Knockdown of polypyrimidine tract binding protein facilitates motor function recovery after spinal cord injury
Ri-Yun Yang, Rui Chai, Jing-Ying Pan, Jing-Yin Bao, Pan-Hui Xia, Yan-Kai Wang, Ying Chen, Yi Li, Jian Wu, Gang Chen, ,
Abstract After spinal cord injury (SCI), a fibroblast- and microglia-mediated fibrotic scar is formed in the lesion core, and a glial scar is formed around the fibrotic scar as a result of the activation and proliferation of astrocytes. Simultaneously, a large number of neurons are lost in the injured area. Regulating the dense glial scar and replenishing neurons in the injured area are essential for SCI repair. Polypyrimidine tract binding protein (PTB), known as an RNA-binding protein, plays a key role in neurogenesis. Here, we utilized short hairpin RNAs (shRNAs) and antisense oligonucleotides (ASOs) to knock down PTB expression. We found that reactive spinal astrocytes from mice were directly reprogrammed into motoneuron-like cells by PTB downregulation in vitro. In a mouse model of compressioninduced SCI, adeno-associated viral shRNA-mediated PTB knockdown replenished motoneuron-like cells around the injured area. Basso Mouse Scale scores and forced swim, inclined plate, cold allodynia, and hot plate tests showed that PTB knockdown promoted motor function recovery in mice but did not improve sensory perception after SCI. Furthermore, ASO-mediated PTB knockdown improved motor function restoration by not only replenishing motoneuron-like cells around the injured area but also by modestly reducing the density of the glial scar without disrupting its overall structure. Together, these findings suggest that PTB knockdown may be a promising therapeutic strategy to promote motor function recovery during spinal cord repair.
Key Words: antisense oligonucleotides; astrocytes; glial scar; motoneuron-like cells; motor function; neurogenesis; neuron-like cells; polypyrimidine tract binding protein; short hairpin RNAs; spinal cord repair 1Department of Histology and Embryology, Medical School of Nantong University, Nantong, Jiangsu Province, China; 2Key Laboratory of Neuroregeneration of Jiangsu Province and the Ministry of Education, Co-innovation Center of Neuroregeneration, Nantong University, Nantong, Jiangsu Province, China; 3Center for Basic Medical Research, Medical School of Nantong University, Nantong, Jiangsu Province, China; 4Department of Anesthesiology, Affiliated Hospital of Nantong University, Nantong, Jiangsu Province, China

Introduction 396 Methods 397 Results 398 Discussion 399
Graphical Abstract
Knockdown of PTB induces reprogramming of spinal astrocytes into motomeuron-like cells in vitro and improves motor function recovery after SCI

Introduction
Spinal cord injury (SCI) is a devastating central nervous system injury that often results in paralysis below the injury site (Sofroniew, 2018; Fouad et al., 2021). After SCI, fibroblasts and microglia form fibrotic scars that fill the lesion core. Simultaneously, astrocytes rapidly activate and proliferate, which results in the formation of a dense glial scar around the lesion core. An increasing number of studies have confirmed that the glial scar has dual effects on SCI repair (Yang et al., 2020a). Favorable effects include alleviating further development of inflammatory responses, limiting the spread of fibrotic scars, and forming astrocytic bridges that contribute to axonal regeneration (Anderson et al., 2016; Sofroniew, 2018). However, the glial scar is also regarded as a physical barrier that hinders axons from regenerating through the lesion site and serves as a chemical barrier because of the secretion of molecules that suppress neural regeneration (Li et al., 2020; Escartin et al., 2021). Therefore, the dual role of glial scars makes SCI repair difficult. Our previous study confirmed that 2 weeks was a suitable time point for manipulating the glial scar after SCI (Yang et al., 2020b). In SCI repair, this time point may alleviate the detrimental effects of the glial scar while supporting its favorable effects. During and after SCI, impaired neurons are lost because of necrosis or apoptosis, and the remaining damaged neurons have little regenerative capacity (O’Shea et al., 2017). These adverse factors coordinate with each other and, ultimately, complicate the SCI repair process. To date, there are no effective approaches for facilitating nerve regeneration and motor function restoration after SCI.
Direct conversion of somatic cells is a promising strategy for clinical use in regenerative medicine. Astrocytes are widely distributed throughout the spinal cord and have high plasticity (Yu et al., 2020). Reactive astrogliosis occurs after SCI, and reactive astrocytes express high levels of the neural stem cell marker nestin and display some characteristics of neural progenitor cells (Liddelow and Barres, 2017). Some studies (Su et al., 2014; Puls et al., 2020; Yang et al., 2020b; Liu et al., 2021) have shown that overexpression of neural transcription factors (TFs) can directly reprogram spinal astrocytes into diverse neuronal phenotypes bothin vitro
andin vivo
. However, these studies mainly used glutamatergic, GABAergic, or mature neurons (Su et al., 2014; Puls et al., 2020; Yang et al., 2020b; Liu et al., 2021). Moreover, these approaches showed limited roles in motor function restoration. Therefore, the strategy for gaining more motor neurons and allowing effective restoration of motor function after SCI requires further investigation.Polypyrimidine tract binding protein (PTB, also known as PTBP1), encoded by the Ptbp1 gene in mice, is an important RNA-binding protein that widely participates in RNA metabolism (Xue et al., 2009). PTB can regulate tau exon 10 inclusion or skipping that leads to neuronal vulnerability (Roussarie et al., 2020). Moreover, PTB plays a key role in neuronal induction. During neurogenesis, the expression level of PTB is naturally decreased (Hu et al., 2018). Studies have demonstrated that knockdown of PTB was able to directly reprogram multiple cell types into neurons or neuron-like cells, including primary mouse embryonic fibroblasts (MEFs), HeLa cells, mouse neural progenitor cells (N2A), human embryonic carcinoma stem cells (NT2), and human retinal epithelial cells (ARPE19) (Xue et al., 2009, 2013). Furthermore, PTB silencing can reprogram midbrain astrocytes into functional dopaminergic (DA) neuronsin vitro
, replenish DA neurons located in the midbrainin vivo
, and effectively reverse the locomotor phenotype of a mouse model of Parkinson’s disease (Qian et al., 2020). In addition, retinal ganglion cells, which are replenished by down-regulation of PTB, promote the recovery of visual responses in mice with retinal injury (Zhou et al., 2020). Maimon et al. (Maimon et al., 2021) confirmed that downregulation of PTB by an antisense oligonucleotide (ASO) significantly reprogrammed astrocytes into neuronsin vitro
. Treatment with a PTB-ASO generated neurons that integrated into the hippocampal circuit and ameliorated the behavioral deficits in aging mice (Maimon et al., 2021).The above studies show that suppression of PTB expression is a powerful strategy for treating various disorders caused by neuronal loss. However, the function of PTB knockdown in SCI repair has not yet been explored. In this study, short hairpin RNAs (shRNAs) and ASOs were used to knock down the expression of PTB to determine its role bothin vitro
in primary murine reactive spinal astrocytes andin vivo
using a mouse model of compressioninduced SCI.Methods
ASOs and viral vectors
ASOs were produced by RIBOBIO Biotechnologies (Guangzhou, China). The sequence of the mouse Ptbp1 ASO (PTB-ASO) was 5′-GGG TGA AGA TCC TGT TCA ATA-3′. The Cy3 fluorochrome was linked to the 5′ end of the ASO.
The shRNA sequence targeting mouse Ptbp1 (shPTB) was 5′-GGG TGA AGA TCC TGT TCA ATA-3′. To construct the lentiviral vector, shPTB was subcloned into the pCLenti-GFAP-shRNA (NC)-CMV-EGFP-WPRE vector (OBiO Biotechnologies, Shanghai, China).
To construct adeno-associated virus (AAV) vectors, the same sequence of shPTB was inserted into the pAAV-GFAP-EGFP-MIR155 vector to construct pAAV-GFAP-EGFP-shPTB-MIR155 (Genechem Co., Ltd.; Shanghai, China) (Additional Figure 1
). Empty vector was used as a control (shCtrl), and the final viral product titers were as follows: lentivirus-shCtrl, pCLenti-GFAP-shRNA(NC)-CMV-EGFP-WPRE (1.31 × 10transduction units (TU)/mL); lentivirus-shPTB, pCLenti-GFAP-shRNA(Ptbp1)-CMV-EGFP-WPRE (4.09 × 10TU/mL); AAVshCtrl, pAAV-GFAP-EGFP-MIR155 (1.75 × 10viral genomes (VG)/mL); and AAV-shPTB, pAAV-GFAP-EGFP-shRNA(Ptbp1)-MIR155 (2.71 × 10VG/mL).Primary murine spinal astrocyte culture and activation
All animal experiments were approved by the Institutional Animal Ethics Committee of Nantong University (approval No. S20201015-402) on October 15, 2020. All experiments were designed and reported according to the Animal Research: Reporting ofIn Vivo
Experiments (ARRIVE) guidelines (Percie du Sert et al., 2020). Primary murine astrocytes were prepared from the spinal cords of 1–2-day-old Institute of Cancer Research (ICR) mice (Animal Center of Nantong University; Nantong, China; license No. SYXK (Su) 2017-0046) and cultured as described previously (Gao et al., 2016). Neonatal mice were euthanized with carbon dioxide (CO). The concentration of COwas continuously increased until respiratory and cardiac arrest occurred, and then a normal supply of oxygen was provided. The COfill rate was 30–70%, which guaranteed that the mice achieved loss of consciousness before causing pain. The intervention time for COwas 50 minutes (Shomer et al., 2020). Then, mice were placed in 75% ethyl alcohol for 5 minutes. The spinal cords of the mice were separated on ice. The dissociated spinal cords were mechanically minced on ice after removal of the meninges and blood vessels. Tissue was digested using 0.25% trypsin-ethylenediaminetetraacetic acid (Beyotime; Shanghai, China) solution in a 37°C incubator for 15 minutes. The cell suspension was dispersed and immediately filtered through a 100-µM pore membrane (Merck; Darmstadt, Germany). The cells were centrifuged at 157 ×g
for 5 minutes and resuspended in Dulbecco’s modified Eagle medium/F12 (DMEM/F12; Gibco; Grand Island, NY, USA) containing 10% fetal bovine serum (Sigma–Aldrich) and 1% penicillin/streptomycin (Gibco). The mixed cells were added to a T-75 flask at a density of 1.0 × 10cells/mL and maintained in an incubator at 37 °C and 5% CO. The conditioned culture medium was replaced every 3 days. After 7 days, primary microglial cells were removed by shaking at 40 ×g
for 16 hours. Primary astrocytes were passaged twice and used for further studies. Lipopolysaccharide (500 ng/mL) was applied for 24 hours to induce astrocyte activation (Agalave et al., 2020).Reactive astrocyte reprogramming
To induce reprogrammingin vitro
, reactive spinal astrocytes from mice were resuspended in DMEM/F12 medium containing 10% fetal bovine serum and lentivirus-shPTB. The mixture of cells and lentivirus was plated in 6-well cell culture plates (four 12-mm poly-L-lysine-coated glass coverslips/well, 20,000 cells/well). The next day, the medium was changed to induction medium, which consisted of equal volumes of DMEM/F12 and neurobasal medium (Gibco) supplemented with sodium selenite (30 nM; Sigma-Aldrich), insulin (25 µg/mL; AbMole, USA), putrescine (100 nM; APExBIO; Houston, TX, USA), progesterone (20 nM; Sigma-Aldrich), 2% fetal bovine serum, 0.4% B-27(Gibco), SB431542 (10 µM; Sigma-Aldrich), ChIR99021 (1 µM; Sigma-Aldrich), dibutyryl-cyclic adenosine monophosphate (1 mM; APExBIO), neurotrophin 3, and 10 ng/mL each of ciliary neurotrophic factor, brain-derived neurotrophic factor, and glial cell-derived neurotrophic factor (Peprotech; Rocky Hill, NJ, USA). One-half volume of the medium was replaced every 3 days.To determine the function of the PTB-ASOsin vitro
, primary spinal cord astrocytes were seeded into 6-well culture plates as described above (20,000 cells/well). The following day, PTB-ASOs or control ASOs (75 pmol/well) were transfected using Lipofectamine RNAiMAX (Invitrogen; Carlsbad, CA, USA). The medium was replaced with induction medium after 48 hours for further transdifferentiation.SCI mouse model
Eight-week-old female ICR mice (specific pathogen-free grade, 23–28 g, Animal Center of Nantong University; license No. SYXK [Su] 2017-0046) were anesthetized throughout the surgery with isoflurane (1–1.5%, flow rate: 200 mL/minute) in oxygenized air supplied by a standard small animal anesthesia device (RWD; Shenzhen, China). SCI leads to urine retention in mice, and male mice are more likely to suffer from urethritis and die than female mice. In addition, the experimental period was up to 12 weeks in this study. Considering the survival rate of mice after SCI, female mice were selected (Wu et al., 2021).
The spinal cord was exposed by a T9–T10 laminectomy. A complete compression injury was induced for 10 seconds by bilateral compression with No. 5 Dumont forceps (Fine Science Tools, Foster City, CA, USA) (Anderson et al., 2018). The overlying muscle was closed with sutures. Wound clips were utilized to close the skin incision. The SCI mice were randomly divided into four groups: AAV-shCtrl, AAV-shPTB, Control ASO, and PTB-ASO groups, each of which included 25 mice.
AAV and ASO injection
AAVs or ASOs may require several days to take effectin vivo
, and 2 weeks after SCI was considered an appropriate time point to manipulate the glial scar (Yang et al., 2020b). Therefore, we performed injections 7 days after SCI. AAV-shCtrl was diluted to the same titer as AAV-shPTB (2.71 × 10VG/mL) with phosphate-buffered saline (PBS). AAVs were injected at two oblique points on opposite sides of the lesion site (1.0 mm caudal and rostral to the lesion core at approximately 45°, 1.5 µL per injection) utilizing a 10 µL microsyringe with a 33G needle (Hamilton, Basel, Switzerland). The method for injecting ASOs (1 µg/µL, 1.0 µL per injection) was the same as that for injecting AAVs. At 12 weeks after SCI, mice were deeply anesthetized with intraperitoneally administered ethyl carbamate (Sinopharm Chemical Reagent Co. Ltd., Shanghai, China) and then sacrificed. The spinal cords of the mice were collected.Western blot analysis and quantitative reverse transcription-polymerase chain reacti
onFor measuring protein expression levels of PTB following PTB silencing in reactive spinal astrocytes, the reprogrammed cells were lysed in cell lysis buffer containing cOmplete™ Protease Inhibitor Cocktail (Roche, Mannheim, Germany). After quantification, equivalent amounts of reprogrammed cell protein from each treatment group were resolved by sodium dodecyl sulfatepolyacrylamide gel electrophoresis. Proteins were then transferred to a polyvinylidene difluoride membrane (Merck Millipore, Billerica, MA, USA). After blocking with 5% skim milk (Beyotime), the membrane was incubated with specific primary antibodies for 18 hours at 4°C. Then, after three washes with Tris-buffered saline containing Tween-20, the membrane was incubated with the corresponding secondary antibody for 2 hours at 25°C. Immunoreactions were visualized with a chemiluminescent substrate (Thermo Fisher Scientific, Waltham, MA, USA) and a fully automated ChemiDoc system (Tanon, Shanghai, China). The primary antibodies and dilutions were as follows: rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:1000; Beyotime, Cat# AF 1186) and rabbit anti-PTB (1:1000; ABclonal, Wuhan, China, Cat# A3487, RRID: AB_2863069). The secondary antibody was horseradish peroxidase-conjugated goat anti-rabbit IgG (1:5000; Thermo Fisher Scientific, Cat# 31466, RRID: AB_10960844). ImageJ 1.47v software (National Institutes of Health, Bethesda, MD, USA) (Schneider et al., 2012) was used to quantify the protein band signals.
Total RNA from reprogrammed cells or spinal cord was extracted using TRI reagent (Sigma-Aldrich). The RNA was reverse transcribed to complementary deoxyribonucleic acid (cDNA) using a reverse transcription kit (Thermo Fisher Scientific). Polymerase chain reaction (PCR) was performed using a QuantiNova SYBR Green PCR Kit (Qiagen, Duesseldorf, Germany) and Bio-Rad CFX96 Real-Time PCR instrument (Hercules, CA, USA). The quantitative reverse transcription PCR mixture (20 µL) contained 6 µL RNase-free water, 10 µL SYBR Green, 1 µL forward primer, 1 µL reverse primer, and 2 µL cDNA. Reaction conditions were: pre-denaturation at 95°C for 2 minutes followed by 40 cycles of denaturation at 95°C for 5 seconds and annealing at 60°C for 10 seconds. The relative expression of target genes was normalized toGapdh
. The primer sequences are listed inTable 1
.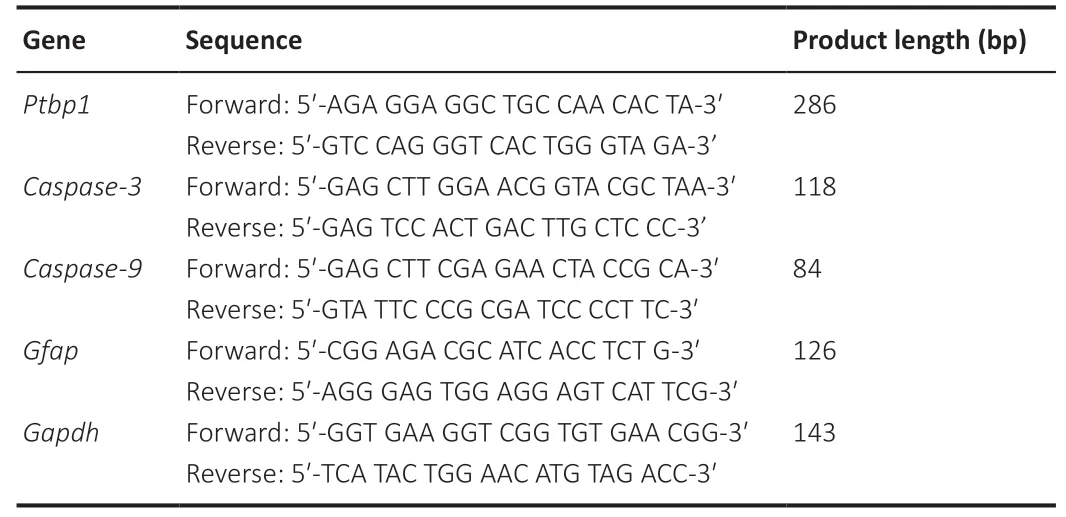
Table 1 |Primers used for quantitative reverse transcription-polymerase chain reaction
Immunocytochemistry
To measure astrocyte transdifferentiationin vitro
, reprogrammed cells were collected after 3 and 4 weeks of AAV-shPTB-mediated PTB knockdown or 5 weeks of PTB ASO-mediated PTB knockdown and fixed with 4% paraformaldehyde for 10 minutes. Cells were permeabilized in PBS containing 1% Triton X-100 for 15 minutes and then washed three times with PBS. Cells were blocked with 3% bovine serum albumin in PBS for 30 minutes and incubated with specific primary antibodies diluted in PBS containing 3% bovine serum albumin for 16 hours at 4°C. The primary antibodies included mouse anti-microtubule-associated protein 2 (MAP2; 1:600; Abcam, Cambridge, MA, USA, Cat# ab11268, RRID: AB_297886), goat anti-green fluorescent protein (GFP; 1:1000; Abcam, Cat# ab5450, RRID: AB_304897), rabbit anti-choline acetyltransferase (ChAT; 1:400; Abcam, Cat# ab181023, RRID: AB_2687983), and goat anti-glial fibrillary acidic protein (GFAP; 1:600; Abcam, Cat# ab53554, RRID: AB_880202). After washing three times with PBS, reprogrammed cells were incubated for 2 hours at room temperature with the following secondary antibodies: Alexa Fluor 488-labeled donkey anti-goat IgG (1:1000; Thermo Fisher Scientific, Cat# A11055, RRID: AB_2534102), Alexa Fluor 488-labeled donkey anti-rabbit IgG (1:1000; Thermo Fisher Scientific, Cat# A21206, RRID: AB_2535792), Alexa Fluor 568-labeled donkey anti-mouse IgG (1:1000; Thermo Fisher Scientific, Cat# A10037, RRID: AB_2534013), or Alexa Fluor 647-labeled donkey anti-rabbit IgG (1:1000; Thermo Fisher Scientific, Cat# A31573, RRID: AB_2536183). Nuclei were labeled with Hoechst 33342 (Beyotime) by incubation for 15 minutes at 37°C. Images were acquired using a confocal microscope (Leica, Wetzlar, Germany). MAP2, GFP, ChAT, GFAP, and Hoechstcells were counted.Immunohistochemistry
To measure the localization of shPTB delivered by AAV in the spinal cord of mice, spinal cord staining was conducted 1 week after injection. To explore the role of PTB knockdownin vivo
, spinal cord staining was conducted 12 weeks after SCI. Mice were anesthetized with isoflurane and perfused transcardially with 0.9% NaCl followed by 4% paraformaldehyde. The T9–T11 vertebrae of the spinal cord were excised and postfixed with 4% paraformaldehyde for 10 hours at 4°C and then incubated for 1 week in 30% sucrose at 4°C. Spinal cord sections (16 µm) were prepared using a freezing microtome (Leica). The spinal cord sections were blocked for 1 hour at 37°C in PBS containing 0.3% Triton X-100 and 3% bovine serum albumin. The steps were similar to those used for staining reprogrammed cells on coverslips as described above. The spinal cord sections were incubated with specific primary antibodies diluted in PBS containing 3% bovine serum albumin for 16 hours at 4°C. The following primary antibodies were used: goat anti-GFAP (1:600, Abcam, Cat# ab53554), mouse anti-GFAP (1:600, Abcam, Cat# ab279289), mouse anti-NeuN (1:600, Abcam Cat# ab104224, RRID: AB_10711040), rabbit anti-ChAT (1:400, Abcam, Cat# ab181023), mouse anti-MAP2 (1:600, Abcam, Cat# ab11268), goat anti-GFP (1:1000; Abcam, Cat# ab5450), and mouse anti-FoxJ1 (1:400, Abcam, Cat# ab220028). After washing three times with PBS, the sections were incubated for 2 hours at 25°C with Alexa Fluor 488-labeled donkey anti-goat IgG (1:1000), Alexa Fluor 568-labeled donkey anti-mouse IgG (1:1000), or Alexa Fluor 647-labeled donkey anti-rabbit IgG (1:1000) as described above.Slices across the spinal cord were sampled at eight slice intervals and subjected to immunofluorescent staining for ChAT, NeuN, MAP2, GFP, and GFAP. Stereological methods were used to count the total number of cells of a given type (Abercrombie, 1946), and the formula utilized was previously described (Qian et al., 2020). The percentages of NeuN, MAP2, and ChATneurons among GFPcells were quantified at 12 weeks after SCI. The immunofluorescence density of the GFAP signal in the glial scar area around the lesion core (Yang et al., 2020b) was determined quantitatively using ImageJ software.
Terminal deoxynucleotidyl transferase dUTP nick-end labeling
To explore the effect of PTB knockdown on apoptosis, sections across the spinal cord were prepared as described above, fixed with 4% paraformaldehyde for 60 minutes, and washed two times with PBS. Sections were permeabilized in PBS containing 0.5% Triton X-100 for 5 minutes at room temperature. After washing two times with PBS, the sections were incubated with the terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) detection liquid (Beyotime) for 1 hour at 37°C. Nuclei were labeled with Hoechst 33342 by incubation for 15 minutes at 37°C. Images were acquired with a confocal microscope, and the numbers of TUNELcells were counted. The percentages were calculated as the number of TUNELcells divided by the total number of Hoechstcells.
Behavioral experiments
Motor recovery was measured at 1 week post-SCI and then weekly for 12 weeks post-SCI.
The motor function of SCI mice was evaluated using the Basso Mouse Scale (BMS) scoring system (Huang et al., 2020). Two trained evaluators scored the mice in a double-blinded manner. Mice were evaluated for at least 5 minutes after scouting the open field environment for 20 minutes. The score was from 0 to 9. A higher score represented improved function of both hindlimbs.
The swimming ability of each SCI mouse was evaluated using the forced swim test. A rectangular plexiglass container (42 cm × 30 cm × 35 cm) filled with water was used for the test with a water depth of approximately 18 cm and temperature at approximately 23°C (Yang et al., 2020b). The swim test was performed for 1 minute each time and repeated three times at 15 minutes intervals. An HD camera was utilized to photograph the swimming process. The swing frequency of the hindlimb was determined every 10 seconds. The maximum swing frequency during each minute was measured, and the mean value of three tests was calculated.
The muscle strength of both hindlimbs of SCI mice was evaluated using the inclined plate test (Yang et al., 2020b), which consisted of a rectangular plexiglass box (42 cm × 7 cm × 8 cm) with a rough wooden surface at the bottom. When the box was inclined, the mice would slide down from the rough surface because of gravity. We gradually raised the tilt of the box until the mice could no longer maintain position for 5 seconds without sliding down. The recorded result was generated from the maximum height of the top of the box perpendicular to the horizontal plane. The test was repeated three times, and the maximum height was used as the result.
The sensory recovery of SCI mice was assessed by the cold allodynia test. Cold allodynia was scored by the acetone solution method (Burton et al., 1999). 50 µL of acetone solution was placed onto the plantar skin surface of the paw through a blunt needle attached to a syringe. The reaction of the mouse was observed within 20 seconds: adiaphoria, scored 0; minor response, scored 1; moderate reaction–the hindpaw was lifted but did not touch any surface when sprayed with acetone, scored 2; strong response–the paw was licked, bit, and shaken, scored 3. The test was repeated three times at 15 minute intervals, and the mean value was recorded.
The hot plate test was used to assess the sensory function of SCI mice (Luszczki and Czuczwar, 2008). Mice were placed in a clear 25 cm × 25 cm chamber with a height of 40 cm. The chamber contained a metal plate, and the surface temperature of the plate was 55 ± 0.5°C. The cumulative time that the mouse lifted its hindpaw from the hot plate and licked the paw within a 30 second interval was calculated. The experiment was repeated three times at 15 minute intervals, and the mean value of the three experiments was calculated.
Statistical analysis
The numbers of repeated samples or mice are listed in the figure legends. Degrees of freedom were used to predetermine sample sizes for thein vivo
study (Charan and Kantharia, 2013), and sample sizes were estimated according to our previous studies for similar behavioral, immunofluorescence, and TUNEL staining analyses (Yang et al., 2020b). Outcome assessors were blinded to the assignment. All data are presented as the means ± standard error of mean (SEM). All statistical analyses were performed with GraphPad Prism 6.01 software (GraphPad Software, San Diego, CA, USA; www.graphpad.com). The Shapiro-Wilk test was utilized to measure data normality. Western blot, RT-PCR, immunocytochemistry, and immunohistochemistry reactivity were analyzed using two-tailed Student’st
-tests. Behavioral data were analyzed using repeated measures two-way analysis of variance with Tukey’spost hoc
tests. A value ofP
< 0.05 was considered statistically significant.Results
ShPTB mediates direct conversion of reactive spinal astrocytes from mice into motoneuron-like cells
Given the demonstrated direct neuronal conversion of cultured mouse cortical astrocytes after shPTB treatment (Qian et al., 2020), we explored whether PTB silencing led to the conversion of reactive mouse spinal astrocytes into motor neuronsin vitro
. In a reactive astrocyte model, we treated primary murine spinal astrocytes with lipopolysaccharide (Additional Figure 2A
andB
). Next, we transduced reactive spinal astrocytes from mice with a lentiviral vector containing aGfap
promoter driving the expression of an shRNA against mousePtbp1
. When astrocytes were transduced with this vector for 2 days, shPTB significantly downregulated both the protein expression of PTB and mRNA expression ofPtbp1
(Figure 1A–C
). At 2 weeks post-reprogramming, shPTBinfected cells showed complex neurite outgrowth, and, by 4 weeks, these cells displayed obvious neuronal morphology, whereas cells transduced with the shCtrl displayed an astrocyte-like flattened and polygonal morphology (Figure 1D
). Three weeks after transduction, approximately 41% of shPTB-transduced GFPcells demonstrated positive staining for the neuronal marker MAP2 (Figure 1E
andF
). At 4 weeks post-conversion, the percentage of GFP/MAP2cells was approximately 58%, and approximately 12% of GFPcells exhibited expression of the motor neuron marker ChAT (Figure 1G
andH
). In contrast, cells transduced with the shCtrl lentiviral vector exhibited positive staining only for GFP (Figure 1E
andG
). These data demonstrate that reactive spinal astrocytes can be reprogrammed into motoneuron-like cells by PTB silencingin vitro
.PTB silencing replenishes motoneuron-like cells around the injured area after SCI
SCI induces the loss of a large number of neurons in the injured area (Anderson et al., 2016). To determine whether PTB silencing replenished neurons, including motor neurons, in an SCI mouse model, we injected AAVs into the oblique opposite sides of the injured spinal area. We used the Gfap promoter to drive shPTB expression. A large number of GFPcells were colocalized with GFAPastrocytes 1 week after injection (Figure 2A
), whereas the GFPcells were rarely colocalized with ChATmotor neurons (Figure 2B
). Quantitatively, approximately 75% of GFPcells were colocalized with GFAPastrocytes, but no GFPcells were colocalized with ChATmotoneurons (Figure
2C
). Moreover, the GFPcells did not colocalize with FoxJ1neural stem cells (Additional Figure 3A
andB
). Eleven weeks after AAV-shPTB injection, some GFPcells were co-labeled with the neuronal marker NeuN or MAP2 around the injured area (Figure 3A
andB
). Quantitatively, approximately 30% and 29% of GFPcells were co-labeled with NeuN and MAP2, respectively (Figure 3C
). Furthermore, approximately 19% of GFPcells expressed the motor neuron-specific marker ChAT at 11 weeks after AAV-shPTB injection (Figure 3A–C
). At this same time point, neither GFPneuron-like cells nor GFPmotoneuron-like cells were detected around the injured area after AAVshCtrl injection (Figure 3A–C
). Quantitative analysis of neuronal-like cell and motoneuron-like cell populations around the lesion area showed that the number of NeuN, MAP2, and ChATcells were more abundant in the AAVshPTB group compared with that in the AAV-shCtrl group (Figure 3D
). In conclusion, these results reveal that shPTB can replenish motoneuron-like cells around the injured area after SCI.PTB knockdown facilitates motor function restoration in SCI mice
We determined whether PTB knockdown could promote functional recovery in SCI mice. We used the BMS score and swim test to evaluate motor recovery. A compression SCI was induced in mice, and AAV-shPTB or AAV-shCtrl was injectedin situ
1 week post-injury (Figure 4
). BMS scores were decreased at 1 week post-SCI. At 6–12 weeks post-injury, AAV-shPTB-injected mice showed higher BMS scores than AAV-shCtrl mice (Figure 5A
). However, there was no obvious difference between the two groups in the swim test (Figure 5B
). AAVshPTB-injected mice maintained position at a higher height than AAV-shCtrl mice at 12 weeks post-SCI in the inclined plate test (Figure 5C
). These results demonstrated improved muscle strength recovery after PTB knockdown. Sensory recovery was assessed by the cold allodynia and hot plate tests. The AAV-shPTB-injected mice did not exhibit any improvement in performance compared with that of AAV-shCtrl-injected mice in either the cold allodynia or hot plate test at 6–12 weeks post-SCI (Figure 5D
andE
). These findings indicate that AAV-shPTB treatment can improve motor recovery after SCI.Conversion of spinal astrocytes into motoneuron-like cells
in vitro
after transfection with PTB-ASOs
Given the demonstrated capability of ASOs to degrade their target mRNAs (Bennett et al., 2019; Qian et al., 2020; Maimon et al., 2021), downregulation of PTB by PTB-ASO suggests a clinically feasible strategy. As shown inFigure 6A–C
, compared with the control ASO, PTB-ASO transfection markedly reduced PTB protein andPtbp1
mRNA expression in astrocytes after 2 days. At 5 weeks post-transfection, we evaluated the morphology of reprogrammed cells and the expression of the mature neuronal marker MAP2 as well as the motor neuron marker ChAT via immunocytochemistry (Figure 6D
). Quantitative analysis of MAP2and ChATcells among total Hoechstcells showed that approximately 64% of the reprogrammed cells exhibited neurite outgrowth and expressed MAP2, and approximately 14% of the reprogrammed cells exhibited positive ChAT staining (Figure 6D
andE
). These results suggest that PTB-ASO significantly induced the conversion of reactive spinal astrocytes into motoneuron-like cellsin vitro
.PTB-ASOs reduce the density of glial scars and replenished motoneuronlike cells in SCI mice
As previously described, the glial scar has a dual role in SCI repair. Some studies have suggested that moderate reduction in the glial scar can play a positive role in functional recovery after SCI (Rodriguez et al., 2014; Hesp et al., 2018; Yang et al., 2020b). We determined the effects of PTB ASOs on glial scar density by GFAP staining of the injured spinal cord. As shown inFigure 7A
, at 12 weeks after SCI, control ASO-injected mice showed typical glial scar morphology with interdigitating astrocytes that displayed a considerable number of parallel processes. PTB-ASO-injected mice also exhibited integrated glial scar structures, which were different from the exhaustive ablation of glial scars in some previous reports. In PTB ASO-injected mice, glial scar-derived astrocytes were loosely distributed with fewer parallel processes (Figure 7A
). Quantitative analysis of the immunofluorescent intensity of GFAP around the lesion area revealed a moderate decrease in the density of glial scars in PTB ASO-injected mice compared with that in control ASO-injected mice (Figure 7B
).SCI results in irreversible loss of neurons in the injured area, and the regenerative capacity of the damaged neurons is limited (Sofroniew, 2018). To explore the potential beneficial function of the PTB-ASO in SCI mice, we determined whether injection of the PTB-ASO into the injured spinal cord replenished neurons, including motor neurons. This strategy may be helpful for promoting functional recovery after massive SCI-associated loss of neurons in the lesion area. We found that control ASO-injected mice exhibited an obvious decrease in NeuNand ChATcells in the region proximal to the lesion area compared with the distal region at 12 weeks after SCI (Figure 7A
). In contrast, PTB-ASO-injected mice retained many NeuNand ChATcells in the region proximal to the lesion at a level similar to that observed in the distal site (Figure 7A
). Quantitatively, the number of NeuN+ and ChATcells in the region proximal to the lesion area was greater in PTB-ASO-injected mice compared with that in control ASO-injected mice (Figure 7C
). TUNEL staining was utilized to detect cell apoptosis around the lesion area at 12 weeks post-SCI. We found that the number of TUNELcells around the lesion area was lower in PTB-ASO-injected mice than in control ASO-injected mice (Figure
8A
andB
). Furthermore, quantitative RT-PCR analysis of mRNA from injured spinal tissue exhibited lower expression levels of the apoptotic markers caspase-3 and caspase-9 in PTB-ASO-injected mice compared with those in control ASO-injected mice (Figure 8C
). These findings revealed that the PTB-ASO reduced glial scar density without disrupting its overall structure, replenished motoneuron-like cells around the SCI lesion area, and decreased apoptotic cell death in the injured spinal cord.PTB-ASOs promote motor function recovery in SCI mice
The function of the PTB-ASO was further evaluated by behavioral tests in SCI mice. Strikingly, compared with the control ASO, the PTB-ASO enhanced motor function recovery from 5–12 weeks post-SCI as determined by BMS scores and swim tests (Figure 9A
andB
). The PTB-ASO-treated mice reached a higher height than control ASO-treated mice in the inclined plate test at 12 weeks post-SCI (Figure 9C
). Compared with control ASO-treated mice, PTB ASO-treated mice tended to use their lower limbs and displayed greater muscle strength after SCI. In the sensory system, cold- and heat-induced sensory responses were not different between the PTB-ASO-treated and control ASO-treated mice from 6–12 weeks post-injury (Figure 9D
andE
). These results demonstrated that PTBASOs promoted motor function recovery in SCI model mice.
Figure 1|Direct reprogramming of primary murine reactive spinal astrocytes into motoneuron-like cells by PTB knockdown in vitro.(A) Western blot bands and (B) quantification of the protein expression levels of PTB following PTB silencing with short hairpin (sh)PTB RNA in reactive mouse spinal astrocytes for 2 days. The results were normalized to the sh control (shCtrl) group. (C) Ptbp1 mRNA levels after PTB knockdown for 2 days. The results were normalized to the shCtrl group. (D) Representative photographs of the morphological changes following PTB silencing for 2 and 4 weeks. After PTB silencing for 2 weeks, shPTB-infected cells showed complex neurite outgrowth, and these cells displayed obvious neuronal morphology by 4 weeks, whereas cells transduced with shCtrl displayed an astrocyte-like flattened and polygonal morphology. Scale bar: 40 µm. (E) Reactive mouse spinal astrocytes were immunostained for the neuronal marker MAP2 (red, Alexa Fluor 568) after PTB knockdown for 3 weeks. There were some shPTB-transduced GFP+ (green, Alexa Fluor 488) cells that stained positive for MAP2, whereas no shCtrl-transduced GFP+ cells were positive for MAP2. Scale bar: 40 µm. (F) Quantification of the number of MAP2+ cells among transduced GFP+ cells at 3 weeks. (G) Reprogrammed motoneuron-like cell morphology and expression of ChAT (cyan, Alexa Fluor 647) after PTB silencing for 4 weeks. There were some shPTB-transduced GFP+ (green, Alexa Fluor 488) cells that stained positive for ChAT, whereas no shCtrl-transduced GFP+ cells stained positive for ChAT. Scale bar: 40 µm. (H) Quantification of the number of MAP2+ cells and ChAT+ cells among transduced GFP+ cells at 4 weeks. Data are shown as the means ± SEM. The experiments were repeated three times. *P < 0.05, vs. shCtrl (two-tailed Student’s t-test). ChAT: Choline acetyltransferase; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; GFP: green fluorescent protein; MAP2: microtubule-associated protein 2; n.d.: not detected; PTB: polypyrimidine tract-binding protein; Ptbp1: polypyrimidine tract-binding protein; w: weeks.

Figure 2|Localization of shPTB delivered by AAV in the spinal cord of mice.At 1 week post-injection; immunohistochemical staining showed that GFP+ (green, Alexa Fluor 488) cells colocalized with GFAP+ (red, Alexa Fluor 568) astrocytes (A) but not with ChAT+ (cyan, Alexa Fluor 647) motor neurons (B). The lower panels are magnifications of the white dashed box areas in the upper panels to improve clarity. The arrowheads in (A) indicate representative co-labeled spinal astrocytes, and the swallowtail arrowheads in (B) denote endogenous motor neurons (n = 3 mice, four sections per mouse). Scale bars: 50 µm. (C) Quantitative analysis of the percentage of GFAP+ and ChAT+ cells colocalizing with GFP+ cells. The data are presented as the means ± SEM (n = 3 mice/group) and were analyzed by two-tailed Student’s t-test. AAV: Adeno-associated virus; ChAT: choline acetyltransferase; GFAP: glial fibrillary acidic protein; GFP: green fluorescent protein; n.d.: not detected; PTB: polypyrimidine tract-binding protein.

Figure 3|PTB knockdown replenished motoneuron-like cells around the injured area after SCI in mice. (A) Longitudinal sections at 12 weeks post-SCI. In the AAV-shPTB group, some of the GFP+ (green, Alexa Fluor 488) cells were colocalized with NeuN+ (red, Alexa Fluor 568) and ChAT+ (cyan, Alexa Fluor 647) cells. The arrows show representative supplementary motoneuron-like cells, and the arrowheads indicate representative endogenous motor neurons. In the AAV-shCtrl group, there were no co-labeled GFP+/NeuN+ cells or GFP+/ChAT+ cells (n = 3 mice, four sections per mouse). Scale bars: 200 µm. In each group, the lower panels are magnifications of the white dashed box areas in the upper panels to improve clarity. (B) Replenished neuron-like cells (GFP+ [green, Alexa Fluor 488] and MAP2+ [red, Alexa Fluor 568]) and motoneuron-like cells (GFP+/ChAT+ [cyan, Alexa Fluor 647]) were detected in the AAV-shPTB group 12 weeks after SCI. The arrows denote representative replenished motoneuronlike cells, and the arrowheads indicate representative endogenous motor neurons. There were no GFP+/MAP2+ or GFP+/ChAT+ cells in the AAV-shCtrl group (n = 3 mice, four sections per mouse). Scale bars: 200 µm. (C) Quantitative analysis of the percentage of NeuN+, MAP2+, and ChAT+ cells among the GFP+ cells (n = 3 mice). (D) Quantitative analysis of total NeuN+, MAP2+, and ChAT+ cells around the injured spinal cord areas in the AAV-shCtrl and AAV-shPTB mouse groups. The data are shown as the means ± SEM (n = 3 mice/group). #P < 0.05, vs. AAV-shCtrl (two-tailed Student’s t-test). AAV: Adeno-associated virus; ChAT: choline acetyltransferase; GFP: green fluorescent protein; MAP2: microtubule-associated protein 2; PTB: polypyrimidine tract-binding protein; SCI: spinal cord injury.

Figure 4|The experimental timelines.AAV: Adeno-associated virus; ASO: antisense oligonucleotide; PTB: polypyrimidine tractbinding protein; RT-PCR: reverse transcription-polymerase chain reaction; SCI: spinal cord injury; TUNEL: TdT-mediated dUTP Nick-End Labeling.
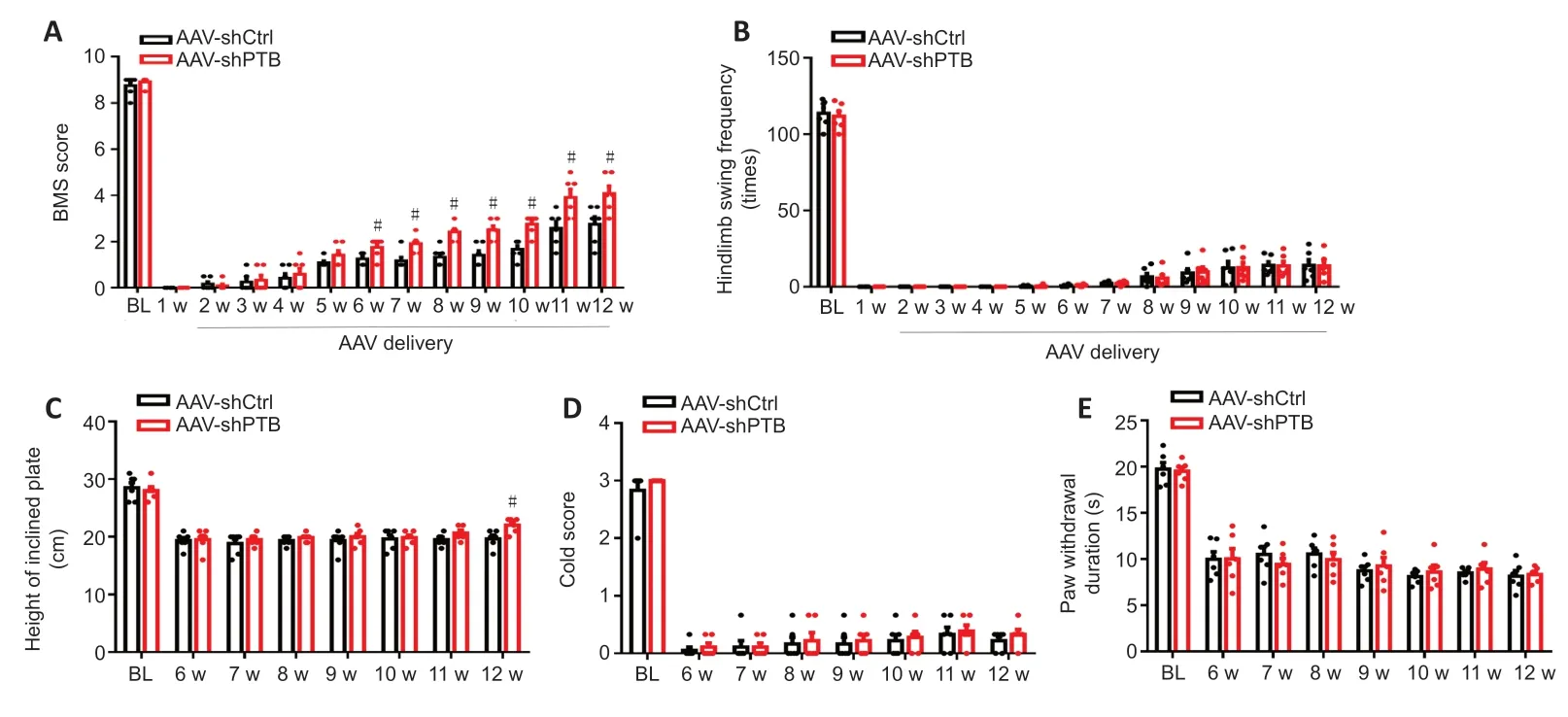
Figure 5|PTB knockdown facilitated motor function recovery after SCI.(A) BMS scores. Motor function recovery was better in mice with AAV-shPTB-mediated PTB knockdown from 6 to 12 weeks after SCI than in mice that received the AAV-shCtrl. (B) The swim, (C) inclined plate, (D) cold allodynia, and (E) hot plate tests. No obvious differences were observed in the swim, cold allodynia, and hot plate tests between the AAV-shCtrl and AAV-shPTB groups. The inclined plate test showed that AAV-shPTBinjected mice maintained their position at a greater height than AAV-shCtrl mice at 12 weeks post-SCI. The data are shown as the means ± SEM (n = 6 mice/group). #P < 0.05, vs. AAV-shCtrl (repeated measures twoway analysis of variance followed by Tukey’s post hoc test). AAV: Adeno-associated virus; BI: baseline; BMS: Basso Mouse Scale; PTB: polypyrimidine tract-binding protein; SCI: spinal cord injury; w: week(s).

Figure 6|The PTB-ASO mediated the reprogramming of murine reactive spinal astrocytes to motoneuron-like neurons in vitro. The protein expression levels of PTB were measured by (A) western blot analysis after PTB-ASOs were transfected into murine reactive spinal astrocytes for 2 days. (B) Quantification of PTB protein. The results were normalized to the Control ASO group. (C) The mRNA expression levels of Ptbp1 following PTB silencing for 2 days. The results were normalized to the Control ASO group. (D) Representative images of the expression of ChAT (green, Alexa Fluor 488) by immunocytochemical staining after PTB-ASO-mediated PTB knockdown for 5 weeks. In the PTB-ASO group, MAP2+ (red, Alexa Fluor 568) cells stained positive for ChAT (green, Alexa Fluor 488), but there were no MAP2+ and ChAT+ cells in the Control ASO group. Scale bar: 40 µm. (E) Quantitatively, the efficiencies of neuron-like and motoneuron-like cell reprogramming were calculated based on the percentages of MAP2+ and ChAT+ cells among total Hoechst+ cells. The experiments were repeated three times. The data are shown as the means ± SEM. †P < 0.05, vs. control ASO (two-tailed Student’s t-test). ASO: Antisense oligonucleotide; ChAT: choline acetyltransferase; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; MAP2: microtubule-associated protein 2; n.d.: not detected; PTB: polypyrimidine tract-binding protein; Ptbp1: polypyrimidine tract-binding protein.

Figure 7|The PTB-ASO reduces the density of the glial scar and replenishes motoneuron-like cells around the spinal cord lesion.(A) At 12 weeks post-SCI, the GFAP (green, Alexa Fluor 488) fluorescence intensity was lower and the number of NeuN+ (red, Alexa Fluor 568) and ChAT+ (cyan, Alexa Fluor 647) cells was much greater around the lesion area in PTB-ASO-injected mice than in the Control ASO group. The arrows indicate the glial scar area. The yellow swallowtail arrowheads and lines surrounding the area denote the NeuN+ cells in the region proximal to the lesion area. The white swallowtail arrowheads and lines surrounding the area denote the ChAT+ cells around the damaged area. The yellow arrowheads and lines surrounding the area indicate NeuN+ cells in the region distal to the damaged area. The white arrowheads and lines surrounding the area denote ChAT+ cells in the region distal to the damaged area (n = 3 mice; four sections per mouse). Scale bars: 200 µm. In each group, the lower panels are magnifications of the white dashed box areas in the upper panels to improve clarity. (B) Quantitative analysis of the fluorescence intensity of GFAP in the glial scar area. (C) Quantitative analysis of the numbers of NeuN+ and ChAT+ cells per 0.1 mm2 in the region proximal to the lesion area. The data are shown as the means ± SEM (n = 3 mice/group). †P < 0.05, vs. control ASO (two-tailed Student’s t-test). ASO: Antisense oligonucleotide; ChAT: choline acetyltransferase; GFAP: glial fibrillary acidic protein; PTB: polypyrimidine tract-binding protein; SCI: spinal cord injury.
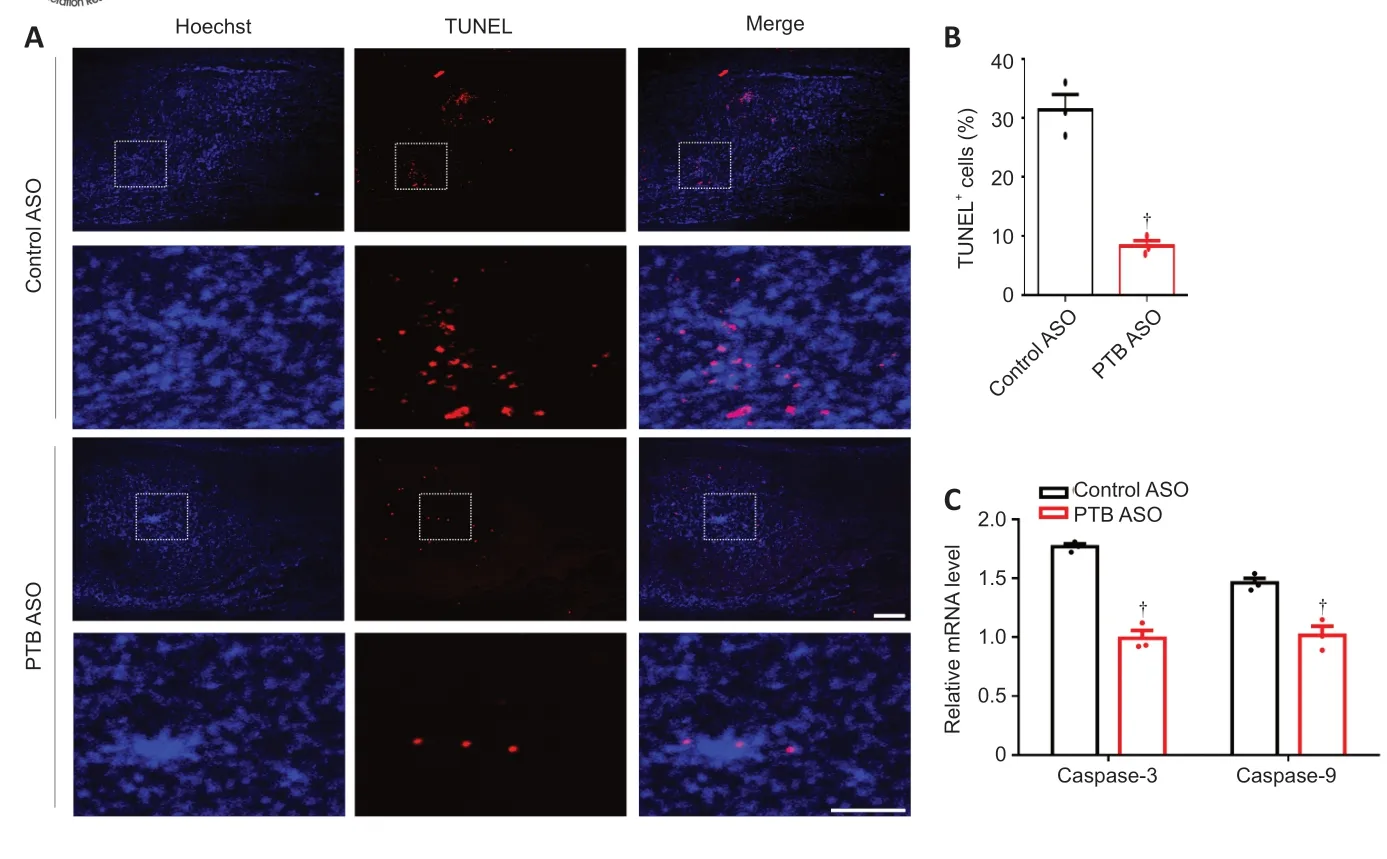
Figure 8|The PTB-ASO prevented apoptosis of injured spinal cord cells. (A) Twelve weeks after SCI, PTB-ASO-injected mice had fewer TUNEL+ cells than control ASO-injected mice around the injured area (n = 3 mice; four sections per mouse). Scale bars: 200 µm. In each group, the lower panels are magnifications of the white dashed box areas in the upper panels to improve clarity. (B) Quantitative analysis of the percentage of TUNEL+ cells. (C) The mRNA expression levels of caspase-3 and caspase-9 in the injured spinal cord of PTB-ASO-injected mice were lower than those of control ASO-injected mice. The data are shown as the means ± SEM (n = 3 mice/group). †P < 0.05, vs. control ASO (two-tailed Student’s t-test). ASO: Antisense oligonucleotide; PTB: polypyrimidine tract-binding protein; SCI: spinal cord injury; TUNEL: TdT-mediated dUTP nick-end labeling.

Figure 9|PTB-ASOs facilitates motor function recovery in SCI mice. After PTB-ASO injection in mice, motor function recovery following SCI was better than that in the control ASO group from 5 to 12 weeks. Motor function recovery was assessed using the BMS score (A) and swim test (B) weekly after SCI. (C) In the inclined plate test, PTB-ASO-treated mice reached a higher height than control ASO-treated mice at twelve weeks post-SCI. (D) The cold allodynia and (E) hot plate tests were used to detect sensory function recovery and indicated that there were no marked differences between the control ASO and PTB-ASO groups. The data are shown as the means ± SEM (n = 8 mice/group). †P < 0.05, ††P < 0.01, vs. control ASO (repeated measures twoway analysis of variance followed by Tukey’s post hoc test). ASO: Antisense oligonucleotide; BI: baseline; PTB: polypyrimidine tract-binding protein; SCI: spinal cord injury.
Discussion
SCI is a serious neurological disorder with the pathological characteristics of fibrotic fibroblast-mediated cavitation, astrogliosis-mediated glial scar formation around the cavity, and loss of major motor neurons, which ultimately causes failure of axonal regeneration in the injured area. At present, research on SCI repair mainly focuses on inhibiting astroglial-fibrous scar formation, supplementing neurons, and improving nerve regeneration. However, many therapeutic approaches, such as signal transducer and activator of transcription 3 knockout, leads to the exhaustive ablation of the glial scar, which results in poor functional recovery after SCI (Anderson et al., 2016; Sofroniew, 2018; Gu et al., 2019). Moreover, many approaches that replenish neurons around the injured area do not achieve satisfactory functional recovery after SCI (Huang et al., 2020; Yang et al., 2020b). Thus, the identification of efficacious therapeutic approaches for SCI is an extremely urgent need. As mentioned earlier, PTB can regulate the inclusion or skipping of tau exon 10, which leads to neuronal vulnerability (Roussarie et al., 2020). In addition, PTB has an important effect on neuronal induction, and its expression level is naturally reduced during neurogenesis (Hu et al., 2018). Our current study is the first to explore the function of silencing PTB in SCI.
As previously described, the glial scar has dual effects on SCI repair. An appropriate method to treat the glial scar needs to be discovered to alleviate the adverse effects of the scar while supporting its favorable role in SCI repair. Our previous studies confirmed that 2 weeks post-SCI is a suitable time point for manipulating the glial scar because the formation of the scar is almost complete by this time after injury (Yang et al., 2020b). Because gene therapies may take additional days to stably alter gene expression, we postponed thein situ
injection for 7 days after SCI. Thus, the effects of gene therapy began around 2 weeks after SCI, which coincided precisely with the appropriate time point to manipulate the glial scar. In our study, we found that PTB knockdown modestly reduced the density of the glial scar and maintained the structure of the scar after SCI in contrast to exhaustive scar ablation.Somatic cell reprogramming is an alternative to cell replacement therapy and shows great potential in SCI repair. As some previous studies have shown, spinal astrocytes can be reprogrammed into neurons. Our team previously revealed that the SOX2 TF can reprogram glial scar-forming astrocytes into neurons in a mouse model of SCI (Yang et al., 2020b). More recently, activation of the endogenous neuron-related genesNgn2
andIsl1
was shown to reprogram astrocytes into motor neurons in the spinal cord of adult mice (Zhou et al., 2021). The proneural ASCL1 TF reprograms spinal astrocytes into neuronsin vitro
(Kempf et al., 2021). TheNgn2
gene reprograms spinal astrocytes into neurons bothin vitro
andin vivo
(Kempf et al., 2021; Liu et al., 2021). The proneural OCT4 and KLF4 TFs can facilitate motor recovery in a mouse model of SCI, and combinations of TFs can reprogram astrocytes into neural stem cell-like cellsin vitro
(Huang et al., 2020).Neurod1
induces resident astrocytes to undergo neuronal reprogramming after SCI (Puls et al., 2020). In SCI, the combination of SOX2 and valproic acid can reprogram astrocytes to neurons (Su et al., 2014). However, to date, onlyNgn2
andIsl1
have been found to reprogram spinal astrocytes into motor neurons, while most other strategies result in astrocyte-derived glutamatergic, GABAergic, or mature neurons. Thus, the identification of new key TFs or genes involved in motor neuron replenishment is expected to be a new breakthrough.PTB participates in the regulation of RNAs involved in neural differentiation. Neurons have a positive RE1-silencing transcription factor (REST)/miR-124 signaling loop related to neural induction. In this loop, miR-124 efficiently inhibits the expression of non-neural genes (including REST). However, in non-neural cells, PTB expression induces a negative feedback REST/miR-124 loop in which REST downregulates the expression of many neural-specific TFs and miR-124 (Xue et al., 2013; Hu et al., 2018). Thus, the regulation of PTB plays a key role in the cell fate decision of the neuronal lineage. Various cells, including MEFs, N2A, NT2, and ARPE19 cells, can be gradually reprogrammed into functional neurons via activation of the REST/miR-124 loop induced by PTB knockdown (Xue et al., 2013). Notably, astrocytes have the same regulated signaling loop for neuronal maturation as neurons; thus, PTB knockdown alone leads to the activation of a positive REST/miR-124 feedback loop (Qian et al., 2020). This characteristic increases the feasibility of one-step reprogramming of astrocytes into neurons by reducing PTB expression. Qian et al. (2020) confirmed that a single one-step strategy that suppressed PTB significantly induced reprogramming of midbrain astrocytes into functional DA neuronsin vitro
. In a mouse model of Parkinson’s disease, after PTB knockdown for 3 months, this strategy improved motor function by not only replenishing DA neurons but also by rebuilding the striatal dopamine circuit (Qian et al., 2020). Maimon et al. (2021) demonstrated that ASO-mediated PTB knockdown reprogrammed astrocytes into neuronsin vitro
. In aging mice, this PTB-ASO replenished neurons in the brain and modified behavioral dysfunction (Maimon et al., 2021). Moreover, many studies have reported that the region-specific status of astrocytes strongly affects the cell conversion process. Astrocytes from different regions have different transcriptional and proteomic environments and, potentially, region-specific neural TFs that lead to conversion into different subtype-specific neurons (Mattugini et al., 2019; Herrero-Navarro et al., 2021; Kempf et al., 2021). The property of regionalization is maintained during astrocyte-to-neuron conversion bothin vitro
andin vivo
. Because the spinal cord contains an abundance of motor neurons, we successfully reprogrammed reactive spinal astrocytes into motoneuron-like cells through PTB knockdownin vitro
. Interestingly, a challenge has recently been raised in the field (Wang et al., 2021), where PTB knockdown failed to reprogram brain astrocytes into neuronsin vivo
over a relatively short period of time, and endogenous neurons were the source of the presumed astrocyte-derived neurons. Some scholars believe that astrocytic reprogramming is a long and gradual developmental process that requires a relatively long time to be observedin vivo
, and the conversion rate may be related to the appropriate virus concentration and type. In our mouse model of compression-induced SCI, we found that PTB silencing for approximately 3 months not only reduced the density of the glial scar without disrupting its overall structure but also replenished motoneuronlike cells around the injured area and decreased apoptotic cell death in the injured spinal cord, which ultimately led to the promotion of motor function recovery. Based on the present studies (Qian et al., 2020; Wang et al., 2021), we speculate that the supplemented motoneuron-like cells may either be induced by astrocytic reprogramming, originate from endogenous neurons because of the neuronal protective effect of PTB knockdown, or are derived from PTB knockdown-mediated residual neural stem cell differentiation. Thus, the origin of the supplemented motoneuron-like cells in SCI mice after PTB silencing requires confirmation by further studies.In this study, we used AAVs and ASOs as gene therapeutics to target Ptbp1. We are the first to use an shPTB and a PTB-ASO to reprogram spinal astrocytes into motoneuron-like cellsin vitro
. In our study, AAV-mediated delivery of shPTB and ASO-mediated knockdown of PTB were shown for the first time to replenish motoneuron-like cells around the injured area after SCI. Moreover, after SCI, the PTB-ASO replenished the motoneuron-like cells in the lesion area, decreased apoptotic cell death in the injured spinal cord, and modestly reduced the density of glial scar without destroying its overall structure. The use of AAVs as viral vectors for gene delivery is clinically feasible because of their minimal immunogenicity, wealth of engineered tissue-specific serotypes, and minimal insertion mutagenesis rate (Wu et al., 2006). Recently, some AAV-mediated clinical gene therapeutic approaches have been verified, such as GlaxoSmithKline’s severe combined immunodeficiency therapy (Naldini, 2015). AAVs are a better choice than other viral vectors for shRNA deliveryin vivo
. Recombinant AAVs provide rapid and steady expression within 1 week (Mason et al., 2010). The formation of glial scars almost completely ceased 2 weeks post-SCI, and, thus, 1 week post-SCI is a better time point for injection of AAVs into the injured area. AAVmediated shRNA expression can be sustained for months or even yearsin vivo
with low toxicity (Aguiar et al., 2017). Moreover, in 2016, an ASO gene technology for spinal muscular atrophy therapy was approved by the U.S. Food and Drug Administration (Tosolini and Sleigh, 2017), which contributes to gene therapy of motor neurons. ASOs are synthetic chemically modified single strands of 15–25 nucleotides that efficiently reduce the expression level of target genes by binding to specific sequences of pre-mRNA or mRNA (Amado and Davidson, 2021). When injected into the central nervous system, ASOs can be distributed widely (Rigo et al., 2014; Tosolini and Sleigh, 2017). Moreover, ASOs have drug-like features and are easy to regulate (Kordasiewicz et al., 2012), and manufacturing of ASOs is simple. All of these properties make ASOs suitable for therapy of some medical diseases. However, ASOs have more off-target effects and cause more inflammatory reactions and toxicity than AAVs (Rao et al., 2009). Although both AAV-mediated shRNA and ASOs are reported to knock down specific genes efficiently, the type of strategy must be carefully selected to improve the therapeutic effect. The determination of efficacy differences between these two strategies for SCI repair will require further investigations.In summary, we found that PTB knockdown replenished motoneuron-like cells around the injured area, decreased apoptotic cell death in the injured spinal cord, and moderately reduced the density of the glial scar without disrupting its overall structure, thereby facilitating motor function recovery after SCI. In addition, a limitation of this approach was that the functional recovery extended only to improved motor ability but did not include improvement in sensory perception. As a result, further improvements of this therapeutic approach will be required. The serious consequences of SCI are a result of many factors. Future studies should consider strategies for SCI repair from multiple aspects, such as combining PTB knockdown strategies with other engineering techniques, with the aim of further enhancing functional recovery after SCI.
Author contributions:
Study design, and manuscript draft: GC, RYY; experiment implementation: RYY, RC, JYP, JYB, PHX, YKW, YC, YL, JW. All authors contributed to the manuscript revision, read, and approved the the final version of the manuscript.
Conflicts of interest:
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:
Lukas Grassner, Trauma Center Murnau, Germany.
Additional files:
Schematic diagram of pAAV-GFAP-EGFP-MIR155(Ptbp1) (left) and pClenti-GFAP-shRNA(Ptbp1)-CMV-EGFP-WPRE.
Establishment of a cell model of mouse reactive spinal astrocytes.
Localization of neural stem cells in the injured spinal cord of mice.
- 中国神经再生研究(英文版)的其它文章
- c-Abl kinase at the crossroads of healthy synaptic remodeling and synaptic dysfunction in neurodegenerative diseases
- The mechanism and relevant mediators associated with neuronal apoptosis and potential therapeutic targets in subarachnoid hemorrhage
- Microglia depletion as a therapeutic strategy: friend or foe in multiple sclerosis models?
- Brain and spinal cord trauma: what we know about the therapeutic potential of insulin growth factor 1 gene therapy
- Functions and mechanisms of cytosolic phospholipase A2 in central nervous system trauma
- Cre-recombinase systems for induction of neuronspecific knockout models: a guide for biomedical researchers

