability. The study of photoluminescence shows that solid complex 4 emits strong red light in the visible region, which indicates that 2,4,6-tmhbas are highly effective sensitizers and are expected to become luminescent materials. The magnetic properties of complex 6 show that there is antiferromagnetic interaction in the synthesized complex.
Supplementary data:Crystallographic data for the structure reported in this paper are deposited in the Cambridge Crystallographic Data Center (CCDC 1906870(1); CCDC 1906869(2); CCDC 1906871(3); CCDC 1906867(4); CCDC 1906868(5); CCDC 1906865(6).)
Supporting Information:Thermal analysis curves of 1–6 are included. This information is available free of chargeviathe internet at http://www.whxb.pku.edu.cn.
References(1)Janicki, R.; Mondry, A.; Starynowicz, P.Coordin. Chem. Rev. 2017,340, 98. doi: 10.1016/j.ccr.2016.12.001(2)Allendorf, M. D.; Bauer, C. A.; Bhakta, R. K.; Houk, R. J.Chem. Soc.Rev.2009,38(5), 1330. doi: 10.1039/b802352m(3)Ahmed, Z.; Iftikhar, K.Inorg. Chem.2015,54(23), 11209.doi: 10.1021/acs.inorgchem.5b01630(4)Bradberry, S. J.; Savyasachi, A. J.; Martinez-Calvo, M.;Gunnlaugsson, T.Coordin. Chem. Rev.2014,273–274, 226.doi: 10.1016/j.ccr.2014.03.023(5)Bunzli, J. C.; Piguet, C.Chem. Soc. Rev.2005,34(12), 1048.doi: 10.1039/b406082m(6)Hiller, M.; Krieg, S.; Ishikawa, N.; Enders, M.Inorg. Chem.2017,56(24), 15285. doi: 10.1021/acs.inorgchem.7b02704(7)Lin, P. H.; Burchell, T. J.; Clerac, R.; Murugesu, M.Angew. Chem.Int. Ed. Engl.2008,47(46), 8848. doi: 10.1002/anie.200802966(8)Reis, S. G.; Briganti, M.; Soriano, S.; Guedes, G. P.; Calancea, S.;Tiseanu, C.Inorg. Chem.2016,55(22), 11676.doi: 10.1021/acs.inorgchem.6b01616(9)Rinehart, J. D.; Long, J. R.Chem. Sci.2011,2(11), 2078.doi: 10.1039/c1sc00513h
(10)Wang, G.; Song, T.; Fan, Y.; Xu, J.; Wang, M.; Wang, L.Inorg. Chem.Commun.2010,13(1), 95. doi: 10.1016/j.inoche.2009.10.026
(11)Niu, Y.; Xu, Q.; Wang, Y.; Li, Z.; Lu, J.; Ma, P.Dalton Trans.2018,47(29), 9677. doi: 10.1039/c8dt01243a
(12)Wang, W.; Wang, X.; Zhou, S.; Xu, X.; Du, J.; Zhang, L.Inorg.Chem.2018,57(16), 10390. doi: 10.1021/acs.inorgchem.8b01556
(13)Li, Y. J.; Yan, B.Inorg. Chem.2009,48(17), 8276.doi: 10.1021/ic900971h
(14)Heffern, M. C.; Matosziuk, L. M.; Meade, T. J.Chem. Rev.2014,114(8), 4496. doi: 10.1021/cr400477t
(15)Bünzli, J. C. G.J. Lumin.2016,170, 866.doi: 10.1016/j.jlumin.2015.07.033
(16)Zhao, Q. Q.; Zhu, M. M.; Ren, N.; Zhang, J. J.J. Mol. Struct.2017,1149, 171. doi: 10.1016/j.molstruc.2017.07.080
(17)Monteiro, J. H. S. K.; Sigoli, F. A.; de Bettencourt-Dias, A.Can. J.Chem.2018,96(9), 859. doi: 10.1139/cjc-2017-0436
(18)Jin, C. W.; Wang, Y.; Ren, N.; Geng, L. N.; Zhang, J. J.J. Chem.Thermodyn.2016,103, 181. doi: 10.1016/j.jct.2016.08.011
(19)Wu, J.; Li, H.; Ren, N.; Zhang, J.; Wang, S.J. Rare Earths2016,34(11), 1083. doi: 10.1016/s1002-0721(16)60138-2
(20)Xia, C. K.; Sun, W.; Min, Y. Y.; Yang, K.; Wu, Y. L.Polyhedron2018,141, 377. doi: 10.1016/j.poly.2017.11.011
(21)Utochnikova, V. V.; Grishko, A.; Vashchenko, A.; Goloveshkin, A.;Averin, A.; Kuzmina, N.Eur. J. Inorg. Chem.2017,2017(48), 5635.doi: 10.1002/ejic.201700896
(22)He, S. M.; Sun, S. J.; Zheng, J. R.; Zhang, J. J.Spectrochim. Acta A Mol. Biomol. Spectrosc.2014,123, 211.doi: 10.1016/j.saa.2013.12.023
(23)Carter, K. P.; Pope, S. J. A.; Cahill, C. L.CrystEngComm2014,16(10), 1873. doi: 10.1039/c3ce42267d
(24)Zapała, L.; Kosińska, M.; Woźnicka, E.; Byczyński, Ł.; Zapała, W.;Kalembkiewicz, J.J. Anal. Appl. Pyrol.2017, 123, 1.doi: 10.1016/j.jaap.2017.01.010
(25)Gao, H. L.; Huang, S. X.; Zhou, X. P.; Liu, Z.; Cui, J. Z.Dalton Trans. 2018,47(10), 3503. doi: 10.1039/c8dt00063h
(26)Kariem, M.; Yawer, M.; Kumar, M.; Nawaz Sheikh, H.; Sood, P.;Kolekar, S. S.J. Solid. State. Chem.2017,255, 61.doi: 10.1016/j.jssc.2017.08.001
(27)Shen, P. P.; Zhu, M. M.; Ren, N.; Zhang, J. J.; Wang, S. P.Appl.Organomet. Chem.2017,31(12), e3886, doi: 10.1002/aoc.3886
(28)Xie, H.; Lu, G.J. Rare Earths2013,31(6), 639.doi: 10.1016/s1002-0721(12)60334-2
(29)Zhao, Y. F.; Chu, H. B.; Bai, F.; Gao, D. Q.; Zhang, H. X.; Zhou, Y. S.J. Organomet. Chem.2012,716, 167.doi: 10.1016/j.jorganchem.2012.06.031
(30)Shen, C. Q.; Yan, T. L.; Wang, Y. T.; Ye, Z. J.; Xu, C. J.; Zhou, W. J.J. Lumin.2017,184, 48. doi: 10.1016/j.jlumin.2016.12.018
(31)Zhu, M. M.; Ren, N.; Zhang, J. J.Inorg. Chim. Acta2018,480, 140.doi: 10.1016/j.ica.2018.05.022
(32)Bünzli, J. C. G.; Chauvin, A. S.; Kim, H. K.; Deiters, E.; Eliseeva, S.V.Coordin. Chem. Rev.2010,254(21–22), 2623.doi: 10.1016/j.ccr.2010.04.002
(33)Wei, X. H.; Yang, L. Y.; Liao, S. Y.; Zhang, M.; Tian, J. L.; Du, P. Y.Dalton Trans.2014,43(15), 5793. doi: 10.1039/c3dt53112k
- 物理化学学报的其它文章
- Hollow Nitrogen-Rich Carbon Nanoworms with High Activity for Metal-Free Selective Aerobic Oxidation of Benzyl Alcohol
- Photocrosslinking-Immobilized Polymer Vesicles for Lowering Temperature Triggered Drug Release
- CO Hydrogenation to Ethanol over Supported Rh-Based Catalyst:Effect of the Support
- CdTeSe合金幻数团簇的室温合成和形成机理研究
- 体相界面导通的复合快离子导体
- 异氰酸苯酯诱导的类胶原多肽自组装

