Lower extremity amputation: the emerging role of targeted muscle reinnervation (TMR) and regenerative peripheral nerve interface (RPNI)
Yoshiko Toyoda, Said Azoury, Andrew Bauder, L. Scott Levin, Stephen Kovach,
1Division of Plastic and Reconstructive Surgery, University of Pennsylvania, Philadelphia, PA 19104, USA.
2Department of Plastic and Reconstructive Surgery, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA.
3Department of Orthopedic Surgery, University of Pennsylvania, Philadelphia, PA 19104, USA.
Abstract Lower extremity amputation is increasingly prevalent in the United States, with growing numbers of patients suffering from diabetes and peripheral vascular disease. Amputation has significant functional sequelae as more than half of patients are unable to ambulate at one year postoperatively. Improving mobility and decreasing chronic post-amputation pain can significantly improve the quality of life for these patients and reduce the cost burden on the healthcare system. Plastic and reconstructive surgery has been at the forefront of “reconstructive amputation”, in which nerve pedicles can be surgically guided to decrease painful neuroma formation as well as provide targets for myoelectric prosthesis use. We herein review post-amputation outcomes, epidemiology of chronic, postamputation pain, and current treatments, including total muscle reinnervation and regenerative peripheral nerve interface, which are at the forefront of multidisciplinary treatment of lower extremity amputees.
Keywords: Lower extremity amputation, post-amputation pain, neuroma, targeted muscle reinnervation, regenerative peripheral nerve interface
INTRODUCTION
Extremity amputation is one of the oldest procedures known to man, with archeological records suggesting purposeful amputations as far back as 45,000 years since Neolithic times[1]. The word “amputation” can trace its origin to the Latin term “amputatio” meaning “to cut around”[1]. Amputation continues to be prevalent in the present day, with approximately 1.6 million people with major limb amputations in the United States in 2005 and 185,000 major limb amputations every year[2,3]. By 2050, the prevalence is estimated to be as large as 3.6 million, given that the most common causes for amputation, especially in the lower extremity, are diabetes and peripheral vascular disease, which continue to uptrend[1,3]. Nontraumatic lower extremity amputation is estimated at only 3/10,000 among the healthy population in 2010, but as high as 28.4-46.2/10,000 among diabetics[4]. Unsurprisingly, significant healthcare costs are associated with these patients. The lifetime all-cause direct cost for lower extremity amputation was estimated to be $35.8 billion for the country[4]. No appropriate literature on the indirect or total cost of amputation currently exists, but it is estimated to be significant as studies have found that after a major lower extremity amputation, up to 53.9% of patients were still nonambulatory at 6-month follow-up after the operation[4]. Another retrospective chart review of 206 patients who underwent major lower extremity amputation demonstrated a one-year postamputation ambulatory rate of 46.1%[5]. Furthermore, nonambulatory rates were higher in those with higher BMI, which portend a poor future as the United States population suffers from increasing rates of obesity[5]. Lower extremity amputation is therefore not only a serious problem for individual patients, but a large - and only increasing - burden on the United States healthcare system. We herein aim to discuss post-amputation outcomes, epidemiology of chronic, post-amputation pain, and current treatments including total muscle reinnervation (TMR) and regenerative peripheral nerve interface (RPNI) at the forefront of multidisciplinary treatment of lower extremity amputees.
Outcomes of amputation
Functional equivalence of lower extremity amputation compared to limb salvage was demonstrated in the seminal Lower Extremity Amputation Prevention (LEAP) study[6]. This was a multicenter, prospective, observational study of 569 severe lower extremity injuries who received either amputation or reconstruction[6]. After two years of follow-up, the functional outcome of amputation and reconstruction as measured by the Sickness Impact Profile was not significantly different[6]. Rather, predictors of poor outcome were more correlated with intrinsic patient factors such as education level, ethnicity, insurance status, and social support[6]. Amputationvs.salvage has been a difficult decision tree branching point even for institutions well versed in critical limb injury. Ultimately, Blacket al.[7]offer some general consensus, but concluded that many patient-specific factors, as well as injury factors, must be considered prior to decide whether to amputate or salvage. Amputation is therefore not equivalent to failure to salvage, but a viable alternative reconstructive option with likely functional equivalence in the appropriate patient population.
In evaluating functionality after amputation, Suckowet al.[8]performed four focus groups in twenty-six patients who had lower extremity amputations from critical limb ischemia. Quality of life was determined mainly by impaired mobility (65%), pain (60%), progression of disease in the remaining limb (55%), and depression/frustration (54%). Sixty-two percent had multiple prior revascularization procedures. Areas of improvement included peer support (88%), extensive rehab/prosthetist involvement (71%), earlier mention of amputation as a possible outcome (54%), and early discontinuation of narcotics (54%). Physiciancontrolled factors such as the timing of amputation, informed decision-making, and postamputation support, play an important role[8]. For amputees, the function of their shortened limb correlates closely with prosthetic use[9]. These results have also been corroborated in the LIMB-Q, a patient-reported outcome instrument in which numerous themes were extracted from direct interviews with patients who suffered high-energy lower extremity trauma[10]. Themes such as physical ability, psychological, and prosthesis were important to patients’ daily quality of life[10]. As expected, a long-term study of patients over 20 years after their amputation demonstrated that higher amputation levels were associated with decreased prosthetic use while less intense residual limb pain was associated with greater daily prosthetic use[11]. Therefore, postamputation pain is not only simply unpleasant, but also directly affects patient function.
POST-AMPUTATION PAIN
Prevalence
Pain significantly impairs the post-amputation patient. In fact, among amputees with chronic pain, it is often not the underlying condition (i.e., amputation of the limb) that primarily limits the individual, but rather the chronic pain they experience[2,9]. Unfortunately, chronic pain is commonplace among amputees. Advances in the measurement of pain, including the validated Patient-Reported Outcomes Measurement Information System (PROMIS) pain interference and pain behavior scores, have been integral in better understanding the significance of pain on patients’ lives[12,13]. PROMIS is a National Institute of Healthfunded initiative to develop and validate patient-reported outcomes, including over 300 different measures of physical, mental, and social health typically used for populations with chronic conditions[13]. There are several types of amputation-related pain, including phantom limb pain (PLP), defined as pain in the limb that is no longer present, phantom limb sensation/telescoping, residual limb pain, and back pain[14]. A crosssectional survey through the Amputee Coalition of America in which 914 amputees were interviewed over the telephone found that 95% of amputees had some daily pain[2]. The most common pain type was phantom limb pain (79.7%), residual limb pain (67.7%), followed by back pain (62.3%)[2]. A systematic review of twelve cross-sectional and three prospective studies of traumatic and atraumatic amputees also reported phantom limb pain incidence as high as 82% at one-year post-amputation and a lifetime prevalence as high as 87%[15]. Even decades after amputation, phantom limb pain, and residual limb pain continue to trouble patients. In a survey of 21 patients who underwent lower limb oncological amputation with a median follow-up duration of 41 years, seventeen reported phantom limb and back pain, fifteen residual limb pain, all with median pain scores five and above on a scale of 1-10[11]. Post-amputation pain is, therefore, a ubiquitous and long-lasting complication of the operation.
Causes of chronic pain
While chronic pain is not yet fully understood, mechanistically, it is due to afferent input from the peripheral nervous system. A randomized, double-blind, placebo-controlled crossover study of lidocaine compared to placebo saline injection in amputees demonstrated expected outcomes of improved pain, especially phantom limb pain, in those who received the lidocaine block[16]. The causes of the pain are thought mainly from chronic nerve compression and neuroma. Chronic nerve compression typically occurs with major nerves at common entrapment sites[17]. Neuromas are classified as neurotmesis or Sunderland 5th degree peripheral nerve injury[18]. When peripheral nerves are injured, the distal end undergoes Wallerian degeneration. The proximal axon is unable to progress to its distal target, and the unorganized fascicular overgrowth results in scarring, thereby forming a neuroma[18]. Psychological factors are also thought to play a major role in both acquisition and maintenance of pain symptoms[19].
Symptomatic neuroma
Neuromas were first described by Abroise Pare in 1634, who treated the symptoms with massage[18]. Neuropathic pain associated with neuroma can be classified into four types: spontaneous pain, pain with pressure over the neuroma, pain on movement of adjacent joints, and dysesthesia or hypersensitivity with light skin touch[20]. Histological features and mechanisms of formation have been explored in several animal studies. A study of Sprague Dawley rat forelimb amputations demonstrated a progression of nerve injury from degenerating axons to axonal spouts to unorganized bundles of axons which grow into muscles and nearby structures and eventually into fibrotic tissue, ultimately forming a neuroma [Figure 1][21]. There are multiple theories on why neuromas cause pain. Proposed mechanisms include repetitive mechanical irritation from tethered scar or compression, myofibroblast proliferation, abnormal accumulation of sodium and potassium channels which cause hyperexcitability, surrounding inflammation that chemically stimulates nearby nociceptors, and release of inflammatory cytokines[18]. The amount of axoplasmic flow, the ratio of fascicles to epineural tissue, and the nutritional status of the peripheral nerve are thought to affect neuroma formation[18].
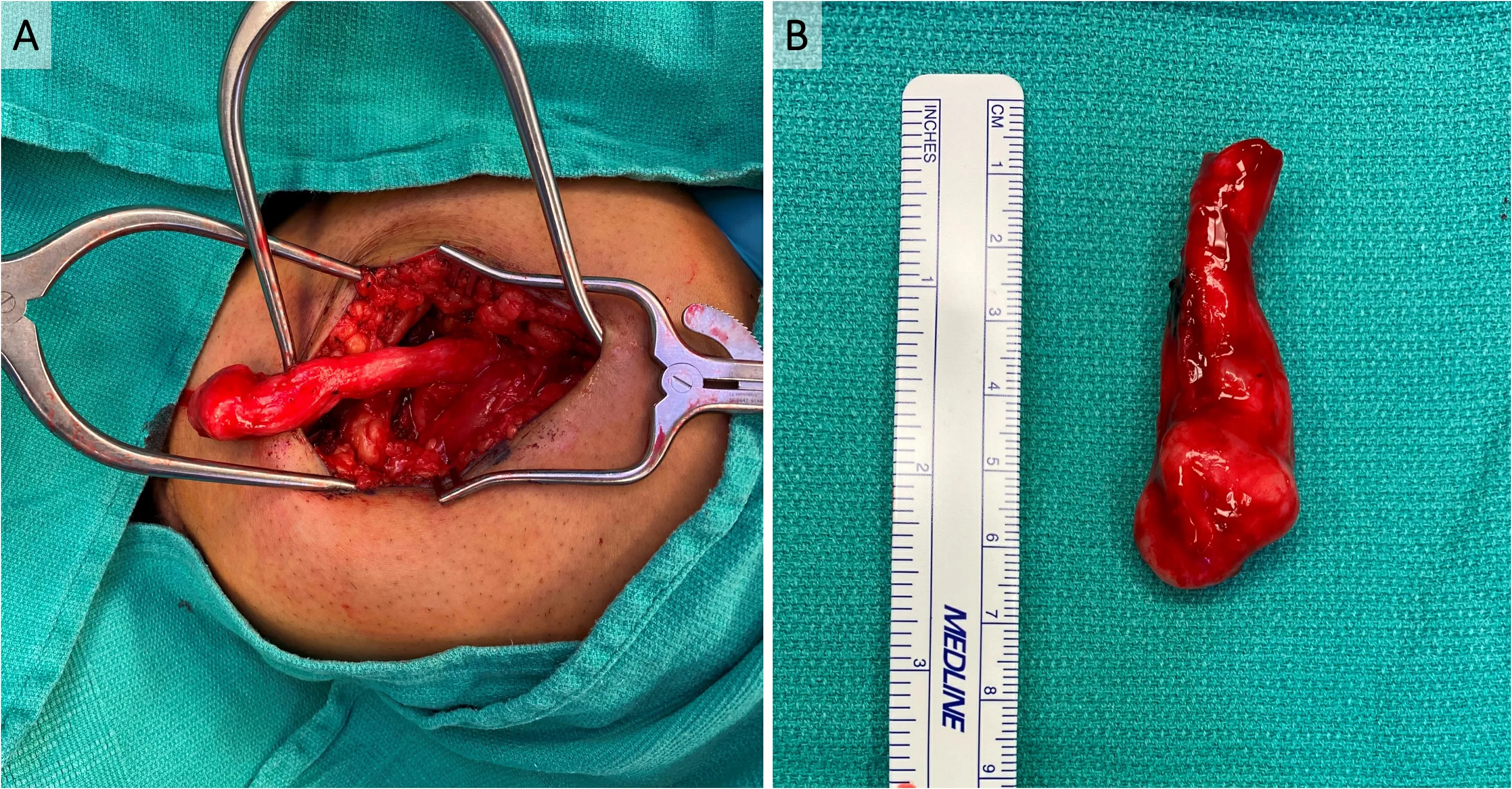
Figure 1. Symptomatic neuroma of sciatic nerve after above-knee amputation (AKA). A 26-year-old man with a history of polyarteritis nodosa who underwent left below-knee amputation as well as right AKA presented with right sciatic nerve symptomatic neuroma approximately 1.5 years after his AKA. The symptomatic neuroma was diagnosed both clinically and radiographically with MRI. (A) In situ sciatic nerve terminal neuroma in his right AKA surgical site. (B) The neuroma was excised, and sciatic nerve cut back to healthyappearing fascicles. The total resected neuroma and pathologic nerve measured roughly 6 cm long.
Diagnosis of the symptomatic neuroma is based on pain with a scar, altered sensation in the nerve distribution, and positive Tinel’s sign[18]. In cases in which more than one nerve is involved, the picture may be muddled, but the sensory issues should present in a dermatomal pattern[17]. Nerve blocks can be diagnostic and therapeutic in these patients.
Nonsurgical treatment of post-amputation pain
Treatment of post-amputation pain typically begins with non-specialized medical management, including a multimodal pain regimen with nonsteroidal anti-inflammatory drugs, opiates, and non-opiate analgesics like acetaminophen. When pain is not controlled, the patients often get referred to pain specialists where they may receive neuropathic pain medication, including gabapentinoids, antidepressants, or NMDA antagonists like ketamine, dextromethorphan, and memantine[1,22]. These are complemented by physical therapy techniques such as massage or injection therapy, including lidocaine and/or corticosteroid[1]. Regional nerve blocks by pain specialists may serve both diagnostic and therapeutic purposes[17].
Given the significant psychological aspect of post-amputation pain, neurologic and psychologic treatment such as biofeedback and cognitive behavioral therapy are trialed as well[1]. Unique therapies, such as mirror therapy may also be effective. A randomized controlled trial of fifteen amputees underwent mirrorvs.control covered mirror or mental visualization therapy[23]. Over four weeks of regular sessions, patients who underwent mirror therapy had a significant decrease in pain scores and daily length of time during which they experienced pain compared to the control group[23]. A systematic review of mirror therapy, motor imagery, and virtual feedback techniques on phantom limb pain after amputation found that while these techniques did seem to reduce pain, the quality of the studies was lacking, and given the limited scientific evidence supporting their effectiveness, no definitive conclusion could be drawn[24]. There has also been active research in neuromodulation techniques. A recent systematic review and meta-analysis of nine randomized controlled trials and five quasi-experimental studies found some hopeful data in transcranial direct current stimulation, although no definitive conclusions were reached yet given the infancy of these techniques for amputees[25].
Multiple disciplines continue to tackle the difficult challenge of post-amputation pain with some exciting preliminary results. However, clinical results of these nonsurgical options continue to be suboptimal, as evidenced by these numerous, diverse, and inconclusive studies. Perioperative management, including assessment of patient and injury risk factors, preoperative consultation of the acute pain service, preoperative loading of neuropathic pain medication, intraoperative blocks, perineural catheter infusion, ketamine infusion, and numerous non-FDA approved postoperative pain management have been proposed, albeit with no conclusive results[14].
Surgical treatment of post-amputation pain
Surgical interventions for symptomatic neuromas can be categorized as either active/reconstructive or passive/ablative[26]. Active/reconstructive measures include TMR and RPNI, which will be discussed in further detail below. Both of these procedures provide the problematic nerve “somewhere to go and something to do”, thereby diverting its natural regenerative processes away from neuroma formation[27].
Passive/ablative procedures include excision of the symptomatic neuroma, burying the problematic nerve in other tissues after neuroma excision, centro-central neurorrhaphy, relocation nerve grafting, nerve capping, and traction neurectomy[26,28-30].
Simple neuroma excision has not been demonstrated to have good long-term efficacy. In a study of patients with upper extremity symptomatic neuromas who underwent surgical treatment by Guseet al.[31], 47% of those who underwent simple neuroma excision required reoperation. Domesheket al.[32]used neuroma excision with proximal transposition in 70 upper and lower extremity neuroma patients and found improved self-reported pain, depression, and quality of life scores. This was corroborated in a more recent meta-analysis by the Washington University group of similar methods, which found that excision with transposition or neurolysis with coverage resulted in a longer reduction in symptomatic neuroma pain compared to other methods, including simple excision[33].
Centro-central neurorrhaphy refers to fascicular dissection and coaptation of the terminal nerve ending with a hollow tube or nerve graft construct[26,29]. Souzaet al.[34]showed that in the foot and ankle, excision of nerve segments causing symptomatic neuroma pain and bridging the gap with nerve allograft resulted in improved pain behavior and pain interference scores on PROMIS.
Relocation nerve grafting refers to the use of nerve allograft on the terminal nerve and redirection away from the painful area. Economideset al.[35]demonstrated effective prophylactic reduction in phantom symptoms, neuroma formation, and improved ambulation rates after coaptation of the tibial and common peroneal nerve, which was then wrapped with collagen. In another study by Prantlet al.[36], in fifteen lower limb amputation patients, the sciatic nerve was split longitudinally, and the two ends were coapted to each other via the epineurium. These patients experienced reduced pain intensity scores as well as a decreased duration of pain attacks[36].
With traction neurectomy, the problematic nerve is held in traction and the terminal end excised so that once relaxed, it is cut in a much more proximal position, thereby relocating the sensitive end to a position that is more likely to be protected by soft tissues. These passive/ablative procedures have so far had mixed results. In a study by Sehirliogluet al.[37]of 75 lower extremity amputees with neuroma pain who were treated with traction neurectomy relatively early in their symptom development, no neuroma recurrence was noted at a mean follow-up of 2.8 years. On the other hand, a retrospective cohort study by Petet al.[38]of 38 patients who were older than the Sehirlioglu study and presented multiple years after symptom development, which had a 42% recurrence rate and 21% required subsequent surgical treatment. Given these mixed results for passive neuroma management, advances in TMR and RPNI for symptomatic neuroma treatment hold hope for these patients.
TARGETED MUSCLE REINNERVATION
History of TMR
TMR is a technique in which the cut ends of the problematic sensory nerves are coapted to nearby motor nerves of redundant muscles. The sensory nerve fascicles are thereby provided with terminal receptors, which are thought to prune axonal sprouting[39]. TMR was originally developed to make use of redundant muscle and nerves in amputees for improved use of upper extremity myoelectric bioprosthetics[39-45]. Myoelectric prostheses use electromyography (EMG) signals from the residual limb to control the motors of the device. TMR, RPNI, and agonist-antagonist myoneural interfaces are the three main means of deriving stable, high signal-to-noise ratio signals from muscles[46]. Ideally, the controls are in independently controlled muscle groups with minimal extraneous EMG noise from nearby musculature, termed muscle cross-talk[42]. Without TMR, there is typically only one pair of independent control sides in the residual limb for prosthesis control (i.e., the extensors and flexors at a joint). However, with TMR, new myoelectric control sites are available, allowing for multiple joint movements while simultaneously bioamplifying the signals to increase signal capture by surface electrodes and reduce relative muscle cross talk, which is especially beneficial for those with very proximal amputations[42,47].
One of the first patients who received TMR had bilateral humeral disarticulations in which the median, radial, ulnar, and musculocutaneous nerves were transferred to the pectoralis major and minor muscles. According to Hijjawiet al.[40], this patient demonstrated significant improvement in myoelectric device use. In another patient with an amputation at the humeral neck, TMR was performed to motor nerves of the pectoral and serratus muscles[43]. The patient reported intuitive control as well as the sensation that the appropriate phantom limb sensations were transferred to her chest upon light touch[43]. Early studies in transhumeral amputees demonstrated signs of reinnervation in 8-12 weeks and sufficient EMG signals for prosthesis operation in 5-7 months[44]. In a study by Milleret al.[48]with six transhumeral or shoulder disarticulation patients, all patients found prosthesis use to be easier with TMR control compared to conventional control, and this was corroborated with improvement in the performance of clothespin and box-and-blocks tests. For upper extremity prostheses, TMR has been described in combination with free muscle transfers such as a free myocutaneous gracilis flap in those lacking adequate soft tissue coverage for prosthesis use[42]. In this patient without biceps muscles, who therefore had a limited number of target muscles, the free gracilis also served as additional nerve targets[42].
TMR has also been studied in conjunction with machine learning algorithms to optimize prosthesis use. Pattern classification techniques could be applied to surface EMG signals of upper extremity amputees so that pattern recognition could be used[49]. A randomized crossover study by Hargroveet al.[50]evaluated direct control compared to pattern recognition myoelectric prosthesis control in nine transhumeral amputees. After 6-8 weeks of at-home trial, most patients preferred pattern recognition control, opening a new frontier for machine learning in the clinical arena[50]. Although not executed clinically, there have also been discussions comparing and contrasting upper extremity transplantation and TMR. There are advantages to both operations, with transplantation classically more beneficial for below mid-forearm amputations and TMR more beneficial for above-elbow amputees[51]. However, the authors conclude that these operations are complementary, not competitive procedures[51]. In fact, the physiology is the same: the proximal arm relies on nerve regeneration to reinnervate the new, distal donor muscles to achieve motor function and sensory recovery. In this sense, one can argue that upper extremity transplantation is perhaps the ultimate TMR.
During these endeavors to treat upper extremity amputees, a retrospective review of fifteen patients who underwent either shoulder disarticulation or transhumeral amputation and had neuroma pain who then received TMR for myoelectric control demonstrated an unexpected finding of fourteen of these patients having complete resolution of pain in the transferred nerves[52]. These results were corroborated in a rabbit forelimb amputation model by Kimet al.[53]. After initial rabbit forelimb amputation, in a secondary procedure, neuromas were excised at the median, radial, and ulnar nerves, and the proximal ends were coapted to muscle nerves on a pedicled rectus abdominis flap[53]. After ten weeks, there was successful coaptation on EMG and better histological features of the neuroma with reduced myelinated fiber counts and larger fascicle diameter[53]. These findings significantly broadened the use of TMR for post-amputation neuroma pain, particularly for lower extremity amputation[54]. The functionality of lower extremity amputees is very different from that of the upper extremity. Myoelectric or active lower limb prosthetics are not as commonly used, and per the LEAP study, patients often regain functionality with standard prosthetic use[6]. However, as noted in numerous studies, prosthetics use is negatively correlated with post-amputation pain. With the expansion of TMR for treatment of residual limb and PLP, its indications for use in the lower extremity exploded.
Surgical technique of TMR
Symptomatic neuromas are diagnosed based on history and physical exam. Intraoperatively, the proximal, cut, mixed motor/sensory, or sensory nerves with symptomatic neuromas are identified. Nerves with neuromas are cut back to a fresh edge unaffected by scar tissue. Small motor nerves of nearby muscles are identified. There are several papers with anatomical roadmaps for both upper and lower extremities to identify the major branch points of motor nerves and motor entry points on muscles[41,55-58]. Handheld neurostimulators can also be used intraoperatively to identify the locations of these nerves[59]. These neurostimulators, such as those from Checkpoint Surgical (Cleveland, OH), do not result in neuronal fatigue despite repeated stimulation when identifying the nerves. Retrograde stimulation to trace the motor nerve entry point is useful[60]. Once these motor nerves are identified, they are dissected and cut proximally. The distal motor nerve and the prepared sensory nerve are coapted usually with 6-0, 7-0, or 8-0 nonabsorbable monofilament suture through the epineurium[60]. Others have described methods in which a single 8-0 suture is placed in the center of the recipient nerve to loosely intussuscept the motor branch into the center of the donor nerve, then reinforcing this with several interrupted 6-0 suture on the epineurium to the fascia and epimysium around the target nerve [Figure 2][59]. The excision of the prior neuroma can be coded asexcision of major peripheral neuroma(64784), and TMR can be coded aspedicle nerve transfers(64905).
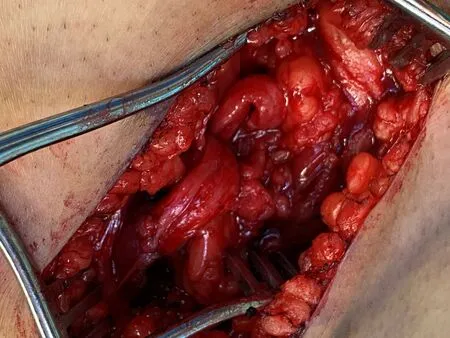
Figure 2. In the same patient as in Figure 1, the peroneal component of the sciatic nerve was coapted to the motor branch of the semimembranosus muscle with interrupted 6-0 prolene sutures.
The authors’ preferred technique for a BKA includes the use of a fishmouth incision with a posterior myocutaneous flap as described by the Attinger group[61]. In brief, the ideal tibial osteotomy is marked on the anterior leg approximately 4 finger breadths or 10-15 cm from the tibial tubercle. The fibular osteotomy should be 1-2 cm shorter than the tibial osteotomy. The anterior skin incision is made 1 cm distal to the planned tibial osteotomy. This line is carried through the lateral axis of the calf, which is defined as connecting the lateral femoral condyle to the lateral malleolus. Posteriorly, the myocutaneous flap is taken distally at the level of the Achilles tendon and trimmed back as needed prior to final closure[61]. The saphenous, sural, superficial peroneal, deep peroneal, and tibial nerves are identified. Common innervation muscle entry points (MEPs) have been defined by previous anatomical studies[55]. In the lateral compartment, the peroneus brevis and longus have MEPs on the medial and deep aspects. In the anterior compartment, the extensor digitorum longus has a readily identifiable MEP on the medial aspect of the muscle. In the posterior compartment, small branches of the tibial nerve can be found by following this nerve. While these branches can often be found by gross examination and knowledge of known anatomical MEPs, the authors utilize the Checkpoint Stimulator to confirm their neural characteristic and TMR is performed with several interrupted 6-0 to 8-0 prolene sutures, depending on the size of the nerves. This coaptation can be reinforced with Tisseel fibrin glue (Baxter International; Deerfield, IL). When nerve targets are not easily identifiable, then the authors quickly turn to RPNI using extraneous nearby muscles as grafts. This technique is described in detail below.
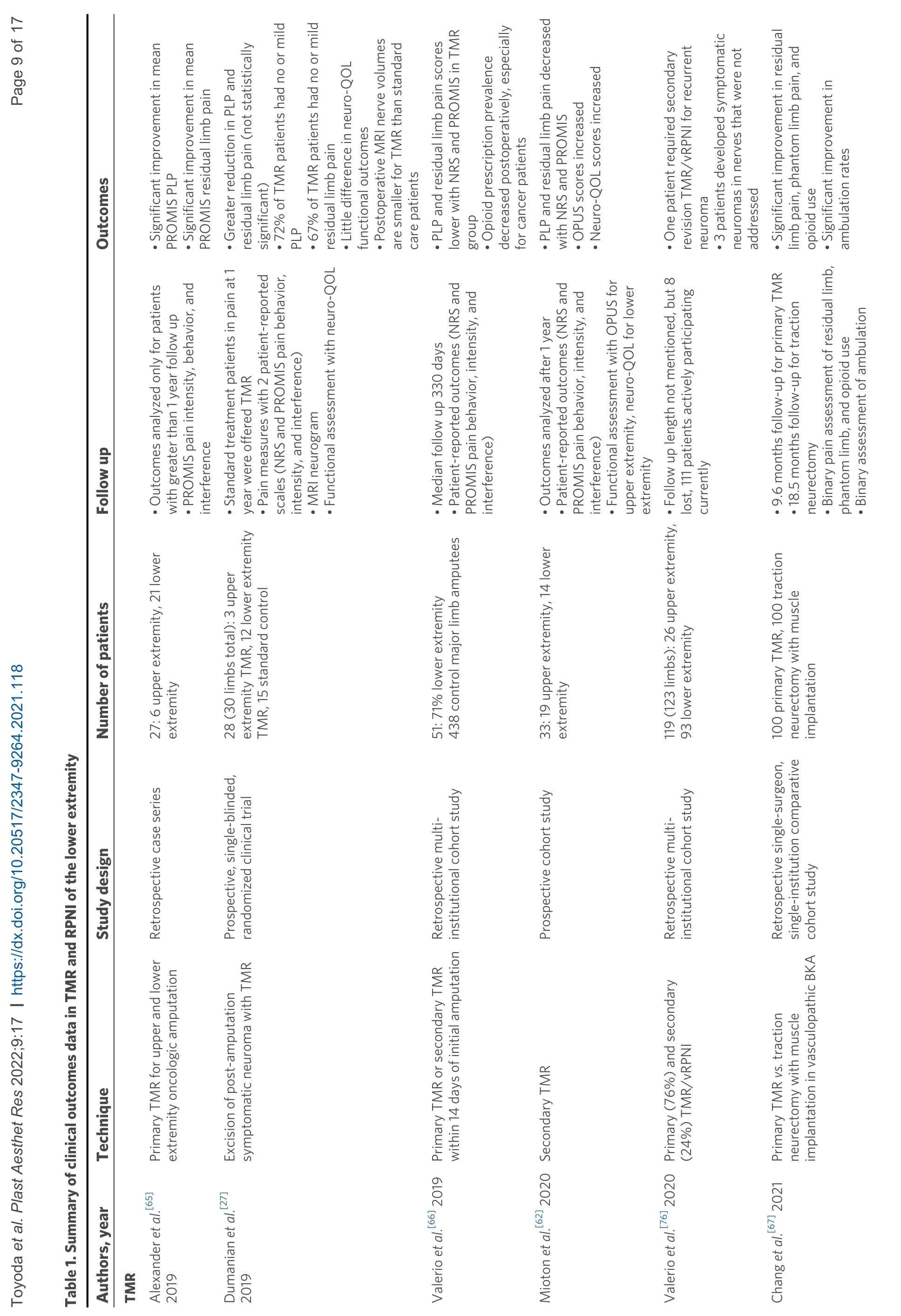
Outcomes of TMR [Table 1]
Despite initial concerns that the nerve transfer may cause new neuropathic pain, most patients find significant improvement in pain with minimal risk. Intraoperatively, identification of the motor entry points is the longest part of this procedure, but facilitated by previously published anatomic guides as well as the use of nerve stimulators. Coaptation of the nerves themselves is quite easily and quickly done, especially by those trained in microsurgery.
?

?
Outcomes research on TMR is still ongoing, but results have been favorable for pain management thus far. Dumanianet al.[27]conducted a prospective, single-blinded, randomized clinical trial at two centers and compared TMR to standard treatment with neuroma excision and burial of the nerve. Twenty-eight major upper and lower limb amputees with neuroma pain were randomized to these two groups. They conducted pain measures with two patient-reported scales, including the numerical rating scale (NRS) and PROMIS pain behavior, intensity, and interference short surveys. They also performed MRI neurograms and functional outcome assessment with the neuro-quality of life (neuro-QOL) measure. At one-year, residual limb and phantom limb pains trended toward improvement with TMR compared to standard treatment, although it did not reach statistical significance. Seventy-two percent of the TMR patients had no or mild phantom limb pain, and 67% had no or mild residual limb pain. Postoperative MRI nerve volumes were smaller for TMR patients. However, there was little difference in the neuro-QOL functional outcomes in this study[27]. Miotonet al.[62]performed a prospective study of 33 upper and lower extremity amputees from 2013-2017. Patient-reported outcomes measures with both NRS and PROMIS pain behavior, intensity, and primary TMR had significantly less frequent and less intense neuroma symptoms[65]. Another multiinstitutional cohort study of major limb amputees (due to any reason) compared 51 patients who had primary TMR to 438 amputees who did not have TMR[66]. Evaluation by Valerioet al.[66]at a median followup of 330 days demonstrated that those with primary TMR had significantly less residual limb and phantom limb pain than the control group. These results were corroborated by a recent cohort study of primary TMR patients. In a single surgeon, single-institution study, vasculopathic patients undergoing BKA were treated with primary TMR or traction neurectomy with muscle implantation[67]. One hundred primary TMR patients underwent TMR of the superficial peroneal nerve and tibial nerve and traction neurectomy with muscle implantation of the remaining major nerves. They were compared to one hundred patients who underwent only traction neurectomy and muscle implantation of all nerves. Baseline patient characteristics were similar, and at nearly 10-month follow-up of the primary TMR group and 18.5-month follow-up for the traction neurectomy group, pain, as defined by residual limb pain and phantom limb pain as well as represented by opioid use, was significantly better in the primary TMR group. In addition, ambulatory rates were significantly higher in the primary TMR group[67]. These results have supported multidisciplinary collaboration between plastic surgeons and surgical groups such as orthopedists and vascular surgeons who perform primary amputations at the time of the index operation. Ideally, this would reduce the need for subsequent operations and reduce total operative time and costs. At the authors’ institution, this multidisciplinary discussion has resulted in routine consult of the plastic surgeon to perform reconstructive lower extremity amputations in collaboration with the vascular or orthopedic teams, if not as the primary surgeon.
REGENERATIVE PERIPHERAL NERVE INTERFACE
History of RPNI
RPNI, similar to TMR, was also originally conceived as a biological interface to harness residual peripheral nerve signal to control neuroprosthetic devices, but was found incidentally to help with symptomatic neuroma pain treatment[68]. The RPNI construct consists of a residual peripheral nerve implanted into a free, autologous muscle graft after excision of the terminal neuroma bulb. The regenerating axons on the proximal nerve ending form new neuromuscular junctions on the denervated muscle graft[68]. Kunget al.[69]demonstrated this with both histology and EMG studies on a rat model in which the extensor digitorum longus (EDL) muscle was removed and used as free muscle grafts. These muscle transfers were later found to be successfully revascularized as well as reinnervated[69]. In another study, some rats underwent sham surgeries of exposure of the soleus muscle while others underwent division of the peroneal nerve and RPNI with or without neurotization[70]. At three months, the neurotized RPNI approached sham subjects in muscle action potential amplitude and area as well as motor unit numbers[70]. Another rat EDL model study by Frostet al.[71]demonstrated that EMG signals could be acquired from RPNIs and translated into realtime, proportional control of neuroprosthetic hands with reduced signal contamination compared to control groups. Implantation of microscale electrodes either within the nerves themselves[72]or within the RPNI muscle grafts[73]were both biocompatible with minimal signal noise in rat hind limb models. In these ways, RPNI bridges the signaling gap between a living peripheral nerve and a mechanical device and facilitates motor function and sensory feedback in prosthetic limbs[47].
Surgical technique of RPNI
Symptomatic neuromas are diagnosed in the usual fashion with history and exams. Intraoperatively, the neuroma is identified and excised. Larger caliber nerves such as the sciatic nerve may require intraneural dissection to isolate individual fascicles. Autologous muscle grafts are harvested from the amputated limb or nearby donor muscles. Grafts are harvested from healthy muscle in the direction of the fibers. Ideally, these grafts are 5-6 mm thick, and they should be without any atrophy, scar, tendons, or signs of infection. The proximal end of the transected nerve is placed parallel to the muscle fibers and secured typically with 6-0 nonabsorbable monofilament sutures in an epimysial-to-epineural fashion. These constructs are located away from the surgical incision and deep to any weight-bearing surface [Figure 3][68].

Figure 3. Reconstructive AKA with primary RPNI. A 75-year-old woman with a history of total left knee arthroplasty complicated by chronic peri-prosthetic infection who underwent multiple surgeries with poor bone and soft tissue availability underwent removal of hardware and left AKA. (A) The tibial and peroneal components of the sciatic nerve were dissected. (B) Small muscle grafts roughly 2 cm × 4 cm were taken from the nearby semitendinosus. (C) The tibial and peroneal components of the sciatic nerve are wrapped with the muscle grafts for RPNI. (D) The AKA incision was closed in layers over a drain. AKA: Above-knee amputation; RPNI: regenerative peripheral nerve interface.
Creating an RPNI construct takes an estimated 7-10 min, according to the inventor group. Surgical creation of RPNI is technically simple and does not require expertise in microsurgery, unlike that with TMR. When, in TMR, the appropriate recipient motor nerves are difficult to identify, there is a limit on the number of independent muscle targets, or the patient is unable to tolerate a longer operation, RPNI is an excellent alternative option which can be elected for intraoperatively. This efficient, reproducible, and effective procedure has the potential to be implemented by surgeons from many disciplines with minimal additional cost[68]RPNI, like TMR, can be coded aspedicle nerve transfer(64905).
Outcomes of RPNI [Table 1]
RPNI has demonstrated promising clinical results in improving neuroma pain and phantom pain. Wooet al.[74]conducted a retrospective case series of sixteen upper and lower extremity amputees with symptomatic neuromas. Patients have interviewed an average of 7.5 months after RPNI surgery using patient-reported pain scores, which were adapted from PROMIS instruments[74]. There was an average 71% reduction in neuroma pain and 53% in that for phantom pain[74]. This was also associated with 56% of patients reporting decreased analgesic use with no patients using more than preoperatively[74]. Aggregate pain interference scores for both neuroma and phantom pain decreased from an average of 4.6 preoperatively to 2.15 postoperatively, and 94% of patients claimed they would choose to undergo surgery again if given the option[74]. Further studies are underway to corroborate these results with a larger sample size as well as a longer follow-up[29].
As with TMR, primary RPNI at the time of amputation has also been increasingly explored with excellent preliminary results. A retrospective case series by Kubiaket al.[75]compared 45 patients who underwent primary RPNI, with both upper and lower extremity amputations to 45 control patients who underwent amputations without interfaces. Operative time was significantly longer in the primary RPNI group, with a mean of 152 min compared to 90 min in the control group who did not require identification of the major nerves. At a mean follow-up of nearly one year, those who had primary RPNI had a significantly fewer incidence of symptomatic neuroma and phantom limb pain. In fact, none of the primary RPNI patients developed symptomatic neuromas[75].
A combination technique of TMR with vascularized RPNI has also been trialed. In this technique, Valerioet al.[76]performed TMR in the usual fashion, and the neurorrhaphy was wrapped with a pedicled, vascularized surrounding muscle cuff from freshly denervated muscle. Formal results are still pending from this study, but so far, only three out of 119 patients have developed symptomatic neuromas with this technique. The theoretical benefits include buffering of any axonal escape after coaptation with TMR given the size mismatch, which is often inevitable when solely performing TMR. This combination technique may also provide additional focal muscle targets for reinnervation and muscle stimulation for functional prosthetics[76].
CONCLUSION
Identification of the significant individual and systems burden of amputation, advances in microsurgery, as well as collaborative efforts with medicine-adjunct fields such as mechanical and bioengineering for prosthetics as well as neuromodulation are what birthed TMR and RPNI. These techniques to treat and, increasingly, prevent post-amputation pain are newer additions to the plastic surgeons’ armamentarium, which have allowed further evolution of the top rungs of the reconstructive ladder in an often challenging patient population[60].
Although these techniques were developed in the realm of plastic surgery, plastic surgery has a collaborative history in which many surgical interventions developed by plastic surgeons are adopted by other specialties. Amputation is performed by multiple surgical disciplines, including general surgery, orthopedic surgery, and vascular surgery, depending on the patient-specific indications as well as institution. Given the significant morbidity that post-amputation pain can have on these patients and the optimistic results with TMR and RPNI, collaboration with amputating surgeons is not only frontier medicine, but also owed to the patient to provide optimal care. In cases of limb salvage, the “orthoplastic approach” is the current gold standard[77]. Collaboration between orthopedists and plastic surgeons has been demonstrated to decrease time to definitive skeletal stabilization and soft tissue coverage, length of hospital stay, postoperative complications, and need for revision procedures, ultimately resulting in improved functional outcomes[78]. Amputation is an important alternative treatment for many in the same patient pool. One can conjecture that collaboration between plastic surgeons, and the primary amputating surgical specialties would result in improved outcomes, as demonstrated in preliminary results of primary TMR and RPNI.
Increasing evidence in the medical literature, as well as anecdotal evidence on a case-by-case basis, have made primary TMR and RPNI the gold standard at our institution and multi-surgical collaboration for these patients commonplace. Barriers to more widespread implementation of these new surgical techniques include awareness by the primary amputating surgeon as well as access to technical instruction[68]. Given how impactful these surgical interventions can be on amputees’ quality of life and function, interinstitutional conversation and live demonstration courses, among other strategies, are needed to increase access for this increasingly growing patient population.
DECLARATIONS
Authors’ contributions
Made substantial contributions to conception and design of the study, performed data analysis and interpretation, wrote and edited the manuscript: Toyoda Y, Azoury S, Bauder A, Levin LS, Kovach S
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
Stephen Kovach is a consultant for Checkpoint Surgical which is mentioned in the manuscript. Other authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2022.
 Plastic and Aesthetic Research2022年3期
Plastic and Aesthetic Research2022年3期
- Plastic and Aesthetic Research的其它文章
- Eyelid defect reconstruction
- Weight stigma mitigating approaches to genderaffirming genital surgery
- Functional reconstruction of lower extremity nerve injuries
- Free tissue transfer for lower extremity trauma in the pediatric patient
- Flaps for bulbar urethral ischemic necrosis in pelvic fracture urethral injury
- Traumatic soft tissue defects: a perspective review on reconstruction and communication priorities from the orthopaedic trauma surgeon as a partner in care
