Call for action: Increased healthcare utilization with growing use of percutaneous cholecystectomy tube over initial cholecystectomy in cirrhotics
Srikanth Vedachalam a , Sajid Jalil a , b , Somashaker G Krishna a , b , Kyle Porter c , Na Li a ,Sean G Kelly a , b , Lanla Conteh a , b , Khalid Mumtaz a , b , *
a Department of Internal Medicine, The Ohio State University Wexner Medical Center, Columbus, OH 43210, USA
b Division of Gastroenterology, Hepatology and Nutrition, The Ohio State University Wexner Medical Center, Columbus, OH 43210, USA
c Center for Biostatistics, Department of Biomedical Informatics, The Ohio State University Wexner Medical Center, Columbus, OH 43210, USA
Keywords:National Healthcare Cholecystectomy Percutaneous cholecystostomy Cirrhosis Decompensated cirrhosis
ABSTRACT
Background: Acute calculous cholecystitis (ACC) is frequently seen in cirrhotics, with some being poor candidates for initial cholecystectomy. Instead, these patients may undergo percutaneous cholecystostomy tube (PCT) placement. We studied the healthcare utilization and predictors of cholecystectomy and PCT in patients with ACC.
Methods: The National Database was queried to study all cirrhotics and non-cirrhotics with ACC between 2010-2014 who underwent initial PCT (with or without follow-up cholecystectomy) or cholecystectomy.Cirrhotic patients were divided into compensated and decompensated cirrhosis. Independent predictors and outcomes of initial PCT and failure to undergo subsequent cholecystectomy were studied.
Results: Out of 919 189 patients with ACC, 13 283 (1.4%) had cirrhosis. Among cirrhotics, cholecystectomy was performed in 12 790 (96.3%) and PCT in the remaining 493 (3.7%). PCT was more frequent in cirrhotics (3.7%) than in non-cirrhotics (1.4%). Multivariate analyses showed increased early readmissions [odds ratio (OR) = 2.12, 95% confidence interval (CI): 1.43-3.13, P < 0.001], length of stay (effect ratio = 1.39, 95% CI: 1.20-1.61, P < 0.001), calendar-year hospital cost (effect ratio = 1.34, 95% CI: 1.28-1.39, P < 0.001) and calendar-year mortality (hazard ratio = 1.89, 95% CI: 1.07-3.29, P = 0.030) in cirrhotics undergoing initial PCT compared to cholecystectomy. Decompensated cirrhosis (OR = 2.25, 95%CI: 1.67-3.03, P < 0.001) had the highest odds of getting initial PCT. Cirrhosis, regardless of compensated(OR = 0.56, 95% CI: 0.34-0.90, P = 0.020) or decompensated (OR = 0.28, 95% CI: 0.14-0.59, P < 0.001),reduced the chances of getting a subsequent cholecystectomy.
Conclusions: Cirrhotic patients undergo fewer cholecystectomy incurring initial PCT instead. Moreover, the rates of follow-up cholecystectomy are lower in cirrhotics. Increased healthcare utilization is seen with initial PCT amongst cirrhotic patients. This situation reflects suboptimal management of ACC in cirrhotics and a call for action.
Introduction
Increased incidence of gallstones and hence acute calculous cholecystitis (ACC) has been reported in patients with cirrhosis as compared to the general population [1] . Cholecystectomy is the definitive therapy for ACC, but it has risks in patients with cirrhosis. Studies have shown that cirrhotic patients tend to have higher rates of morbidity and mortality after cholecystectomy with up to 12 times increased mortality in decompensated cirrhotics [ 2 , 3 ]. However, medical optimization in decompensated cirrhosis patients with ACC that undergo cholecystectomy may reduce high morbidity and mortality rates [3] . Moreover, advances in laparoscopic surgeries in cirrhotics have shortened operative times,length of hospital stay, and resulted in more rapid recovery [4] .
Biliary decompression with percutaneous cholecystostomy tube(PCT) placement is indicated in cirrhotic patients who are not medically fit for immediate cholecystectomy. This technique has traditionally been used in poor surgical candidates as a bridge to cholecystectomy for patients who are able to recover from their acute illnesses [5-7] . PCT has additionally been shown to be effective in cirrhotics with ascites, which was considered a relative contraindication in the past [8] . This procedure can provide patients with decompression of their biliary system, typically within a few days, reduce overall complications of ACC, and allow time to optimize cirrhotic patients for definitive surgery [7-9] . However, in the general population, PCT typically leads to multiple readmissions,prolonged hospital stays, and higher hospital costs when it is not followed by definitive management [8-10] . Pavurala et al. [10] has recently reported the predictors of PCT vs. cholecystectomy in patients with ACC using a national database. Presence of cirrhosis was identified as a risk factor for not getting initial cholecystectomy in patients with ACC [10] .
In patients with cirrhosis, healthcare utilization, predictors and outcomes of cholecystectomy vs. PCT in the management of ACC has not been addressed at a national level. Therefore, we aimed to study healthcare utilization including early readmission, length of stay, hospital cost and mortality of cholecystectomy vs. PCT in patients with cirrhosis. We also studied the predictors of initial PCT and predictors of not getting subsequent cholecystectomy after initial PCT in ACC patients with cirrhosis.
Methods
Database information
We utilized the Nationwide Readmission Database (NRD) to study the aims described above. The NRD is the largest all-payer inpatient care, administrative claims database in the United States.The NRD is designed to analyze national readmission rates for hospitals across all age groups. The 2010-2014 NRD is drawn from the State Inpatient Databases (SIDS). The database was constructed from 22 state databases and accounted for 51.2% of total US population and 49.3% of all hospitalizations [11] . All hospital admissions within the states are tracked with special patient linkage numbers and hospital identifiers, but not across state lines. Up to 25 diagnoses codes, 15 procedure codes and 30 Agency for Healthcare Research and Quality (AHRQ) comorbidity variables were included in each hospitalization with a total of more than 100 patient- and hospital-related variables. All data were de-identified and couldnot be tracked to individual patients. Data were constructed using the NRD from 2010 to 2014 with pre-validatedInternationalClassificationofDiseases,NinthRevision,ClinicalModification(ICD-9 CM)codes for diagnoses and procedures.
The Ohio State University’s Institutional Review Board (IRB)deemed this study ‘exempt’ from IRB approval, as the NRD is a publicly available database of de-identified patients.
Study population
Patients with a primary diagnosis of ACC and having procedure codes (Table S1) for either cholecystectomy or PCT were identified for our study ( Fig. 1 ). Patients under the age of 18 years, had a hepatobiliary or intestinal malignancy, or had an autoimmune hepatobiliary condition were excluded. Patients with index discharges in December were excluded due to the inability to track a full 30 days for readmissions, as NRD cannot track across calendar years.
Patients were considered to have cirrhosis if they have a secondary diagnosis of liver cirrhosis during their index admission.Patients with no cirrhosis were considered as a reference group.We defined “the cases” as cirrhotic patients with ACC who underwent PCT with or without subsequent cholecystectomy during index admission. Cirrhotic patients with ACC who underwent cholecystectomy without preceding PCT were considered as “the controls” in this study. Cirrhotic patients were further categorized into compensated and decompensated based on Baveno IV criteria. According to Baveno IV criteria, stages I through IV are defined as: Ino varices or ascites; II- varices without ascites; III- ascites with or without varices; and IV- bleeding varices with or without ascites,respectively [12] . Therefore, we identified decompensated cirrhosis through the combination of a cirrhosis code and at least one code for ascites, hepatic encephalopathy, variceal bleeding, or spontaneous bacterial peritonitis. The ICD-9 CM diagnosis and procedure codes used in this study are presented in Table S1.
Patient and hospital characteristics
Demographic data collected from the NRD for each patient included age, sex, primary payer, income quartile of patient based on the zip code, and length of stay. Procedures included cholecystectomy and PCT. cholecystectomy was further differentiated by open vs. laparoscopic (Table S1). Comorbidities were assessed using the Elixhauser comorbidity index and stratified into 0-4 and ≥5(modified to exclude liver disease) [13] . The Elixhauser comorbidity index uses ICD-9 CM diagnosis codes to stratify comorbidities to predict hospital resource use and in-hospital mortality. It has been validated for use with administrative hospital databases [14] .In addition, we analyzed a broad list of medical comorbidities to further characterize patients’ level of health, which can be found in Table S1. We also collected data on the type and size of hospitals. Hospital type was categorized as metropolitan, teaching;metropolitan, non-teaching; and non-metropolitan. Hospital bed size was stratified as small, medium and large based on the algorithm developed by Healthcare Cost and Utilization Project(HCUP) [11] .
Our group has recently validated the ICD-9 codes for the diagnosis of ACC and interventions (cholecystectomy and PCT) at the Ohio State University, Columbus, Ohio, USA [10] . We used the same validated codes for this study. All codes are presented in Table S1.
Outcomes and definitions
Primary outcome of our study was healthcare utilization including early readmission, length of stay, hospital cost and mortality with initial cholecystectomy vs. PCT placement. Decompensated and compensated cirrhosis patients were combined to assess primary healthcare utilization outcomes between patients receiving initial PCT vs. those receiving initial cholecystectomy given relatively lower numbers of decompensated cirrhosis.
Secondary outcomes studied were the predictors of initial PCT placement and predictors of not receiving subsequent cholecystectomy after initial PCT placement at index admission.
The initial qualifying admission was designated the index admission. “Early readmission” was defined as admission within 30 days of the index discharge. In patients with multiple readmissions within a month, the earliest admission was included as the “early readmission”. Readmission occurring in the same state and same calendar year is identifiable in the NRD.
Calendar-year mortality was defined as in-hospital mortality at any time within the calendar year of the index admission (index mortality + any readmission mortality). Patients who died at index admission were excluded from readmission analysis.
Hospital cost was summarized at the index admission and as the sum of all readmissions within 30 days of index discharge and the sum of all admissions throughout the calendar year. Hospital cost was estimated by multiplying the NRD hospital charge by the hospital-specific cost-to-charge ratio provided by the HCUP [14] .Hospital costs were adjusted for inflation to the 2014 basis using the Personal Health Care (Hospital Care) Price Indices from the Medical Expenditure Panel Survey (MEPS).

Fig. 1. Study design flowchart. PCT: percutanous cholecystostomy tube; CCY: cholecystectomy.
Statistical analysis
All statistical analyses were weighted and accounted for the NRD sampling design (discharge weights, stratification, and clustering). Hospital cost (index and calendar-year admissions) and index length of stay were tested by linear regression using logtransformed outcomes to meet normality assumptions. Among patients receiving a cholecystectomy subsequent to PCT, healthcare outcomes were compared between no cirrhosis and cirrhotic patients using the linear regression analysis for initial PCT and initial cholecystectomy.
Logistic regression models identified predictors of receiving PCT and predictors of having a subsequent cholecystectomy following PCT. The subsequent cholecystectomy model only used data from patients having an initial PCT. Backward selection with a threshold ofP<0.05 was used to select predictors included in each of the final models.
Calendar-year mortality was tested by Cox proportional hazards.Patients who died at index admission were considered to die one day after discharge and patients not having in-hospital mortality within the calendar year were considered censored based on the discharge month of the NRD record.
Admissions with missing charges (n= 233) were excluded from the cost analyses. The only other missing data were for the primary payer and zip code income quartile variables; for the primary payer the missing values (n= 19; 0.1%) were categorized with the“other/unknown” admissions and for income quartile missing values (n= 14 731; 1.6%) were categorized as a separate “missing”category for analysis. All analyses were performed using SAS 9.4(SAS Institute Inc., Cary, NC, USA).
Results
Patient and hospital characteristics
A total of 919 189 patients with ACC underwent cholecystectomy or PCT. Of these, 905 906 were no cirrhosis (98.6%) and 13 283 (1.4%) were cirrhotics; out of those with cirrhosis, 11 093(83.5%) were compensated cirrhosis and 2190 (16.5%) were decompensated cirrhosis. Baseline demographics are presented in Table S2. Increased number of patients with cirrhosis (493/13 283, 3.7%)underwent initial PCT as compared to no cirrhosis (13 016/905 906, 1.4%) on index admission. Among those who underwent initial PCT, subsequent cholecystectomy was performed in 40.0%(n= 5195), 28.7% (n= 93) and 16.0% (n= 27) of patients with no cirrhosis, compensated cirrhosis and decompensated cirrhosis,respectively.
Comparison of initial PCT vs. initial cholecystectomy
Table 1 compares the two intervention arms—cholecystectomy and PCT. There were more patients with decompensated cirrhosis underwent PCT than cholecystectomy (34.3% vs. 15.8%,P<0.01)Patients undergoing initial PCT had an increased Elixhauser comorbidity scores ≥5 (44.0%) than those undergoing initial cholecystectomy (22.9%). Initial PCT was performed more frequently in metropolitan teaching (60.9% vs. 41.4%,P<0.01) and large bedsized hospitals (79.0% vs. 62.9%) than initial cholecystectomy.
Healthcare utilization in ACC patients with cirrhosis
Among all cirrhotic patients (compensated cirrhosis and decompensated cirrhosis combined), initial PCT was performed in 493 (3.7%) and initial cholecystectomy was performed in 12 790(96.3%). Increased healthcare utilization including early readmissions (35.0% vs. 17.0%; odds ratio (OR) = 2.12, 95% CI: 1.43-3.13,P<0.001), length of stay [median: 7 vs. 4 days; effect ratio(ER) = 1.39, 95% CI: 1.20-1.61,P<0.001], calendar-year hospital cost (median: $27 292 vs. $16 400; ER = 1.34, 95% CI: 1.28-1.39,P<0.001) and calendar-year mortality (12.4% vs. 3.8%; hazard ratio = 1.89, 95% CI: 1.07-3.29,P= 0.030) were observed in cirrhotic patients undergoing initial PCT compared to cholecystectomy on multivariate analysis ( Table 2 ).
Predictors of initial PCT placement in all ACC patients
One point four percent patients with no cirrhosis underwent initial PCT as compared to 2.9% with compensated cirrhosis and 7.7% with decompensated cirrhosis. Independent predictors of initial PCT placement were presence of decompensated cirrhosis(OR = 2.25, 95% CI: 1.67-3.03,P<0.001), but not compensated cirrhosis (OR = 1.15, 95% CI: 0.92-1.43,P= 0.230). Older age(age ≥65 years: OR = 3.88, 95% CI: 3.29-4.58,P<0.001; age 45-64 years: OR = 2.19, 95% CI: 1.94-2.48,P<0.001), increased score of Elixhauser comorbidity (Elixhauser ≥5: OR = 5.68, 95%CI: 4.84-6.66,P<0.001; Elixhauser 1-2: OR = 1.65, 95% CI:1.42-1.90,P<0.001; Elixhauser 3-4: OR = 3.24, 95% CI: 2.83-3.81,P<0.001), Medicare (OR = 1.26, 95% CI: 1.11-1.43,P<0.001)& Medicaid insurances (OR = 1.41, 95% CI: 1.21-1.63,P<0.001),metropolitan teaching (OR = 3.62, 95% CI: 2.92-4.49,P<0.001)and metropolitan non-teaching (OR = 1.46, 95% CI: 1.19-1.79,P<0.001) compared to non-metropolitan hospitals, large bed size hospitals (OR = 1.44, 95% CI: 1.23-1.70,P<0.001), CAD (OR = 1.37,95% CI: 1.25-1.50,P<0.001), atrial fibrillation (OR = 1.46, 95%CI: 1.33-1.60,P<0.001), sepsis (OR = 3.95, 95% CI: 3.42-4.57,P<0.001), diastolic and systolic heart failure (systolic: OR = 1.79,95% CI: 1.50-2.13,P<0.001; diastolic: OR = 1.54, 95% CI: 1.31-1.81,P<0.001) were also associated with PCT placement. On the other hand, female patients (OR = 0.75, 95% CI: 0.70-0.80,P<0.001),hypertension (OR = 0.80, 95% CI: 0.74-0.86,P<0.001), type 2 diabetes (OR = 0.86, 95% CI: 0.80-0.93,P<0.001) and mechanical ventilation (OR = 0.56, 95% CI: 0.44-0.72,P<0.001) were less likely to undergo initial PCT ( Table 3 ). Initial PCT was performed in 4.2% of patients requiring mechanical ventilation (n= 9506)as compared to 1.4% who did not require mechanical ventilation(n= 13 110).
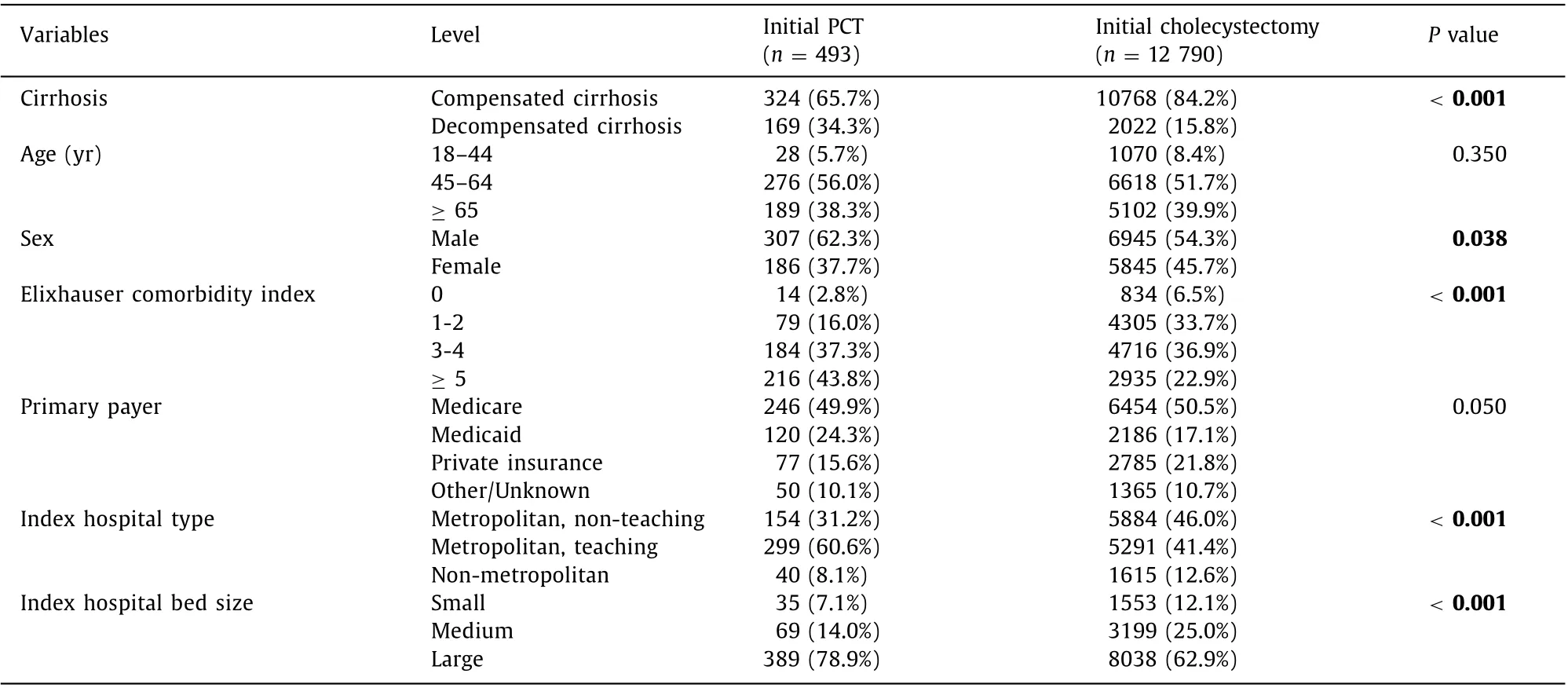
Table 1 Demographic and hospital characteristics of patients with cirrhosis receiving PCT vs. cholecystectomy on index admission.

Table 2 Healthcare utilization among patients with cirrhosis receiving PCT vs. cholecystectomy on index admission.
Predictors of failure to undergo subsequent cholecystectomy in all ACC patients
Overall, 39.3% (n= 5315/13 510) of all patients receiving initial PCT underwent follow-up cholecystectomy within one calendar year. Follow-up cholecystectomy was performed in 40.0%, 28.7%and 16.0% patients with no cirrhosis, compensated cirrhosis and decompensated cirrhosis, respectively. Presence of cirrhosis, regardless of compensated cirrhosis (OR = 0.56, 95% CI: 0.34-0.90,P= 0.020) or decompensated cirrhosis (OR = 0.28, 95% CI: 0.14-0.59,P<0.001), was an independent predictor of not getting subsequent cholecystectomy. Other independent predictors of not getting subsequent cholecystectomy were older age (age ≥ 65 years: OR = 0.38, 95% CI: 0.30-0.48,P<0.001; age 45-64 years:OR = 0.61, 95% CI: 0.48-0.77,P<0.001), Elixhauser comorbidity score (Elixhauser ≥5: OR = 0.57, 95% CI: 0.43-0.75,P<0.001;Elixhauser 3-4: OR = 0.69, 95% CI: 0.53-0.91,P= 0.010), receiving hemodialysis (OR = 0.40, 95% CI: 0.22-0.72,P= 0.002), and diastolic heart failure (OR = 0.64, 95% CI: 0.46-0.90,P= 0.010).Contrary to that, index admission to metropolitan, non-teaching(OR = 1.54, 95% CI: 1.31-1.81,P<0.001 vs. metropolitan, teaching hospitals) and mechanical ventilation (OR = 1.63, 95% CI: 1.03-2.56,P= 0.040) were predictive of follow-up cholecystectomy after initial PCT ( Table 4 ). Subsequent cholecystectomy after initial PCT was performed in 45.8% of patients who underwent mechanical ventilation at index admission (n= 400) as compared to39.1% of those with no mechanical ventilation at index admission(n= 13 110).

Table 3 Predictors of initial percutaneous cholecystostomy in all acute calculous cholecystitis patients.
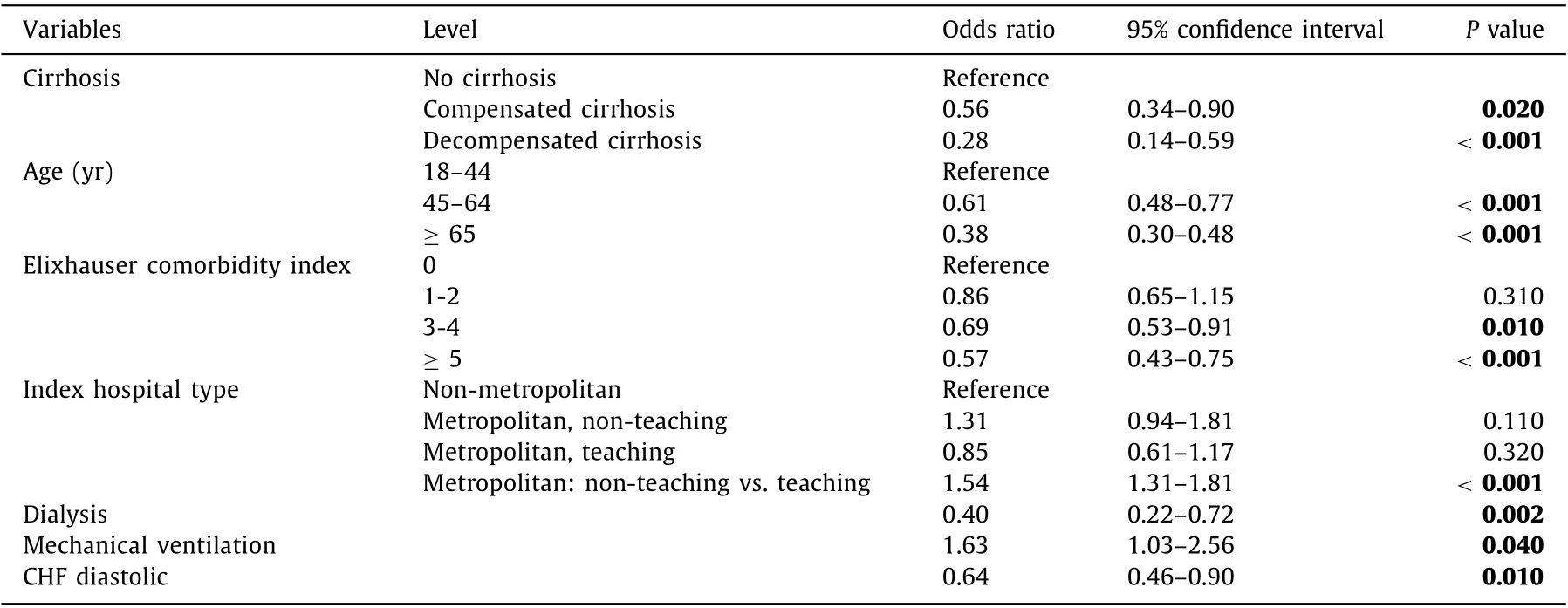
Table 4 Predictors of subsequent cholecystectomy following percutaneous cholecystostomy in all acute calculous cholecystitis patients.
Discussion
In this national analysis of patients with ACC, we found that the rates of initial PCT instead of cholecystectomy were higher among patients with cirrhosis as compared to patients with no cirrhosis.Comparatively patients with compensated cirrhosis had twice the rate, and decompensated cirrhosis had five-times the rate of initial PCT. This procedure in cirrhotics was associated with increased healthcare utilization as compared to initial cholecystectomy. Elderly patients with decompensated cirrhosis, possessing medicaid or medicare insurance, admitted in large and metropolitan hospitals, with cardiac risk factors have increased chances of getting initial PCT. Moreover, presence of cirrhosis was associated with lower rates of subsequent cholecystectomy during one-calendar year of follow-up, especially in decompensated cirrhosis. Other predictors of failure to undergo subsequent cholecystectomy were older age,increased Elixhauser comorbidity scores, hemodialysis, and diastolic heart failure. This situation reflects suboptimal management of ACC in cirrhotics and a call for action.
Previous studies have shown that PCT typically leads to multiple readmissions, prolonged hospital stays, and higher hospital costs [ 1 , 9 , 10 ]. In one study, despite adjusting for multiple variables including age, race, sex, comorbidity, teaching hospital status and year, this still holds true [9] . PCT also has been reported by others to have an adverse event rate of 10%-17% [ 8 , 10 , 15 ]. Like all other patients, cirrhotics seem to fall within this category of increased healthcare utilization with PCT, including higher 30-day readmission, length of stay, cost of care, and calendar year mortality. We found that these increased rates of healthcare utilization are likely driven by relatively more decompensated cirrhosis having initial PCT, as well as having more individuals with higher Elixhauser comorbidity scores undergoing the procedure. We had also found that large, metropolitan teaching hospitals correlate with these findings, which was likely due to their handling of more ill patients.
Our study showed that 7.7% of decompensated cirrhosis undergoing PCT compared to 1.4% of no cirrhosis. This is expected, as decompensated patients are generally poor surgical candidates due to coagulopathy and portal hypertension. Common complications from cholecystectomy in this population include gallbladder bed bleeding, peri- or post-operative decompensation and systemic infection [6] . While PCT has been shown to increase healthcare utilization, it remains the standard of care in patients deemed to be poor surgical candidates. There is growing literature that supports the use of endoscopic ultrasound with gallbladder drainage (EUSGBD) or endoscopic retrograde cholangio-pancreaticography (ERCP)with cystic duct stenting for treatment of patients not amenable to surgery.
EUS-GBD is an emerging intervention where drainage of gallbladder is achieved through the stomach or duodenum using a combination of an endoscopic needle, guidewire, and plastic or metal stent. EUS-GBD has been shown to be superior to PCT in previous studies [ 14 , 15 ]. One study comparing EUS-GBD vs. PCT has noted that clinical success rate, defined as resolution of cholecystitis, was 95% with EUS-GBD, while it was only 86% with PCT [14] .Additionally, repeat interventions were more common in PCT than those with EUS-GBD (24% vs. 10%) [14] . It showed high clinical and technical success rates with EUS-GBD of 93.3% each (14/15 patients) with EUS-GBD in cirrhotic populations [15] . Pelavski et al.reported their experience of four severe decompensated cirrhosis with ACC managed with EUS-GBD. They showed 100% technical and clinical success with all patients [16] . ERCP with cystic duct stenting in patients with ACC is another less invasive option. Success rate of this intervention has been reported similar to EUSGBD. However, it may be technically challenging given the tortuosity of some cystic ducts, which may be common in cirrhotics [15] .
In addition to cirrhosis, other factors that were found in our study to be predictive of PCT placement included older age, Medicaid or Medicare insurance, type and size of hospital and cardiac risk factors. Advanced age is reported to be correlated to frailty and malnutrition [16] , which is many fold higher in cirrhotics as compared to general population. Medicaid and Medicare correlation with PCT placement may speak to the generally “sicker” and older population that receive these insurance benefits. In terms of hospital type we saw in our study, metropolitan, teaching hospitals performed more PCT than non-teaching hospitals. It is difficult to determine the exact reason, but could be due to “sicker” patient requiring care at major tertiary teaching centers.
Rates of following cholecystectomy after PCT ranged from 21%-39% in our study patient population with no cirrhosis [ 6 , 8 ]. Lower rates were seen in cirrhotics, especially decompensated cirrhotics.While many may never be deemed safe surgical candidates, regular reassessments should be made for definitive, surgical therapy given the potential for increased healthcare utilization with PCT.Apart from presence of cirrhosis, older age, Elixhauser comorbidity score>3, receiving hemodialysis and diastolic heart failure predicted failure to undergo cholecystectomy after PCT. Older age again was likely a barrier to surgery given issues with frailty and malnutrition. The remaining risk factors appeared to designate a“sicker” population.
There were several limitations in our study inherent to administrative nature of database. The NRD depends on the accuracy of billing codes used for the diagnosis and may underrepresent certain populations despite the usage of validated coding throughout the study. In our study this is specifically pertinent with needing accurate billing codes for esophageal varices, spontaneous bacterial peritonitis, hepatic encephalopathy and/or gastrointestinal bleeds in order to classify cirrhotic patients as decompensated vs. compensated. Additionally, we were restricted to not being able to use patient level data given the nature of our database, such as individual laboratory, procedural results, and cause of death. This precluded us from important granular information, including assessing liver disease severity using model for end-stage liver disease or Child-Turcotte-Pugh scores. However, the Baveno criteria used for stratifying patients into compensated cirrhosis and decompensated cirrhosis have been used successfully in previous NRD based study [12] . The NRD is limited to data collected during inpatient admission/procedures with the help of ICD coding. We performed a sensitivity analysis of ICD-9 codes, using The Ohio State University Wexner Medical Center database and demonstrated that patients who received PCT did not undergo subsequent outpatient cholecystectomy [10] . This is similar to our observation based on NRD analyses. However, despite the limitations of our study, the data collected from the NRD are large, robust and provided us with a representation of national trends of PCT and cholecystectomy in patients with cirrhosis.
In conclusion, presence of cirrhosis is found out to be a risk factor for undergoing initial PCT over initial cholecystectomy, and failure to undergo subsequent cholecystectomy. We found that at national level PCT have poor longitudinal outcomes and increased healthcare utilization. Our study results advocate for cholecystectomy, whenever feasible, given its improved outcomes and definitive interventions. However, PCT remains standard of care when cholecystectomy is not feasible. Keeping in mind our results, reflecting currently available suboptimal management of ACC in cirrhotics, a call for action in the form of alternative intervention is required.
Acknowledgments
None.
CRediT authorship contribution statement
Srikanth Vedachalam : Conceptualization, Writing - original draft. Sajid Jalil : Supervision, Writing - review & editing. Somashaker G Krishna : Conceptualization, Writing - review &editing. Kyle Porte r: Data curation, Formal analysis, Software. Na Li : Writing - review & editing. Sean G Kelly : Writing - review& editing. Lanla Conteh : Writing - review & editing. Khalid Mumtaz : Conceptualization, Project administration, Supervision,Writing - review & editing.
Competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Funding
None.
Ethical approval
Not needed.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.hbpd.2021.07.008 .
 Hepatobiliary & Pancreatic Diseases International2022年1期
Hepatobiliary & Pancreatic Diseases International2022年1期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Primary pancreatic lymphoma diagnosed by endoscopic ultrasound-guided fine needle biopsy
- A stable and reliable animal model for hepatocellular carcinoma with portal vein tumor thrombus
- Expert consensus on the application of the magnetic anchoring and traction technique in thoracoscopic and laparoscopic surgery
- Diverse and precision therapies open new horizons for patients with advanced pancreatic ductal adenocarcinoma
- SNHG16 promotes hepatocellular carcinoma development via activating ECM receptor interaction pathway
- Predictors of recurrent bile duct stone after clearance by endoscopic retrograde cholangiopancreatography: A case-control study
