Application of topical gentamicin-a new era in the treatment of genodermatosis
Shan Wang · Zhou Yang · Ying Liu · Mu-Tong Zhao · Juan Zhao · Huan Zhang · Zong-Yang Liu ·Xiao-Ling Wang · Lin Ma · Yong-Hong Yang
Abstract
Keywords Epidermolysis bullosa · Gentamicin · Nagashima-type palmoplantar keratosis · Nonsense mutation ·Readthrough
Introduction
There is no ideal curative treatment for most genetic skin diseases at present. Current disease management strategies are mainly symptomatic treatment and supportive treatment. Other therapies include bone marrow stem cell transplantation, cell therapy, and protein replacement therapy, which are often associated with huge side effects and high costs. Nowadays,there are new treatment advances in genetic diseases caused by nonsense mutations. Nonsense mutations account for approximately 30% of disease-causing gene variations, lead to 11% of human inherited diseases [ 1, 2]. It occurs when a premature termination codon (PTC)-UGA, UAG, or UAA is introduced in the DNA sequence, resulting in stopping protein synthesis and leading to truncated nonfunctional proteins. Recent findings indicate some drugs such as gentamicin can cause cells to bypass stop codons, insert random amino acids, and express full-length proteins, which is called “readthrough”[ 3, 4]. In the recent decade, systemic gentamicin has given promising results in some genetic disorders by promoting PTC readthrough, including cystic fibrosis (CF) [ 5], Duchenne’s muscular dystrophy (DMD) [ 6], leukocyte adhesion deficiency[ 7], hemophilia [ 8], and retinitis pigmentosa. There are also applications in genetic skin diseases such as Nagashima-type palmoplantar keratosis (NPPK), epidermolysis bullosa (EB),Hailey-Hailey disease (HHD), and others. In this review,we will focus on the application of topical gentamicin in the treatment of genodermatosis and summarize in the following section.
Mechanism of action
Because PTC translational termination is not always 100%efficient, there is natural suppression of PTCs decoding by a near-cognate transfer RNA at low frequency, thus forming”readthrough", which can lead to the production of a full-length protein [ 3]. Aminoglycoside antibiotics (gentamicin, geneticin/G418, paromomycin, and amikacin), tetracycline antibiotics, and other compounds such as ataluren/PTC124 can enhance the PTC readthrough process. Among those, gentamicin is the most promising drug with both in vitro and in vivo efficient readthrough abilities of PTCs [ 4, 9].
Gentamicin, first discovered in 1963 [ 10], is a classical broad-spectrum antibiotic that belongs to the aminoglycosides category. They act to inhibit bacterial protein synthesis by irreversibly binding to the bacterial 30S ribosomal subunit at the amino-acyl-transfer RNA (tRNA) acceptor A site causing a conformational change in the tRNA, and thereby leading to codon misreading and translocation inhibition [ 11].Gentamicin can enhance PTC readthrough by similar mechanisms that binding to mammalian ribosomal RNA, impairing codon-anticodon recognition at the amino-acyl-tRNA acceptor A site. This procedure could lead to the mismatch of tRNA,reduce the f idelity of the normal translation process, and insert random amino acids instead, thus appear to bypass PTC and restore the full-length functional proteins [ 3, 12] (Fig. 1).
Gentamicin is a complex of different closely related aminoglycoside sulfates, including gentamicins C1, C2, C1a,and C2a as major components, C2b as a minor component,as well as other related substances, such as sisomicin, garamine, gentamicin B, gentamicin B1, and 2-deoxystreptamine[ 13]. Among these components, gentamicin B1 and X2 are more potent than other components in inducing PTC readthrough [ 4, 14, 15].
Topical gentamicin in the treatment of genodermatosis
Previous clinical application of gentamicin for genetic diseases remains limited, mainly for the concerns regarding nephrotoxicity and ototoxicity through systemic administration. Also, antibiotic resistance is warranted. The feasibility of topical application for genodermatosis is an advantage in gentamicin clinical practice. Several in vivo and in vitro studies have been conducted in recent years,here we search the MEDLINE through PubMed, EMBASE databases, and the Clinical Trials Registry Platform ( https://clini caltr ials. gov/ ) from January 1960 to July 2020 using the key search terms “gentamicin, topical gentamicin, genodermatosis, genetic skin diseases”. We reviewed the literature and summarized as below. We also filtered seven clinical studies and listed in Table 1.
Nagashima-type palmoplantar keratosis
NPPK is the most common type of palmoplantar keratodermas in Asian populations. It is an autosomal recessive skin disease caused by mutations inSERPINB7, a member of the serine protease inhibitor superfamily, characterized by diffuse non-epidermolytic palmoplantar keratosis and erythema on palms and soles, extends to the dorsal surfaces of the hands and feet, inner wrists, ankles, indicating a "transgressive" appearance. It is a distinct but much milder clinical entity than Mal de Meleda (mutations in theSLURP1)[ 23]. NPPK has only been reported in Japanese and Chinese populations, and the nonsense mutation c.796C > T(p.Arg266Ter) ofSERPINB7being the most frequent [ 23].Although there are symptomatic treatments such as topical vitamin D3, topical keratolytic (salicylic acid, urea),and topical retinoids available for NPPK, they are usually unsatisfactory.
NPPK is an ideal and typical research model for studying readthrough because > 90% of patients carry the homozygous TGAA nonsense mutation c.796C > T [ 24]. In 2017,Ohguchi et al. demonstrated that gentamicin could induce dose-dependent readthrough in cells transfected withSERPINB7cDNA with r.796C > U mutation, and promoted fulllength SERPINB7 protein production in NPPK keratinocytes [ 16]. They also treated five NPPK patients harboring c.796C > T mutations in a 4-week investigator-blinded,randomized, bilaterally controlled compassionate use study with 0.1% topical gentamicin ointment. Results showed significant improvement of hyperkeratosis on the gentamicin side than the control side by patients’ self-reported, and two out of five improvements of hyperkeratosis were determined by a blinded-investigator assessment. Reduced scaling and smoothening of palmar skin surfaces were also observed,except for changes in erythema. Meanwhile, mutantSERPINB7mRNAs harboring r.796C > U were degraded by nonsense-mediated mRNA decay (NMD), and the truncated SERPINB7 protein was degraded via the proteasomemediated pathway [ 16]. This was also the first randomized controlled clinical trial performed using topical readthrough agents.
(一)荆轲感燕丹之义,函匕首入秦劫始皇,将以存燕宽诸侯,事虽不成,然亦壮士也。惜其智谋不足以知变识机。始皇之道,异于齐桓。曹沫功成,荆轲杀身,其所遭者然也。及欲促槛车,驾秦王以如燕,童子妇人且明其不能,而轲行之,其弗就也,非不幸。燕丹之心,苟可以报秦,虽举燕国犹不顾,况美人哉!轲不晓而当之,陋矣。[9]

Fig. 1 Mechanism of gentamicin readthrough effect. a During a normal translation process, codon-anticodon recognition of mRNA and the amino-acyl-tRNA occurs in the A site of the rRNA, peptidyl-tRNA bounds in the P site, ribosome moves along the mRNA,generating functional full-length proteins; b when a PTC occurs,there is no corresponding tRNA, resulting in translation termination and release of the polypeptide, leading to truncated nonfunctional proteins; c gentamicin could bind to mammalian rRNA, impairing codon-anticodon recognition at the amino-acyl-tRNA acceptor site A,lead to the mismatch of tRNA, reduce the fidelity of the normal translation process, and insert random amino acids instead, thus appear to bypass PTC and restore the full-length functional proteins. PTC premature stop codon, rRNA ribosome ribonucleic acid, tRNA transfer ribonucleic acid, mRNA messenger ribonucleic acid
Epidermolysis bullosa
EB is a group of rare genodermatosis characterized by the excessive increase of skin and mucosa fragility and the formation of mechanical blisters and bullae, which seriously affect the daily life and life quality of children and their families. According to the place at which blistering takes place in the skin, four broad categories are determined: EB simplex, junctional EB (JEB), dystrophic EB (DEB), and Kindler syndrome. DEB is further divided into an autosomal dominant DEB or an autosomal recessive DEB (RDEB) by inherited trait. There is no ideal treatment for EB until now.According to the literature, the application of gentamicin has been reported in some subgroups of JEB and RDEB.
Therapeutic exploration in autosomal recessive dystrophic epidermolysis bullosa
RDEB is one of the most severe types of EB, caused by mutations in the COL7A1 gene, coding the α1 chain of type VII collagen, the main constituent of anchoring fibrils (AF),which are located below the basal lamina at the basement membrane zone that mediate the adherence of the epidermis to the dermis. More than 800 kinds of mutations have been reported to cause RDEB, in which the nonsense mutations account for approximately 30% of RDEB [ 25]. Early in 2014, Cogan et al. proved that aminoglycosides induced PTC readthrough and restored functional, full-length type VII collagen in keratinocytes and fibroblasts from RDEB patients with nonsense mutations, as well as in HEK293 cells transfected with 22 different known RDEB nonsense mutations. These in vitro results provide the proof of concept for using aminoglycosides in nonsense mutation-suppression therapy for RDEB. Then in 2017, the same research group Woodley et al. attempted treatments in 5 RDEB patients harboring nonsense mutations by two methods-topical 0.1%gentamicin ointment three times per day for two weeks and intradermally injected gentamicin (8 mg) for 2 days, through a double-blind, placebo-controlled clinical trial [ 17]. They found gentamicin treatment by both methods could induce new type VII collagen by direct immunofluorescence (DIF)staining detection and new AFs by immunoelectron microscopy. It also showed improved wound closure effect compared with placebo-treated sites by clinical assessment in two evaluation dimensions (the percentage of wound closure and new blister formation). There was no evidence of systemic toxicity or side effect associated with gentamicin use by neither method during the study. There were also concerns about the possibility of generation of autoantibodies against type VII collagen because the newly produced fulllength type VII collagen, including new domains, are first exposed to the patients' immune system, and the generation of antibodies would lead to a secondary autoimmune bullous disease, indeed epidermolysis bullosa acquisita. However,results showed no detectable anti-type VII collagen antibodies in blood or skin, by neither method.
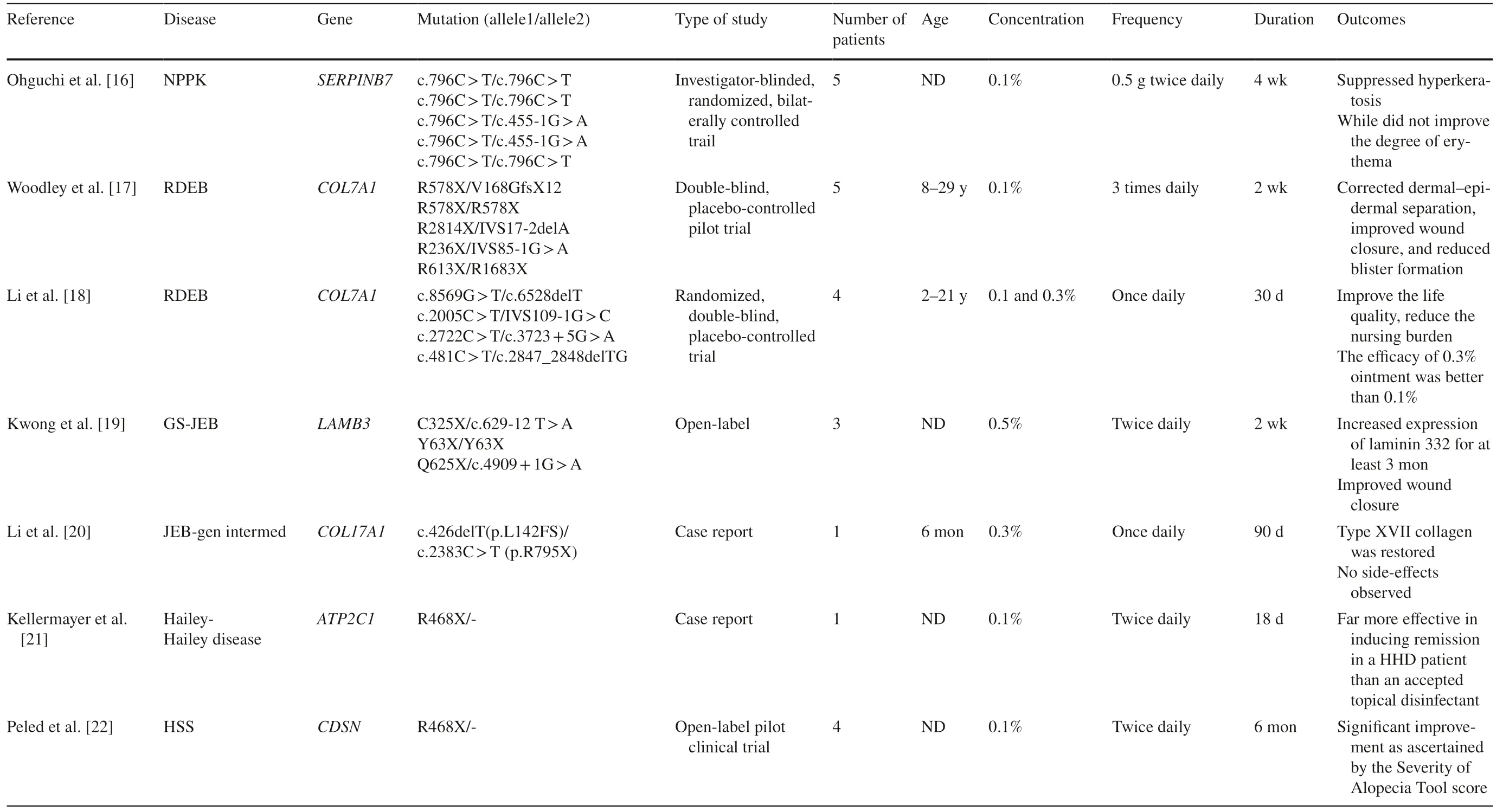
Table 1 Clinical studies of topical gentamicin application in genodermatosis
In 2019, Vishwanath et al. reported an intrabullous injection technique for gentamicin delivery. They drained the bulla of the DEB patient followed by an intrabullous injection of gentamicin solution (80 mg/2 mL). Thus the occluded environment delivers a high concentration and amount of gentamicin locally, which promotes the absorption effect [ 26]. In 2020, Li et al. use gentamicin ointment to treat four RDEB patients and found it could improve the life quality of patients while reducing the nursing burden, the efficacy of 0.3% ointment was better than that of 0.1% [ 18].
Therapeutic exploration in junctional epidermolysis bullosa
The exceedingly rare generalized severe JEB (GS-JEB, also called Herlitz type JEB) is caused by autosomal recessive loss-of-function mutations inLAMA3, LAMB3, or LAMC2genes that encode the laminin α3, β3, or γ2 subunits of laminin-332, respectively.LAMB3nonsense mutations account for approximately 80% of all GS-JEB cases which lead to reduced production of functional laminin 332 protein. Thus PTC readthrough may provide a readily available treatment. In 2018, Lincoln et al. firstly illustrated in vitro gentamicin capable ofinducing dose-dependentLAMB3nonsense mutation readthrough and in GS-JEB cultured keratinocytes, producing full-length laminin β3 then restoring functional laminin 332 protein [ 27]. Therapeutic exploration of gentamicin in GS-JEB patients is reported in recent 2 years. In 2019, Hammersen et al. conducted the first study using systemic gentamicin in infants to assess its clinical effects with GS-JEB [ 28]. The study results showed that systemic gentamicin, even in infants with GSJEB, is well-tolerated and showed a positive impact on skin fragility and quality of life in four out of five patients. In 2020, Kwong et al. reported that gentamicin could induce functional laminin 332 using primary keratinocytes from GS-JEB patients [ 19]. They also found topically 0.5% gentamicin ointment twice a day at open skin wounds for two weeks could promote PTC readthrough, create new laminin 332 at the target skin, and improve wound healing through an open-label trial in the same patients. There were nevertheless no related side effects reported. Because there was a similar concern to autoantibodies' development against new domains of laminin 332 as previously described in RDEB,they also performed enzyme linked immunosorbent assay(ELISA) and DIF and found no anti-laminin 332 autoantibodies exist in either the patients’ blood or skin. The lack of autoantibodies is possibly due to the occurrence of basal readthrough, which could be a protection mechanism for patients from developing autoimmune conditions. For the discussion of how long the effect of newly produced laminin 332 persists, the study of Kwong et al. found increased expression of laminin 332 lasted for at least 3 months and was associated with clinical improvement of wound closure[ 19]. Since the laminin 332 has a relatively long half-life,similar to type VII collagen, about 30-60 days according to previous research [ 17], they supposed that gentamicin could be applied as short-term pulse therapy in the treatment of DEB.
In 2020, Li et al. reported a 6-year-old boy of JEB, generalized intermediate (JEB-gen intermed) topically treated with gentamicin ointment [ 20]. JEB-gen intermed is caused by mutations inCOL17A1that encoding the hemidesmosomal protein collagen XVII, with most mutations are nonsense or insertion/deletion mutations. The boy carried compound heterozygous mutation c.426delT (p.L142FS)and c.2383C > T (p.R795X) of theCOL17A1gene, and was undergone 0.3% gentamicin ointment once a day for 90 days.The results showed gentamicin improved wound closure and reduced blister formation compared with the placebo, with no side effects observed.
Advantages of readthrough therapy in epidermolysis bullosa treatment
Except for daily wound care and prevention of new wounds creation, there are some previous therapeutic strategies in the treatment of EB, including protein replacement therapy,bone marrow stem cells transplantation, cell therapy, and gene-corrected keratinocyte autografts transplantation,which are not consistently effective, also associated with huge side effects and high costs [ 17]. On the contrary,readthrough therapy has the advantages of not exposing patients to live cells, exogenous DNA or RNA, or viral vectors, thus reducing the relative risk. The application is also simple and patient-operable, which does not require surgery for grafting or immunosuppression for transplantation. It indicates a better and convenient way for EB patients and families to manage at home. Most importantly, topical gentamicin is a readily available treatment that is inexpensive and relatively safe, with low cost [ 17, 19].Attenuation of EB symptoms may be due to the readthrough of the PTCs that lead to the induction of functional protein at the epidermal-dermal junction. The other reason lies in their antimicrobial activity because skin wounds of EB are mostly colonized with various bacteria[ 28].
Hailey-Hailey disease
Hereditary hypotrichosis simplex of the scalp (HSS)
HSS patients are usually born with normal hair, with a progressive scalp hair loss with age since early childhood and throughout adolescence, leading to significant scalp loss by the third decade of life. All the other parts of hair, teeth,nails, and sweat glands remain unaffected. HSS was an autosomal dominant hereditary hair loss caused by heterozygous mutations in theCDSNgene, encoding corneodesmosin[ 29]. Nonsense mutation is one of the most often cause.The mutations ofCDSNlead to truncated corneodesmosin formation, which is hypothesized to be toxic and aggregates around hair follicles. This mechanism explains the delayed onset and progressive manner of HSS [ 29].
There was no effective treatment except wearing wigs.In 2020, Peled et al. attempted to apply topical gentamicin on HSS patients after the inspiration of the experiences in NPPK and RDEB [ 22]. They first confirmed in vitro that gentamicin restores full-length corneodesmosin translation in HEK293 cells, then recruited four HSS patients and healthy relatives, and applied 0.1% gentamicin ointment twice daily to the scalp for 6 months. Results showed that gentamicin might restore full-length corneodesmosin protein biosynthesis in patients' keratinocytes, clinically improved hair growth, and reduced Severity of Alopecia Tool score.Gentamicin blood levels were detected consistently at extremely low levels (< 0.5 μg/mL) in all patients during treatment. This study indicates a promising future in further research in hereditary alopecia.
Xeroderma pigmentosum group C (XP-C)
Xeroderma pigmentosum (XP) is a rare autosomal recessive disorder of DNA repair characterized by increased photosensitivity, early development of pigmentary changes, and increased occurrence of skin cancers caused by mutations in genes involved in nucleotide excision repair of ultraviolet radiation (UV)-induced DNA damage. Nonsense mutations have been reported in 15% of XP-C patients [ 30]. In 2013, Kuschal et al. found that treatment with gentamicin could result in stabilized XPC mRNA, which was degraded by NMD, increased expression of XPC protein, recruitment of XPB and XPD proteins to UV DNA damage sites,and increased repair of 6-4 photoproducts and cyclobutane pyrimidine dimers [ 30 ]. Later in 2015, Kuschal et al.reported that gentamicin could achieve PTC readthrough in cells from XP-C patients and detectable XPC protein after gentamicin treatment. Treatment with the NMD inhibitor cycloheximide increased XPC mRNA, indicating that NMDprone XPC mRNA could be restored. They evaluated the toxicity of several aminoglycosides and their readthrough efficiency. The response depended on the PTC sequence,its location within the gene, and the aminoglycoside [ 31].Although there is no topical application in patients' skin yet,this way can be investigated as a personalized treatment in the future.
Potential adverse effect of topical gentamicin
Adverse events of topical gentamicin are relatively mild.According to previous clinical observations mentioned above, there was no evidence of systemic adverse events associated with drug use except for one patient that reported pruritic erythema and vesicles in the study of Ohguchi et al. [ 16] The possibility of irritation including erythema and pruritus, is emphasized according to Food and Drug Administration label of 0.1% gentamicin sulfate cream, but only in a small number of patients. There is also allergic potential mentioned in the literature [ 32].
The other common concerns of gentamicin administration are nephrotoxicity and ototoxicity as side effects of aminoglycoside. These concerns are generally not a problem since the daily topical dose of gentamicin is much lower than systemic use in previously reported clinical studies (7.5-10 mg/kg in DMD, CF, JEB), which exhibited no toxicity [ 6, 28, 33]. A renal function and hearing test could be conducted and followed up when needed.The fact that chronic exposure to topical gentamicin could enhance bacterial resistance is confirmed. However, it is not comparable with the benefits [ 24, 34].
Conclusions
Topical readthrough therapy heralds a new era in the treatment of genodermatosis. When a genodermatosis is suspected, gene sequencing is necessary not only for precise diagnosis but also for the hope of treatment options. For those caused by a nonsense mutation, topical gentamicin is a convenient, targeted, inexpensive, and relatively safe option with a promising therapeutic effect in comparison with systemic treatment and other conventional treatments.Besides NPPK, EB, HHD, HSS, and XP-C, readthrough therapy in more available skin diseases with nonsense mutations awaits exploration in the future.
Author contributions ML and YYH have contributed equally to this work. The article was conceived on a professional discussion network.WS contributed to writing of original draft, data curation and resources.YZ, LY, ZMT, and ZJ contributed to data curation and resources. ZH,LZY, and WXL contributed to review and editing. ML contributed to review and editing, funding acquisition, and supervision. YYH contributed to review and editing, and supervision. All authors contributed to the development of ideas, editing and approved the final manuscript.
Funding This work was supported by Children’s Medicine Research Project of Beijing Children’s Hospital, Capital Medical University(YZZD202002).
Data availability Not applicable for review article.
Declarations
Ethical approval Not required for this review article.
Conflict of interest No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article. The authors have no conflict of interest to declare.
 World Journal of Pediatrics2021年6期
World Journal of Pediatrics2021年6期
- World Journal of Pediatrics的其它文章
- Muscle strength and its association with cardiometabolic variables in adolescents: does the expression of muscle strength values matter?
- Vestibular function of pediatric patients with sudden sensorineural hearing loss: based on vertigo symptom and vestibular function testing
- Evaluation of a new frequency-volume chart for children with primary monosymptomatic nocturnal enuresis: a prospective, comparative study
- Consensus statement on the epidemiology, diagnosis, prevention,and management of cow's milk protein allergy in the Middle East:a modified Delphi-based study
- Treatment of pediatric mild persistent asthma with low-dose budesonide inhalation suspension vs. montelukast in China
- Haploidentical hematopoietic stem cell transplantation for pediatric patients with chronic active Epstein-Barr virus infection:a retrospective analysis of a single center
