氮表面改性非晶碳基涂层的摩擦及腐蚀行为
李昊,郑贺,李淑钰,郭鹏,孙丽丽,柯培玲,汪爱英
氮表面改性非晶碳基涂层的摩擦及腐蚀行为
李昊1,2,郑贺3,李淑钰1,2,郭鹏1,孙丽丽1,柯培玲1,2,汪爱英1,2
(1.中国科学院宁波材料技术与工程研究所 a.中国科学院海洋新材料与应用技术重点实验室 b.浙江省海洋材料与防护技术重点实验室,浙江 宁波 315201;2.中国科学院大学 材料与光电研究中心,北京 100049;3.宁波甬微集团有限公司,浙江 宁波 315033)
在本征无氢非晶碳涂层表面进行掺N表面改性处理,研究其摩擦性能与海水腐蚀行为的演变规律,为海洋防护非晶碳涂层应用提供新思路。采用直流磁控溅射固体石墨靶制备非晶碳涂层,并在顶层进行N掺杂表面改性。改变Ar/N2气流量比来控制顶层掺N量,调控沉积时间,控制涂层厚度一致。SEM用于观测涂层厚度与截面形貌,XPS和Raman光谱仪分别用于表征涂层N掺杂量和碳键结构。涂层力学性能和动态摩擦因数则通过连续刚度模式纳米压痕仪和球盘式摩擦实验机测试得到。采用含有三电极体系的Gamry电化学工作站测量涂层的动电位极化曲线、电化学交流阻抗谱等电化学性能。对无氢非晶碳涂层进行表面改性,随顶层改性N含量的增加,sp2—C易与N结合,导致sp2相含量降低。随着N含量的增加,涂层的力学性能逐渐提升,当N质量分数为21%时,硬度与弹性模量达到最大值,分别为11.71 GPa和284.28 GPa;但当N质量分数最小(12%)时,涂层的断裂韧性与抗弹塑性变形能力最优。由于顶层引入掺N层后,sp2润滑相减少,涂层摩擦因数显著上升,且随N含量的增大逐渐增大。在顶层引入N掺杂量较少的改性层有利于提高非晶碳的耐蚀性,但随N含量的增大,涂层表面的孔隙增多,腐蚀溶液渗透加速,涂层的耐蚀性迅速恶化。顶层少量N掺杂有利于改善非晶碳基涂层的力学性能和耐蚀性能。N含量过高时,涂层性能随着N含量的升高逐渐恶化。在海洋关键零部件表面制备微量N掺杂改性的非晶碳涂层有利于提高其防护性能。
非晶碳;摩擦;腐蚀;磁控溅射;氮掺杂;表面改性
目前,海洋的开发和利用在全球经济发展中发挥着越来越重要的作用。随着海洋经济的快速发展,对高耐久性和可靠性的海洋基础设施和重大装备的需求迅速增加[1-3]。尤其是海洋装备的船舶动力装置、潮汐能发电装置、海水液压传动装置、深潜器浮力调节装置、深海勘探和开采设备等[4-5],将直接暴露于海洋多重复杂耦合环境,如紫外线辐射、氯化物盐、频繁的干湿循环、高湿度以及低温等[6],因此会加速金属等结构材料的降解和失效。采用表面防护涂层技术,可以在不影响金属等基材固有优异性能的基础上,突破其防护性能的不足,延长关键部件的使用寿命,保障其可靠运行[7]。
非晶碳(Amorphous Carbon,a-C)涂层具有高硬度、低摩擦、优异的化学稳定性和生物相容性,在金属材料的海洋腐蚀防护领域备受关注[8-14]。Radi等[15]发现,在人工海水中浸泡14 d后,相比于不锈钢基板,涂覆有非晶碳涂层的不锈钢表面锈点更少,且具有更低的开路电位。同时,为进一步提高非晶碳涂层海水工况下的服役性能,不少学者尝试在非晶碳涂层中掺杂适量异质原子,来提高膜基结合力及涂层的耐蚀性能、力学性能和摩擦学性能[16-19]。Sui等[20]在镍钛合金表面制备氟掺杂非晶碳涂层来提高其耐蚀性,腐蚀电流密度降低1个数量级。Dhandapani等[21]发现Ag掺杂可以有效改善非晶碳涂层在3.5% NaCl溶液中的耐蚀性。Zhang等[22]制备的硅掺杂非晶碳涂层在720 h的盐雾试验中未出现点蚀和剥落。但是,随着金属掺杂量的上升,涂层的耐蚀性往往提高有限甚至出现下降,磨损率也呈上升趋势,其原因主要源于金属与非晶碳之间的电偶作用会加剧腐蚀速率,而生成的腐蚀产物多为金属氧化物硬质颗粒,又在磨擦过程中加剧涂层损伤[23-25]。与金属不同,研究发现,N作为非金属元素掺杂到非晶碳涂层中不会引入新的电偶对,而且含氢非晶碳涂层中掺杂N元素有利于提高涂层的韧性和膜基结合强度,同时由于N掺入使得涂层sp2相增多,从而降低残余应力并起到减摩润滑作用,还可降低摩擦因数[26-28]。虽然含氢非晶碳涂层的硬度大、耐磨性好,但是在水环境下表面悬挂氢键易发生钝化,导致摩擦因数增大,而无氢非晶碳涂层在多环境适应性方面较为优异[14,29-31]。因此,N掺杂或改性无氢非晶碳涂层在涉海工况下可能具有更优异的防护性能,但相关改性非晶碳的摩擦及腐蚀行为尚未开展深入研究。
本文以N掺杂无氢非晶碳涂层为研究对象,选用前期团队优化非晶碳涂层思路,在其表面层继续制备不同N掺杂量的非晶碳[14,30-31],系统研究表层不同N改性掺杂量的非晶碳表面改性涂层的摩擦行为,以及在模拟海水环境中的腐蚀性能变化。相关结果将为提高非晶碳涂层的抗磨蚀防护性能,并用于海洋装备关键零部件防护提供新思路。
1 试验
1.1 涂层制备
非晶碳涂层采用直流磁控溅射(Direct-current Magnetron Sputtering,DCMS)石墨靶制备,其中石墨靶(纯度99.99%)尺寸为380 mm×100 mm×7 mm。基片选用P(100)硅片和316L不锈钢(15 mm × 3 mm),分别观察其微观结构,并测试其摩擦及腐蚀性能。首先,将准备好的基体依次放入丙酮和乙醇中超声清洗15 min,然后用干燥氮气吹干后置于转架上,基架与靶材之间相距约150 mm。待本底真空达到4×10‒6Pa时,通入Ar气,在0.266 Pa气压下,以3 A的电流溅射清洗石墨靶15 min。然后,采用线性离子束技术,利用Ar等离子体对基体表面刻蚀30 min以去除表面氧化层。随后,采用DCMS在偏压–200 V、溅射功率1.2 kW的参数下制备非晶碳层,沉积时间约1 h,接着通入氮气在表层引入N原子,通过调节Ar/N2流量比来改变N掺杂量,调节时间将所有涂层总厚度控制在200 nm左右,其中作为对照的本征非晶碳涂层N-0的沉积时间增加10 min,以确保所有样品厚度一致,具体参数如表1所示。
表1 N掺杂表面改性层沉积参数
1.2 性能测试
扫描电子显微镜(SEM,Verios G4 UC,US)用于观测涂层厚度、横截面微观结构。X射线光电子能谱仪(XPS,Axis ultradld,JP)用于表征涂层中的元素组成和原子键合状态。使用共焦显微拉曼光谱仪(Renishaw-inVia Reflection,UK),在532 nm激发波长下表征碳键结构。使用双高斯函数拟合拉曼数据,以获得G峰位置和峰强度比(D/G)。使用纳米压痕设备(MTS NANO200,US)测量涂层的硬度和弹性模量值,测试采用连续刚度法,使用金刚石压头,压入深度为100 nm,测试6个点以保证数据的准确性。
使用球盘式摩擦试验机(Rtec,US)在大气环境室温下进行摩擦试验。以Al2O3陶瓷球(1 800HV,6 mm)为对磨副,滑动速率为20 mm/s,摩擦总长度为72 m,载荷为5 N。为了分析表层N掺杂改性非晶碳涂层的耐蚀性,使用Gamry电化学工作站(Reference 600+,US),采用传统的三电极体系在3.5% NaCl溶液中进行电化学性能测试。其中以Ag/AgCl电极为参比电极,涂层样品为工作电极,铂片为对电极。在电化学腐蚀试验之前,持续运行开路电位(Open Circuit Potential,OCP)1 h,以确保整个测试系统的电化学稳定性[6,29-30]。随后,以105~ 10‒2Hz的频率、10 mV的正弦扰动测量电化学阻抗谱(Electrochemical Impedance Spectroscopy,EIS)。以0.5 mV/s的扫描速率,扫描范围从–0.2 V到+1.2 V(vs. Ag/AgCl),测量动电位极化曲线。
2 结果和讨论
2.1 涂层的组分结构
图1为不同顶层掺N量的非晶碳涂层的SEM截面形貌。由图1可知,所有涂层的总厚度都控制在(210±10) nm,且涂层整体连续致密,无明显缺陷。总体上看,所有涂层样品在截面形貌上无明显差异,这是由于表层的掺N层是原位生长的,在沉积过程中不存在间断,且下层非晶碳的沉积环境一致,这使得顶层掺N层沿着下层非晶碳的生长取向,避免了明显的界面分层现象。
图2为不同顶层掺N量的非晶碳涂层的XPS能谱图。从全谱(图2a)中可以看出,涂层中主要含有C、O、N 3种元素。氧元素与N-0样品中的少量N元素,主要是由于沉积过程中真空室残留空气,或取样后吸附空气所致[32]。如图2b所示,随N2/Ar流量比增大,顶层N含量(原子数分数,下同)由1.4%逐渐增加,增大幅度逐渐减小,趋于饱和值24%。图2c为C 1s精细谱,拟合分析表明,主要存在4种C原子化学杂化状态,即sp2、sp3、C—N以及C—O,谱峰位置分别位于284.6、285.4、286.4、286.6 eV[33-35]。采用洛伦兹函数(20%)和高斯函数(80%)对C 1s峰进行分峰拟合处理,并对峰面积进行积分,可以得到不同杂化状态的碳键含量。如图3d所示,随着N掺杂量不断增加,sp3—C键含量几乎不变,而sp2—C键含量从66.3%降低到30.7%,该结果与拉曼分析结果相一致。这是因为sp2—C的成键轨道上s轨道占据的比例比sp3—C的多,隧穿效应更强,因此sp2—C键的形成能比sp3—C键低,引入N原子更容易与sp2—C发生键合,导致sp2—C键的含量降低[36]。图2e为N 1s精细谱,其中主要含有3种N原子化学状态,即位于398.6 eV附近的N—C键,400.4 eV附近的N=C键,402.2 eV附近的N—C=O键。N 1s峰的分峰拟合结果如图2f所示,随着N掺杂量的增加,N—C键含量基本不变,而N=C键含量逐渐增加,从47.4%增加到56.1%,这表明大量sp2—C键被N原子取代形成了N=C键。

图1 表层不同掺N量非晶碳涂层的SEM截面形貌

图2 表面N掺杂改性非晶碳涂层的XPS能谱
拉曼光谱用于进一步分析涂层碳键结构的变化。如图3a所示,所有涂层拉曼光谱均显示出非晶碳的典型拉曼峰,且经过高斯拟合后,每个样品中均出现位于1 350 cm‒1附近的D峰和1 560 cm‒1附近的G峰[37-39]。其中,G峰对应碳环和碳链中C—C键的伸缩振动,而D峰对应碳环的呼吸振动[35]。G峰的半高宽(G- FWHM)、G峰位置和D峰与G峰面积比D/G值可以反映非晶碳碳键结构[40]。如图3b所示,随着顶层N含量的增多,D/G逐渐降低,从2.88降到1.82,这表明N掺入量增大不利于sp2团簇的进一步长大。同样,G峰位置向高波数方向偏移,从1 547 cm‒1增加到1 559 cm‒1,也表明氮元素引入阻碍了sp2团簇的形成。G峰半高宽呈现先增大再减小的趋势,从最初的171.5 cm‒1(N-0)增大到178.8 cm‒1(N-2),再减小到172.9 cm‒1(N-4),这表明随着氮原子的掺入,使得涂层键长、键角的扭曲程度以及结构无序度均出现先增大再减小的变化趋势。

图3 表面N掺杂改性非晶碳涂层的拉曼光谱
2.2 涂层的力学性能
表2列出了非晶碳涂层的硬度()和弹性模量(),以及相应的/和3/2结果。可以看出,引入顶层N改性层可以显著提高涂层的硬度和弹性模量,且随着N含量的升高,硬度与弹性模量都呈现先升高后降低的趋势。这是由于随着N含量的增加,sp3—C呈现增大的趋势,通常情况下增大sp3—C含量可以有效地增加碳基涂层的硬度。当N含量增大到21.1%以上时,sp3—C含量不再明显增大,而N=C含量增大会导致硬度略微下降[26-28]。因此,当N掺杂量为21.1%(N-3)时,硬度和弹性模量达到最大值,分别为11.71 GPa和284.28 GPa。/和3/2是反映材料抗弹塑性变形能力的关键参数,与材料的断裂韧性和耐磨性密切相关[41-47]。一般来说,/和3/2的值越高,材料断裂韧性越高,耐磨性越好。随着顶层N含量的增加,3/2呈现先减小后增大的趋势,其中N-3样品的断裂韧性以及抗弹塑性变形能力最差,尽管它的硬度和弹性模量最大。而掺N量最少的样品(N-1)表现出最强的断裂韧性以及抗弹塑性变形能力。
表2 涂层力学性能汇总
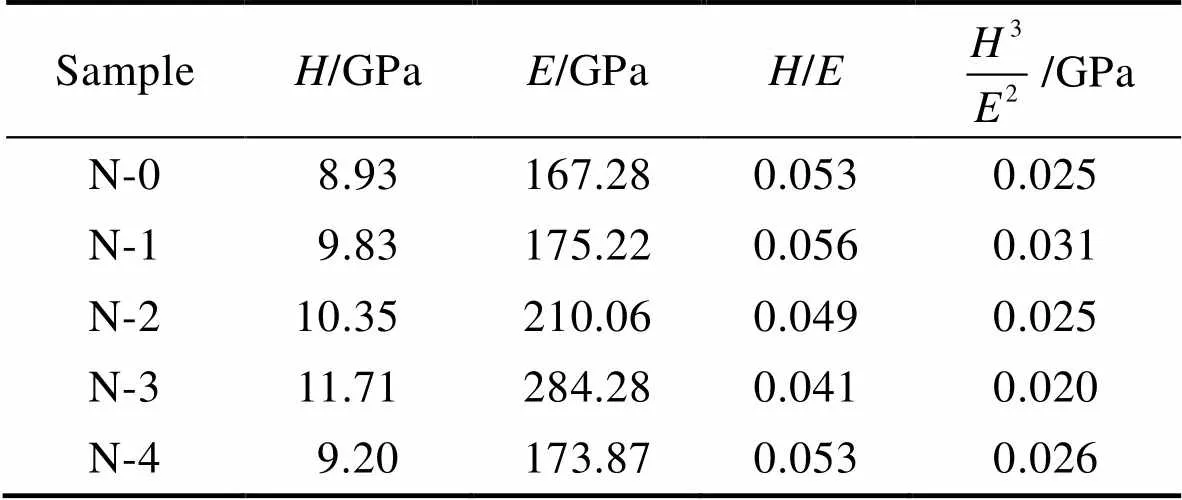
Tab.2 Summary of the mechanical properties of the coatings
2.3 涂层的摩擦性能
图4为所有涂层样品的摩擦因数随时间的变化曲线。所有摩擦因数数据均为通过力学传感器在每0.05 s内采集的平均摩擦因数。由图4可以看出,顶层N掺杂显著增大了涂层的摩擦因数(Coefficient of Friction,COF),且随着N含量的增大,稳态平均摩擦因数从~0.24增大到~0.35。这可能是由于N掺杂导致sp2润滑相减少,从而使涂层的摩擦因数增大。同样地,随着N掺杂量的增大,涂层的摩擦因数更加不稳定,这可能是由于摩擦过程中涂层碎裂导致的,尤其是断裂韧性最差的N-3样品,其摩擦因数的起伏最明显。

图4 所有涂层样品的摩擦因数随时间的变化曲线
2.4 涂层的耐蚀性能
图5a和表3列出了316L基体和所有涂层样品在3.5%NaCl溶液中的动电位极化曲线和相应的分析结果。与316L基体相比,所有涂层样品的动电位极化曲线显示出更高的自腐蚀电位(corr)、更低的腐蚀电流密度(corr)和更高的点蚀电位,这表明所有涂层样品都显著提高了316L基体的耐蚀性。此外,除了顶层掺N量最少的样品(N-1)外,其他顶层掺N样品的耐蚀性均显著下降,且随着N含量的增大,自腐蚀电位由0.382 V显著降低至0.142 V,腐蚀电流密度由0.178 μA/cm2显著增大至0.719 μA/cm2。这说明随着N含量的增加,涂层的腐蚀倾向和腐蚀速率都增大,腐蚀抗力下降。相比于本征非晶碳涂层,只有掺N量为11.9%的样品(N-1)的自腐蚀电位最高,腐蚀电流密度最低。涂层腐蚀动力学的差异与涂层内部缺陷密切相关,因此根据表2中列出的阳极Tafel斜率(a)和阴极Tafel斜率(c),通过公式(1)—(2)计算极化电阻(p)和孔隙率值。其中,p(substrate)和p(coating)分别是基体和薄膜的极化电阻,Δcorr是涂层和基体之间的腐蚀电位差,式(2)中a是基体的阳极Tafel斜率[48-50]。


极化电阻和孔隙率的计算结果见图5b。所有涂层样品的极化电阻都要高出基体1个数量级,表明所有涂层样品均可以对基体起到良好的保护作用,这也与动电位极化测试得到的结果一致。但是,所有顶层掺N样品的极化电阻都要高于本征非晶碳涂层,这可能是由于涂层的孔隙渗透所致。因此,进一步对所有涂层样品的孔隙率进行计算,发现顶层渗N处理后,涂层的孔隙率增大,且随着N含量的增大,孔隙率从~0.4%上升至~1.4%,只有掺N量最低的N-1样品的孔隙率与本征非晶碳涂层相近,均为~0.4%。这说明顶层掺N量的增加会导致较低的极化电阻与高的孔隙率,促进腐蚀溶液通过涂层孔隙进行渗透,从而加速局部腐蚀,导致腐蚀电流密度发生变化。因此,从耐蚀性能上看,顶层微量N掺杂有利于降低腐蚀倾向,延缓腐蚀速率,顶层N含量过高会导致较多的孔隙缺陷,反而不利于腐蚀防护。
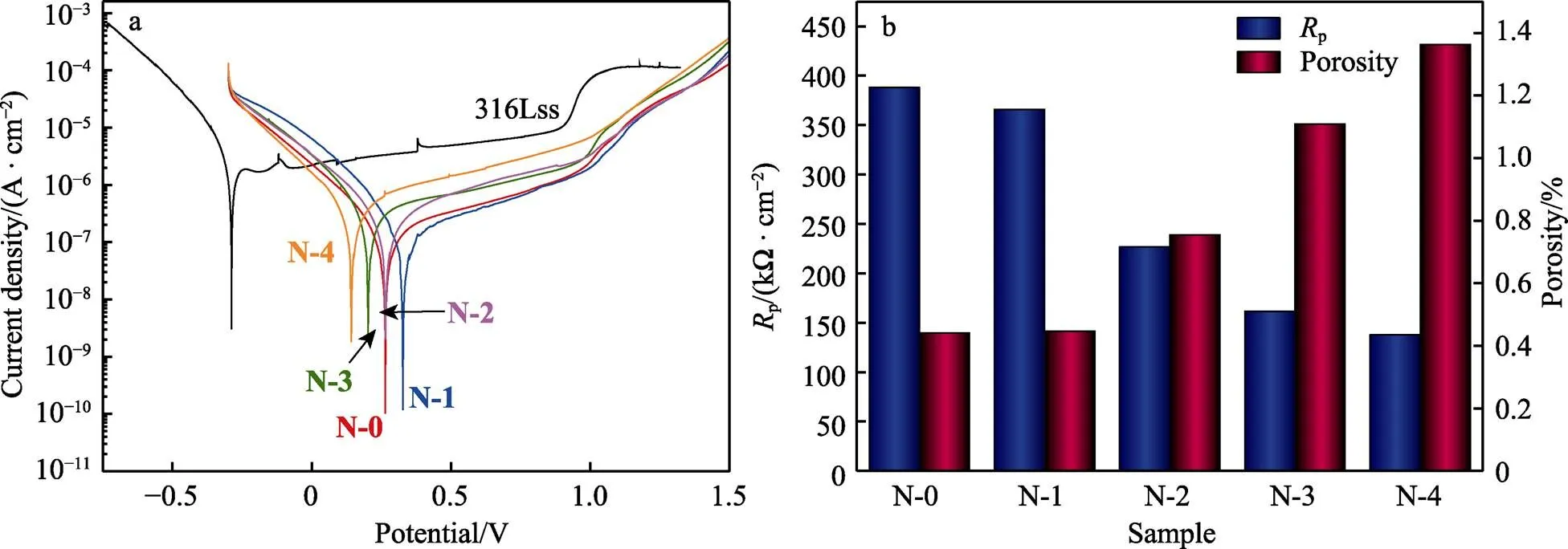
图5 316L基体与所有样品在3.5% NaCl溶液中的动电位极化曲线与相应的极化电阻和孔隙率
表3 3.5%NaCl溶液中动电位极化测试结果分析
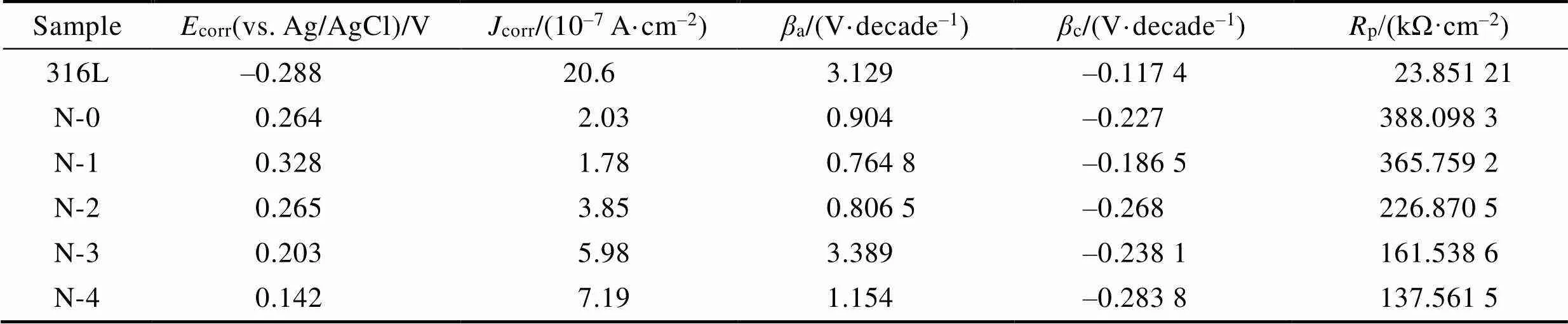
Tab.3 Analysis results of potentiodynamic polarization test in 3.5wt.% NaCl
图6为所有涂层样品和316L基体的EIS测试结果。与316L基体相比,所有涂层样品在Nyquist图和Bode图中表现出更大的容抗弧半径、更高的低频阻抗和更宽的相角平台,这表明所有涂层样品都大大提高了316L基体的耐蚀性。其中,顶层掺N量最少的样品N-1具有最大的容抗弧半径以及最高的低频阻抗,这说明其耐蚀性最佳。而随着顶层掺N量的增大,涂层的耐蚀性逐渐下降,且相角平台出现的位置明显向低频区偏移,这表明N含量过高会导致涂层在腐蚀萌生的初始阶段就具有较高的腐蚀速率和腐蚀倾向。

图6 3.5%NaCl溶液中涂层和316L基体的EIS测试结果
3 结论
1)采用直流磁控溅射技术制备了一系列顶层掺N改性非晶碳涂层,并通过改变沉积过程中Ar/N2的流量比实现了N含量从1.4%到23.8%的大范围调控。结果显示,不同于通常的N掺杂含氢非晶碳,在无氢非晶碳中,随着N含量的增大,更多的sp2—C与N键合,间接导致sp2—C含量由66.3%减少至30.7%。
2)随着顶层掺N量的增大,涂层硬度与弹性模量都有所提高,当掺N量达到21.1%时,硬度与弹性模量达到最大值,分别为11.71 GPa和284.28 GPa。但是,从断裂韧性与抗弹塑性变形能力来看,掺N量最小(11.4%)的样品具有最佳的力学性能。
3)摩擦测试结果显示,由于sp2润滑相减少,在顶层引入掺N层后,涂层的摩擦因数由~0.17显著上升至~0.24,且随着N含量的增大,摩擦因数进一步增大至~0.35,这也与其断裂韧性下降导致的磨粒磨损有关。
4)腐蚀测试结果显示,在顶层引入N掺杂量较少的改性层有利于提高非晶碳涂层的耐蚀性。掺N量为11.9%的样品具有最高的自腐蚀电位(0.382 V)和最低的腐蚀电流密度(0.178 μA/cm2)。但是,随着顶层N含量继续增大,耐蚀性迅速恶化,自腐蚀电位由0.382 V显著降低至0.142 V,腐蚀电流密度由0.178 μA/cm2显著增大至0.719 μA/cm2。这是由于N含量由11.9%升高到23.8%时,表面孔隙缺陷增多(孔隙率从~0.4%上升至~1.4%),加速腐蚀溶液渗透所致。
[1] 王颖, 韩光, 张英香. 深海海洋工程装备技术发展现状及趋势[J]. 舰船科学技术, 2010, 32(10): 108-113, 124.
WANG Ying, HAN Guang, ZHANG Ying-xiang. The Development of Deep Water Ocean Engineering Equipments and Technology[J]. Ship Science and Technology, 2010, 32(10): 108-113, 124.
[2] ARMADA S, TILSET B G, PILZ M, et al. Sealing HVOF Thermally Sprayed WC-CoCr Coatings by Sol-Gel Methods[J]. Journal of Thermal Spray Technology, 2011, 20(4): 918-926.
[3] 周国平. 海洋工程装备关键技术和支撑技术分析[J]. 船舶与海洋工程, 2012, 28(1): 15-20, 37.
ZHOU Guo-ping. Key Technologies and Supporting Technologies of Offshore Engineering Equipment[J]. Naval Architecture and Ocean Engineering, 2012, 28(1): 15-20, 37.
[4] 董从林, 白秀琴, 严新平, 等. 海洋环境下的材料摩擦学研究进展与展望[J]. 摩擦学学报, 2013, 33(3): 311-320.
DONG Cong-lin, BAI Xiu-qin, YAN Xin-ping, et al. Research Status and Advances on Tribological Study of Materials under Ocean Environment[J]. Tribology, 2013, 33(3): 311-320.
[5] 王伟, 文怀兴, 陈威. 海水环境下材料摩擦学行为研究现状[J]. 材料导报, 2017, 31(11): 51-58.
WANG Wei, WEN Huai-xing, CHEN Wei. Research Status on Tribological Behaviors of Materials under Seawater Environment[J]. Materials Review, 2017, 31(11): 51-58.
[6] ARSLAN E, TOTIK Y, EFEOGLU I. The Investigation of the Tribocorrosion Properties of DLC Coatings Deposited on Ti6Al4V Alloys by CFUBMS[J]. Progress in Organic Coatings, 2012, 74(4): 768-771.
[7] 李淑钰, 刘应瑞, 郭鹏, 等. 海洋环境下物理气相沉积氮/碳基抗磨蚀涂层的研究进展[J]. 表面技术, 2021, 50(7): 44-56.
LI Shu-yu, LIU Ying-rui, GUO Peng, et al. Research Progress of Nitrogen/Carbon-Based Anti-Tribocorrosion Coatings by Physical Vapor Deposition for Marine Applications[J]. Surface Technology, 2021, 50(7): 44-56.
[8] LIU Zhe, CHU Zhen-hua, CHEN Xue-guang, et al. Electrochemical Impedance Studies on Tribocorrosion Behavior of Plasma-Sprayed Al2O3Coatings[J]. Journal of Thermal Spray Technology, 2015, 24(5): 878-884.
[9] 徐丽萍, 毛杰, 张吉阜, 等. 表面工程技术在海洋工程装备中的应用[J]. 中国材料进展, 2014, 33(1): 1-8, 31.
XU Li-ping, MAO Jie, ZHANG Ji-fu, et al. Applications of Surface Engineering Technology in Marine Engineering Equipment[J]. Materials China, 2014, 33(1): 1-8, 31.
[10] WU Zhi-wei, ZHOU Fei, MA Qiang, et al. Tribological and Electrochemical Properties of Cr-Si-C-N Coatings in Artificial Seawater[J]. RSC Advances, 2016, 6(80): 76724- 76735.
[11] TOTOLIN V, PEJAKOVIĆ V, CSANYI T, et al. Surface Engineering of Ti6Al4V Surfaces for Enhanced Tribocorrosion Performance in Artificial Seawater[J]. Materials & Design, 2016, 104: 10-18.
[12] WEI Jing, GUO Peng, LIU Lin-lin, et al. Corrosion Resistance of Amorphous Carbon Film in 3.5wt% NaCl Solution for Marine Application[J]. Electrochimica Acta, 2020, 346: 136282.
[13] WEI Jing, GUO Peng, LIU Lin-lin, et al. Tailored Electrochemical Behavior of Ta-C Film by Glancing Angle Deposition[J]. Applied Surface Science, 2020, 516: 146115.
[14] LI Lei, GUO Peng, LIU Lin-lin, et al. Structural Design of Cr/dLC Films for High Tribological Performance in Artificial Seawater: Cr/dLC Ratio and Multilayer Structure[J]. Journal of Materials Science & Technology, 2018, 34(8): 1273-1280.
[15] RADI P A, VIEIRA A, MANFROI L, et al. Tribocorrosion and Corrosion Behavior of Stainless Steel Coated with DLC Films in Ethanol with Different Concentrations of Water[J]. Ceramics International, 2019, 45(7): 9686-9693.
[16] LI Xiao-wei, KE Pei-ling, LEE K R, et al. Molecular Dynamics Simulation for the Influence of Incident Angles of Energetic Carbon Atoms on the Structure and Properties of Diamond-Like Carbon Films[J]. Thin Solid Films, 2014, 552: 136-140.
[17] LI Xiao-wei, GUO Peng, SUN Li-li, et al. Ti/Al Co- Doping Induced Residual Stress Reduction and Bond Structure Evolution of Amorphous Carbon Films: An Experimental and Ab Initio Study[J]. Carbon, 2017, 111: 467-475.
[18] LI Xiao-wei, ZHANG Dong, LEE K R, et al. Effect of Metal Doping on Structural Characteristics of Amorphous Carbon System: A First-Principles Study[J]. Thin Solid Films, 2016, 607: 67-72.
[19] LI Xiao-wei, KE Pei-ling, WANG Ai-ying. Probing the Stress Reduction Mechanism of Diamond-Like Carbon Films by Incorporating Ti, Cr, or W Carbide-Forming Metals: Ab Initio Molecular Dynamics Simulation[J]. The Journal of Physical Chemistry C, 2015, 119(11): 6086-6093.
[20] SUI J H, ZHANG Z G, CAI W. Surface Characteristics and Electrochemical Corrosion Behavior of Fluorinated Diamond-Like Carbon (F-DLC) Films on the NiTi Alloys[J]. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 2009, 267(15): 2475-2479.
[21] DHANDAPANI V S, THANGAVEL E, ARUMUGAM M, et al. Effect of Ag Content on the Microstructure, Tribological and Corrosion Properties of Amorphous CarbonCoatings on 316L SS[J]. Surface and Coatings Technology, 2014, 240: 128-136.
[22] ZHANG Ming-jiong, WU Gui-zhi, LU Zhi-bin, et al. Corrosion and Wear Behaviors of Si-DLC Films Coated on Inner Surface of SS304 Pipes by Hollow Cathode PECVD [J]. Surface Topography: Metrology and Properties, 2018, 6(3): 034010.
[23] XU Xiao-wei, ZHOU Yong, LIU Lin-lin, et al. Corrosion Behavior of Diamond-Like Carbon Film Induced by Al/Ti Co-Doping[J]. Applied Surface Science, 2020, 509: 144877.
[24] XU Xiao-wei, GUO Peng, ZUO Xiao, et al. Understanding the Effect of Al/Ti Ratio on the Tribocorrosion Performance of Al/Ti Co-Doped Diamond-Like Carbon Films for Marine Applications[J]. Surface and Coatings Technology, 2020, 402: 126347.
[25] 王永欣, 王立平, 薛群基. 金属掺杂高硬度类石墨薄膜结构及其摩擦学性能研究[J]. 摩擦学学报, 2011, 31(1): 72-77.
WANG Yong-xin, WANG Li-ping, XUE Qun-ji. Microstructure and Tribological Performances of Highly Hard Graphite-Like Carbon Films Doped with Different Metals [J]. Tribology, 2011, 31(1): 72-77.
[26] RAY S C, PONG W F, PAPAKONSTANTINOU P. Iron, Nitrogen and Silicon Doped Diamond Like Carbon (DLC) Thin Films: A Comparative Study[J]. Thin Solid Films, 2016, 610: 42-47.
[27] 杨满中, 彭继华, 韦宇冲. 低氮掺杂对含氢类金刚石结构和力学性能的影响[J]. 哈尔滨工业大学学报, 2019, 51(5): 38-43.
YANG Man-zhong, PENG Ji-hua, WEI Yu-chong. Effect of Low Nitrogen Doping on the Microstructure and Mechanical Properties of Hydrogenated Diamond-Like Carbon Films[J]. Journal of Harbin Institute of Technology, 2019, 51(5): 38-43.
[28] 薛群基, 王立平. 类金刚石碳基薄膜材料[M]. 北京: 科学出版社, 2012.
XUE Qun-ji, WANG Li-ping. Diamond Like Carbon Films [M]. Beijing: Science Press, 2012.
[29] WEI Jing, GUO Peng, LI Hao, et al. Insights on High Temperature Friction Mechanism of Multilayer Ta-C Films [J]. Journal of Materials Science & Technology, 2022, 97: 29-37.
[30] 李蕾, 郭鹏, 刘林林, 等. 金属过渡层类型对非晶碳膜结构性能的影响[J]. 无机材料学报, 2018, 33(3): 331-338.
LI Lei, GUO Peng, LIU Lin-lin, et al. Metal Buffer Layer on Structure, Mechanical and Tribological Property of GLC Films[J]. Journal of Inorganic Materials, 2018, 33(3): 331-338.
[31] LI Lei, LIU Lin-lin, LI Xiao-wei, et al. Enhanced Tribocorrosion Performance of Cr/GLC Multilayered Films for Marine Protective Application[J]. ACS Applied Materials & Interfaces, 2018, 10(15): 13187-13198.
[32] NAKAO S, YUKIMURA K, NAKANO S, et al. DLC Coating by HiPIMS: The Influence of Substrate Bias Voltage[J]. IEEE Transactions on Plasma Science, 2013, 41(8): 1819-1829.
[33] GUO Peng, LI Xiao-wei, SUN Li-li, et al. Stress Reduction Mechanism of Diamond-Like Carbon Films Incorporated with Different Cu Contents[J]. Thin Solid Films, 2017, 640: 45-51.
[34] DÍAZ J, PAOLICELLI G, FERRER S, et al. Separation of the sp3and sp2Components in the C1s Photoemission Spectra of Amorphous Carbon Films[J]. Physical Review B, Condensed Matter, 1996, 54(11): 8064-8069.
[35] MOHAGHEGHPOUR E, RAJABI M, GHOLAMIPOUR R, et al. Correlation Study of Structural, Optical and Electrical Properties of Amorphous Carbon Thin Films Prepared by Ion Beam Sputtering Deposition Technique [J]. Applied Surface Science, 2016, 360: 52-58.
[36] KRAUSER J, NIX A K, GEHRKE H G, et al. Conductivity Enhancement of Ion Tracks in Tetrahedral Amorphous Carbon by Doping with N, B, Cu and Fe[J]. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 2012, 272: 280-283.
[37] ITO A M, TAKAYAMA A, SAITO S, et al. Formation and Classification of Amorphous Carbon by Molecular Dynamics Simulation[J]. Japanese Journal of Applied Physics, 2013, 52(1): 104.
[38] LI H X, XU T, CHEN J M, et al. The Effect of Applied Dc Bias Voltage on the Properties of A-C: H Films Prepared in a Dual Dc-Rf Plasma System[J]. Applied Surface Science, 2004, 227(1-4): 364-372.
[39] FERRARI A C, ROBERTSON J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon[J]. Physical Review B, 2000, 61(20): 14095-14107.
[40] ARENAL R, LIU A C Y. Clustering of Aromatic Rings in Near-Frictionless Hydrogenated Amorphous Carbon Films Probed Using Multiwavelength Raman Spectroscopy[J]. Applied Physics Letters, 2007, 91(21): 211903.
[41] REN Ping, ZHANG Kan, HE Xin, et al. Toughness Enhancement and Tribochemistry of the Nb-Ag-N Films Actuated by Solute Ag[J]. Acta Materialia, 2017, 137: 1-11.
[42] ZHANG Shi-dong, YAN Mu-fu, YANG Yang, et al. Excellent Mechanical, Tribological and Anti-Corrosive Performance of Novel Ti-DLC Nanocomposite Thin Films Prepared via Magnetron Sputtering Method[J]. Carbon, 2019, 151: 136-147.
[43] LI Ling-ling, BAI Wen-qi, WANG Xiu-li, et al. Mechanical Properties and in Vitro and in Vivo Biocompatibility of A-C/a-C:Ti Nanomultilayer Films on Ti6Al4V Alloy as Medical Implants[J]. ACS Applied Materials & Interfaces, 2017, 9(19): 15933-15942.
[44] REN Ping, WEN Mao, ZHANG Kan, et al. Self-Assembly of TaC@Ta Core-Shell-Like Nanocomposite Film via Solid- State Dewetting: Toward Superior Wear and Corrosion Resistance[J]. Acta Materialia, 2018, 160: 72-84.
[45] CHEN Xin-jie, DU Yao, CHUNG Y W. Commentary on Using/and3/2as Proxies for Fracture Toughness of Hard Coatings[J]. Thin Solid Films, 2019, 688: 137265.
[46] LIN Jian-liang, WEI Rong-hua, BITSIS D C, et al. Development and Evaluation of Low Friction TiSiCN Nanocomposite Coatings for Piston Ring Applications[J]. Surface and Coatings Technology, 2016, 298: 121-131.
[47] YANG Yang, YAN M F, ZHANG Y X, et al. Catalytic Growth of Diamond-Like Carbon on Fe3C-Containing Carburized Layer through a Single-Step Plasma-Assisted Carburizing Process[J]. Carbon, 2017, 122: 1-8.
[48] CHOI J, NAKAO S, KIM J, et al. Corrosion Protection of DLC Coatings on Magnesium Alloy[J]. Diamond and Related Materials, 2007, 16(4-7): 1361-1364.
[49] LIU C, BI Q, LEYLAND A, et al. An Electrochemical Impedance Spectroscopy Study of the Corrosion Behaviour of PVD Coated Steels in 0.5 N NaCl Aqueous Solution: Part II: EIS Interpretation of Corrosion Behaviour[J]. Corrosion Science, 2003, 45(6): 1257-1273.
[50] AHN S H, LEE J H, KIM H G, et al. A Study on the Quantitative Determination of Through-Coating Porosity in PVD-Grown Coatings[J]. Applied Surface Science, 2004, 233(1-4): 105-114.
Effect of Nitrogen Surface Modification on Tribology and Corrosion Behavior of Amorphous Carbon Coating
1,2,3,1,2,1,1,1,2,1,2
(1. a. Key Laboratory of Marine Materials and Related Technologies, b. Zhejiang Key Laboratory of Marine Materials and Protective Technologies, Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences, Zhejiang Ningbo 315201, China; 2. Center of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing 100049, China; 3. Yongwei Group Co., Ltd., Zhejiang Ningbo 315033, China)
N-doped surface modification was carried out on the surface of intrinsic hydrogen free amorphous carbon coating, and the evolution law of its friction properties and seawater corrosion behavior was studied, which provided a new idea for the application of marine protective amorphous carbon coating. Amorphous carbon coating was prepared by DC magnetron sputtering on solid graphite target, and n-doped surface modification was carried out on the top layer. Change the Ar/N2gas flow ratio to control the N content in the top layer, adjust the deposition time and control the coating thickness. SEM was used to characterize the coating thickness and cross-section morphology, XPS and Raman spectra were used to characterize the N-doping content and carbon bond structure of the coating, respectively. The mechanical properties and dynamic friction coefficient of the coating were measured by continuous stiffness mode nano indentation instrument and ball disc friction tester. Gamry electrochemical workstation with three electrode system was used to measure the electrochemical properties of the coating, such as potentiodynamic polarization curve and electrochemical AC impedance spectroscopy.
amorphous carbon; friction; corrosion; magnetron sputtering; nitrogen doping; surface modification
TG172
A
1001-3660(2022)05-0061-09
10.16490/j.cnki.issn.1001-3660.2022.05.007
2021–09–29;
2022–01–11
2021-09-29;
2022-01-11
中国科学院A类战略性先导科技专项(XDA22010303);中国科学院-韩国国家科技理事会协议项目(174433KYSB20200021);王宽诚率先人才计划卢嘉锡国际团队(GJTD-2019-13);中科院创新团队(292020000008)
A-class Pilot of the Chinese Academy of Sciences (XDA22010303); CAS-NST Joint Research Project (174433KYSB20200021); K. C. Wong Education Foundation (GJTD-2019-13); CAS Interdisciplinary Innovation Team (292020000008)
李昊(1993—),男,博士研究生,主要研究方向为海水环境中碳基涂层的磨蚀性能。
LI Hao (1993-), Male, Doctoral student, Research focus: tribocorrosion properties of carbon-based coatings in seawater.
汪爱英(1975—),女,博士,研究员,主要研究方向为表面强化涂层材料与功能改性。
WANG Ai-ying (1975-), Female, Doctor, Researcher, Research focus: strengthening and functional modification for surface coating materials.
李昊, 郑贺, 李淑钰, 等. 氮表面改性非晶碳基涂层的摩擦及腐蚀行为[J]. 表面技术, 2022, 51(5): 61-69.
LI Hao, ZHENG He, LI Shu-yu, et al. Effect of Nitrogen Surface Modification on Tribology and Corrosion Behavior of Amorphous Carbon Coating[J]. Surface Technology, 2022, 51(5): 61-69.
责任编辑:万长清

