Identification of novel biomarkers and therapeutic target candidates for stasis-heat symptom pattern of acute intracerebral hemorrhage by quantitative plasma proteomics
WEI Lexin,LI Weiyi,TIAN Ting,ZHANG Ning,YANG Shijing,YANG Dongqing,LI Guochun,YE Fang
WEI Lexin,LI Weiyi,ZHANG Ning,YANG Shijing,YANG Dongqing,LI Guochun,Department of Public Health,School of Medicine and Holistic Integrative Medicine,Nanjing University of Chinese Medicine,Nanjing 210023,China
TIAN Ting,Emergency Department,Nanjing Hospital of Chinese Medicine affiliated to Nanjing University of Chinese Medicine,Nanjing 210001,China
YE Fang,the Key Laboratory Department of Stasis-heat Pathogenesis of Traditional Chinese Medicine,the First Clinical Medical College,Nanjing University of Chinese Medicine,Nanjing 210001,China
Abstract OBJECTIVE:To explore the novel biomarkers and therapeutic target candidates related to the stasis-heat syndrome of acute intracerebral hemorrhage (AICH).METHODS:Applying an isobaric tagging for relative and absolute quantitation-(iTRAQ-) based quantitative proteomic approach,plasma samples from AICH patients with stasis-heat,and AICH patients with non-stasis-heat and healthy control subjects were collected and analyzed to distinguish differentially expressed proteins (DEPs)correlated to AICH with stasis-heat in this block design.The standard Western blot was applied to verify DEPs.Additionally,DEPs were analyzed via bioinformatic platforms and further approved via Ingenuity Pathway Analysis (IPA). RESULTS:A total of 26 DEPs were found among AICH with the stasis-heat,AICH with non-stasis-heat,and healthy control group.The seven DEPs compared with the non-stasis-heat group are closely related to the pathogenesis of stasis heat.These proteins showed three different protein expression patterns.The alpha-1-b glycoprotein (A1BG) and copper-protein (CP) were upregulated in the stasis-heat group,but down-regulated in the non-stasis-heat group.Compared with the non-stasisheat group,the expression abundance of actinin,alpha 1(ACTN1),carbonic anhydrase I (CA1),peroxiredoxin 2(PRDX2),and vinculin (VCL) is higher in the stasis-heat group,while the CD44 is the opposite.These differences reflect that stasis-heat syndrome has more severe inflammatory immune response,coagulation disorders and damage.Bioinformatics analysis revealed that a wide variety of cellular and metabolic processes and some signaling pathways were involved in the pathophysiology of AICH with stasis-heat.AICH with stasis-heat syndrome showed more severe inflammatory reactions,tissue damage,and coagulation disorders than non-stasis heat syndrome.CONCLUSIONS:There are differences in the protein expression patterns between the stasis-heat syndrome and non-stasis-heat syndrome.These differences reflect that stasis-heat syndrome has more severe damage.CD44,CP,ACTN1,CA1,VCL,PRDX2,and A1BG could be the potential biomarkers and therapeutic target candidates of the stasis-heat subtype.This study provides a reasonable explaination for Liangxue Tongyu decoction through anti-inflammatory and brain protection treatment.
Keywords:cerebral hemorrhage;stasis-heat syndrome;biomarkers;therapeutic target;quantitative plasma proteomics
1.INTRODUCTION
Spontaneous,nontraumatic intracerebral hemorrhage(ICH),accounting for approximately 20% to 35% of all strokes in China (only 10% to 20% in western countries),is a significant cause of morbidity and mortality throughout the world.1,2Although a breakthrough in the treatment of stroke has been made over the past decade,ICH remains a devastating disease and current treatment options lag far behind those for ischemic stroke treated by antithrombotic drugs and mechanical intravascular thrombectomy.3Numerous candidate neuroprotectants or targets for ICH showed beneficial effects in animal experimental stroke studies,however,none of these agents were tested successfully in phase III clinical trials.4Current ‘one drug–one target–one disease’approaches in drug discovery have become increasingly inefficient for the high unmet medical need indication stroke.Network pharmacology defines disease mechanisms as networks best targeted by multiple,synergistic drugs.Its idea originated from the formula treatment of Traditional Chinese Medicine (TCM).5,6
Now more evidences show that TCM is a very promising alternative therapy for AICH.Although there are many herbal remedies for ICH,Liangxue Tongyu prescription,coming from the experience of famous Chinese physicians named Zhou Zhongying,has received great attention.7This prescription is guided from the pathogenesis of stasis-heat which is a therapeutic target of the syndrome.8-10Professor Zhou found the stasis-heat,indicating the severity and prognosis of the disease,is the key pathogenic unit for AICH.11
LTP has been used as combination therapy for AICH in China,and can improve the clinical efficacy.Randomized controlled trials have shown that LTP can significantly improve the curative effect and outcomes for AICH,the total effective rate was 88.0%.12The systematic review also indicates that LTP combined with western conventional medicine has some benefits in treating AICH.7“Stasis-heat” is the main pathogenesis and key targets of LTP.13Research on basic mechanisms of “stasis-heat” is crucial for future advancement,for it helps reveal the pahtgenesis and the molecular targets of LTP.8Our previous clinical trials indicated that the treatment targets of stasis-heat pathogenesis named cooling blood and promoting blood circulation for removing blood stasis can reduce mortality and improve the prognosis of patients with AICH,14however,biomarkers of stasis-heat and underlying molecular mechanisms of efficacy are unknown,which may be explained by systems biology that benefits from the rapid development of multi-omics.
In this study,iTRAQ liquid chromatography tandemmass spectrometry (LC-MS/MS) proteomics was used to detect plasma differential proteins as potential biomarkers of stasis-heat for AICH.We conducted a comparative proteomics study among patients with heatstasis,non-stasis-heat,and healthy people to explore possible mechanisms and potential therapeutic targets involved in stasis-heat of AICH.Furthermore,via the Western blotting,we verified several dysregulated proteins as they were able to distinguish the stasis-heat group with non-stasis-heat group,which may act as biomarkers of stasis-heat or trerapeutic targets.
2.METHODS
2.1.Ethics statement
Institutional Review Board and Ethics Committee of Nanjing University of Chinese Medicine approved this study which was performed in compliance with the Declaration of Helsinki (grant No.2014 NL-037-02).Written informed consent was given by all participates or legal guardians.
2.2.Study design and subjects
To control the confounding factors,8 patients and 4 healthy controls (HC) were invited to participate in this study according to a 2:1 matching design of gender and age.Among them,AICH patients were divided into two subtypes according to the pathogenesis theory of Traditional Chinese Medicine:4 AICH patients with stasis-heat (SH) and 4 patients AICH with non-stasisheat (nonSH).These patients have similar bleeding sites.Three individuals (the patient with SH or nonSH,and HC)in each block met inclusion and exclusion criteria for the corresponding group (Table 1).The inclusion criteria applied to the patients of the study were as follows:Patients who have participated in this study consistent with a diagnosis of AICH by computed tomography or magnetic resonance imaging,admitted within 48 hours of stroke onset,and willing and able to timely be followed up for further survey according to the requirements of the program.The intracerebral hematoma volume was measured according to the ABC/2 formula:V (mL)=length (cm) × width (cm) ×π/6 × number of layers.15Patients were excluded if they were complicated by transient cerebral ischemia,brain infarction,or subarachnoid hemorrhage. Also,participants were excluded if their cerebral hemorrhage resulted from blood diseases,tumors,or trauma;patients who died within 24 h after admission or merged with serious primary diseases would be excluded as well.If patients could not cooperate with the examination due to disabilities included in the legislative framework,they were also excluded.At the same time,patients were classified by the diagnosis of TCM into two subtypes:12SH and nonSH.Besides,the syndrome of stasis-heat was further defined by the assessment of the scale of symptom scores for stasis-heat,such as vitality,fever,face and lip color,symptoms of the abdomen,abnormalities of stool,the tongue coating,and body,mouthfeel,pulse condition.This scale is shown in Table S10 in the supplementary file 3.The patient was diagnosed as stasis-heat syndrome when the scale of stasis-heat was assessed to be greater than or equal to 10 points.16
2.3.Sample collection and preparation
The fasting blood samples were collected from 8 ICH patients on admission at Affiliated Hospital of Jiangsu University and Affiliated Hospital of Nanjing University of Chinese Medicine (Jiangsu Province Hospital of Chinese Medicine) in China.At the same time,the other 4 blood specimens were collected from subjects of the healthy control group.Also,whole blood (2 mL) was collected from study subjects.Using K2-EDTA as the anti-coagulant agent,a total of 12 plasma samples were collected.Then we followed the methods of Liet al17to implement the preparation.
2.4.iTRAQ-based quantitative proteomics
Proteomic measurements were conducted according to our previously published article.17Samples from four three groups and a reference sample using the internal standard method,which is a mixture of plasma samples from three subjects in order to quantify protein relative to each group on the same baseline,were marked by fourchannel isobaric tagging reagents (iTRAQ,AB Sciex LLC,Framingham,MA,USA) and analyzed by strong cation-exchange fractionation,followed by reversephase liquid chromatography,which was online to a QStar XL quadrupole time-of-flight mass spectrometer.MaxQuant (vers 1.2.2.5,Computational Systems Biochemistry,Berlin,Germany) were used to identify and quantify proteins.The ratios of relative protein abundance between two groups were calculated by intensities of tandem mass tags (TMT) reagent reporter ion from HCD spectra.
2.5.Statistical analysis
SPSS 22.0 (IBM Corp.Released 2013.IBM SPSS Statistics for Windows,Version 22.0.Armonk,NY,USA) was adopted for all statistical analyses.Univariate analysis of variance (ANOVA) (two-way analysis of variance with block design) was used to compare the protein abundance among three groups.Once this test showed that differences exist among the means,Bonferroni for post hoc range tests and pairwise multiple comparisons was selected to determine which means differ.Protein abundance of the HC or nonSH was used for a reference to calculate the fold-change;changes of 1.2 or higher and theP-value less than or equal to 0.05 were considered statistically significant.
2.6.Bioinformatics analysis
Ingenuity Pathway Analysis (IPA,QIAGEN,Redwood,CA,USA) was used to analyze and integrate canonical pathways and to further interpret the biological significance of differentially expressed plasma proteins.To determine the significance of each canonical pathway,Fisher’s exact test was used to calculateP-value which would be statistically significant ifP-value ≤ 0.05.Diseases,functional protein networks,and upstream regulator analysis with differently expressed proteins were presented by Z-score.The Z-score ≥ 2 or ≤ -2 was considered as significant activation or significant inhibition respectively.The function annotation of these expressed proteins was assessed by PANTHER (vers 13.1,Thomas lab,LosAngeles,CA,USA) with Fisher’s exact test and false discovery rate (FDR) ≤ 0.05 for multiple test correction.18
2.7.Western blotting analyses
Western blot was used to examine and explore the protein expression levels in this studyviaa standard experimental method.Protein of SH (n=4) and nonSH(n=4) were separated from frozen tissues and the protein concentration was measured by bicinchoninic acid (BCA) assay kit (Nanjing Sai yan Biotechnology Co.Ltd.,Nanjing,China).The protein samples were subjected to 10% SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride(PVDF) membranes (Pall Corporation,New York City,NY,USA).The membranes were incubated either with the monoclonal antibodies (Abcam,Boston,MA,USA)of anti-A1BG,anti-ACTN1,anti-CA1,anti-CD44,anti-CP,anti-PRDX2,or anti-VCL.Then these membranes were incubated with the appropriate HRP-conjugated secondary antibodies (1∶2500) and then visualized with an ECL kit (Thermo Fisher Scientific,Waltham,MA,USA).
3.RESULTS
3.1.Characteristics and relative quantification of plasma proteins of the subjects
Twelve subjects were screened 4 blocks from 190 AICH patients and 62 healthy controls,and each matched with AICH (the patient with SHor nonSH) and a HC by demographical characteristics. The experimental workflow of research is illustrated in Figure 1.Among the 12 participants that 50% were male,the mean age of the SH was 63.5 with a standard deviation (SD) of 5.5 years,63.5 (SD 4.1) years in the nonSH and 63.8 (SD 5.0)years inHC.The mean ICH volume of the SH was 28.0 mL with a standard deviation (SD) of 17.2 mL;27.6 mL(SD 25.4 mL) in nonSH.The mean score of the stasisheat scale was 15.5 (SD 3.7) for patients of the SH,whereas it was only 7.3 (SD 2.4) in nonSH (Table 1).Based on reporter ions with iTRAQ,411 proteins and 3165 peptides were quantified.414 proteins and 3886 peptides were identified in total.
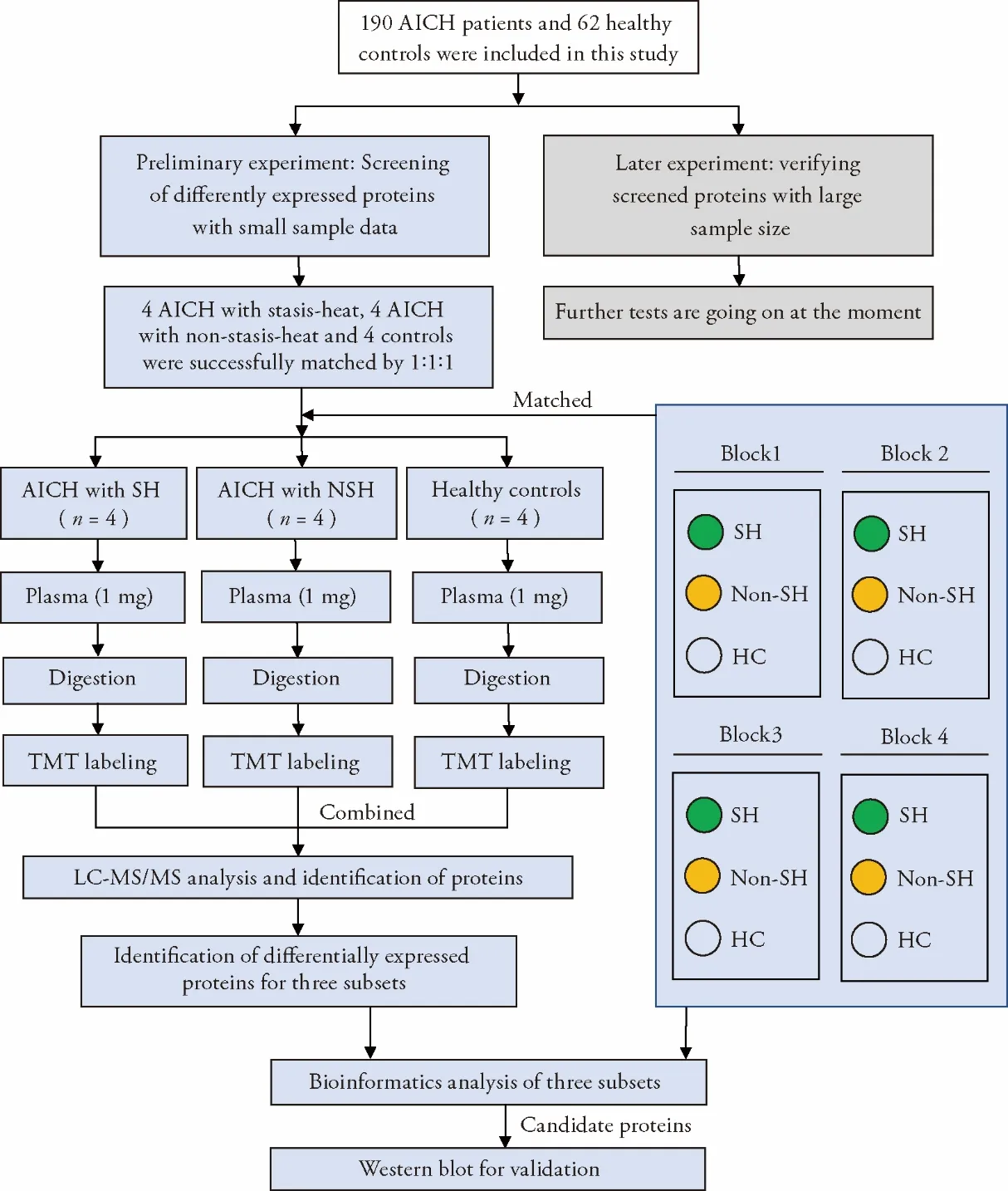
Figure 1 Experimental profile
3.2.Identification and analysis of dysregulated proteins in three subsets
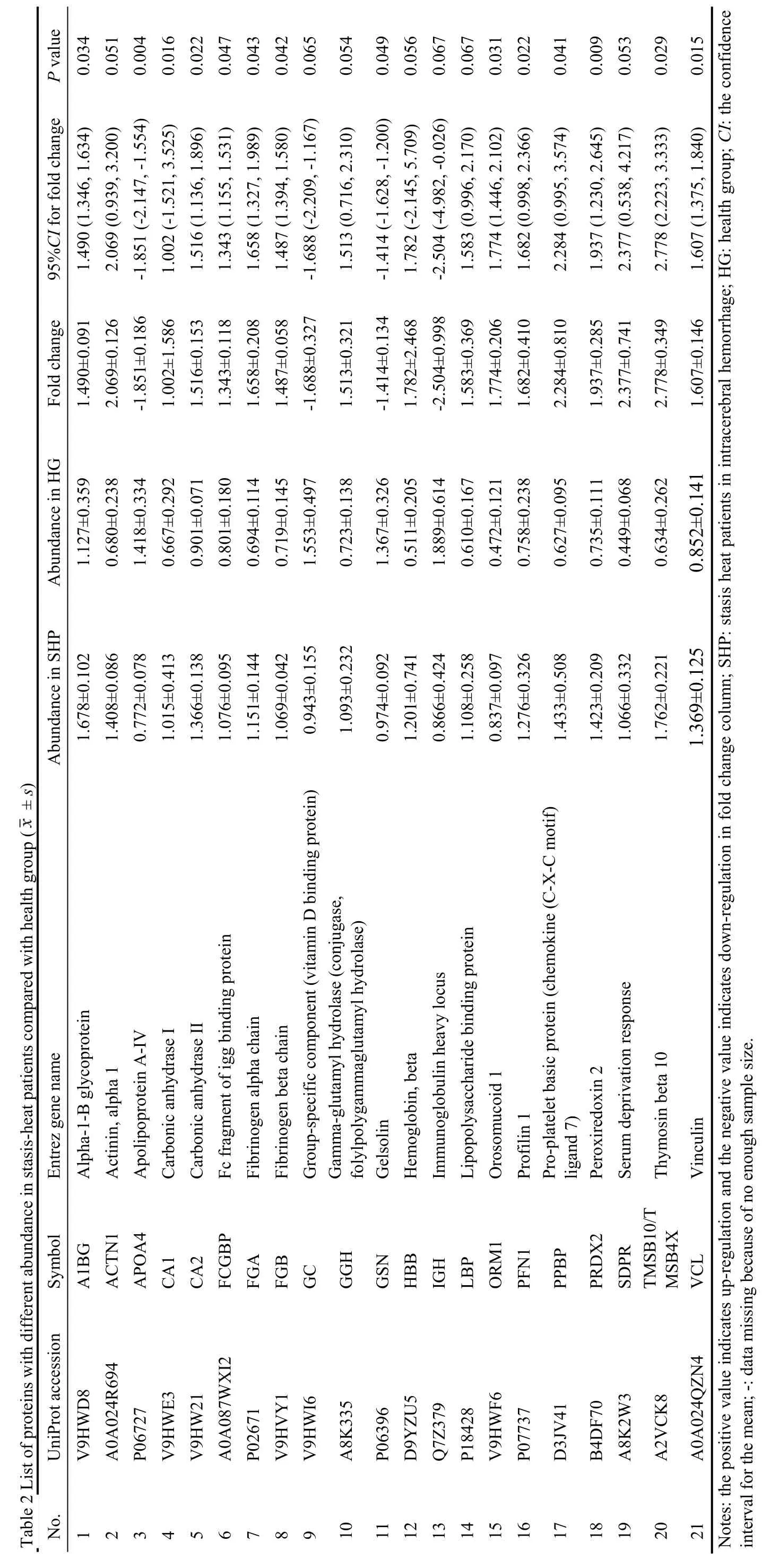
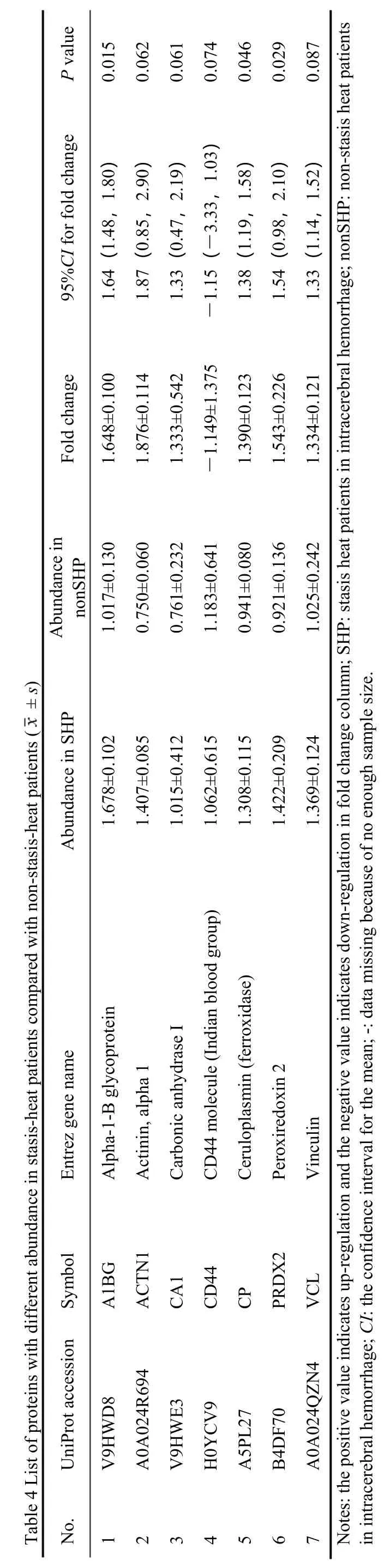
ANOVA was utilized to screen significant proteins according to the abundance of about 411 proteins in the plasma of every participant.Of all proteins,30 proteins were detected to be statistically different among SH,nonSH,and HC.Bonferroni test for the paired sample was applied to determine DEPs,which means the difference among three subsets:21 proteins between SH and HC (Table 2),11 proteins between nonSH and HC(Table 3),and 7 proteins between SH and nonSH (Table 4).
3.3.Differential proteins between stasis-heat patients and health group
Based on the standards for identifying significantly different proteins between different groups,21 DEPs were found in the SH compared with the HC (Table 2).
These proteins were parsed into PANTHER functional classes and GO terms.The major PANTHER protein classes are distributed in signaling molecule (representing 9.5% of annotated hits),cytoskeletal protein(9.5%),and enzyme modulator (9.5%) (Supplementary 1 Tables S1 and S2).
According to IPA,the 21 DEPs were involved in 31 canonical pathways and 2 significant protein interaction networks.IPA analysis revealed that 6 proteins (PFN1,VCL,LBP,GSN,TMSB10/TMSB4X,ACTN1) were in the positive actin cytoskeleton signaling pathway among the first five pathways:liver X receptor and retinoic acid X (LXR/RXR) activation (APOA4,ORM1,LBP,FGA,GC,A1BG),actin cytoskeleton signaling(PFN1,VCL,LBP,GSN,TMSB10/TMSB4X,ACTN1),FXR/RXR activation (APOA4,ORM1,FGA,GC,A1BG),acute phase response signaling (ORM1,FGB,LBP,FGA) and extrinsic prothrombin activation pathway (FGB,FGA).These proteins were also associated with two functional networks:one was involved in cellular assembly and organization,cellular function and maintenance,protein trafficking(A1BG,ACTN1,APOA4,FGA,FGB,GC,GSN,HBB,LBP,ORM1,PFN1,PRDX2,SDPR,TMSB10/TMSB4X,VCL),and the other was in connective tissue disorders,hematological disease,and hereditary disorder(ACTN1,CA1,CA2,FCGBP,GGH,IGH,ORM1).The molecules in the network and the score of network clustering were reported in Supplementary 1 Table S3.
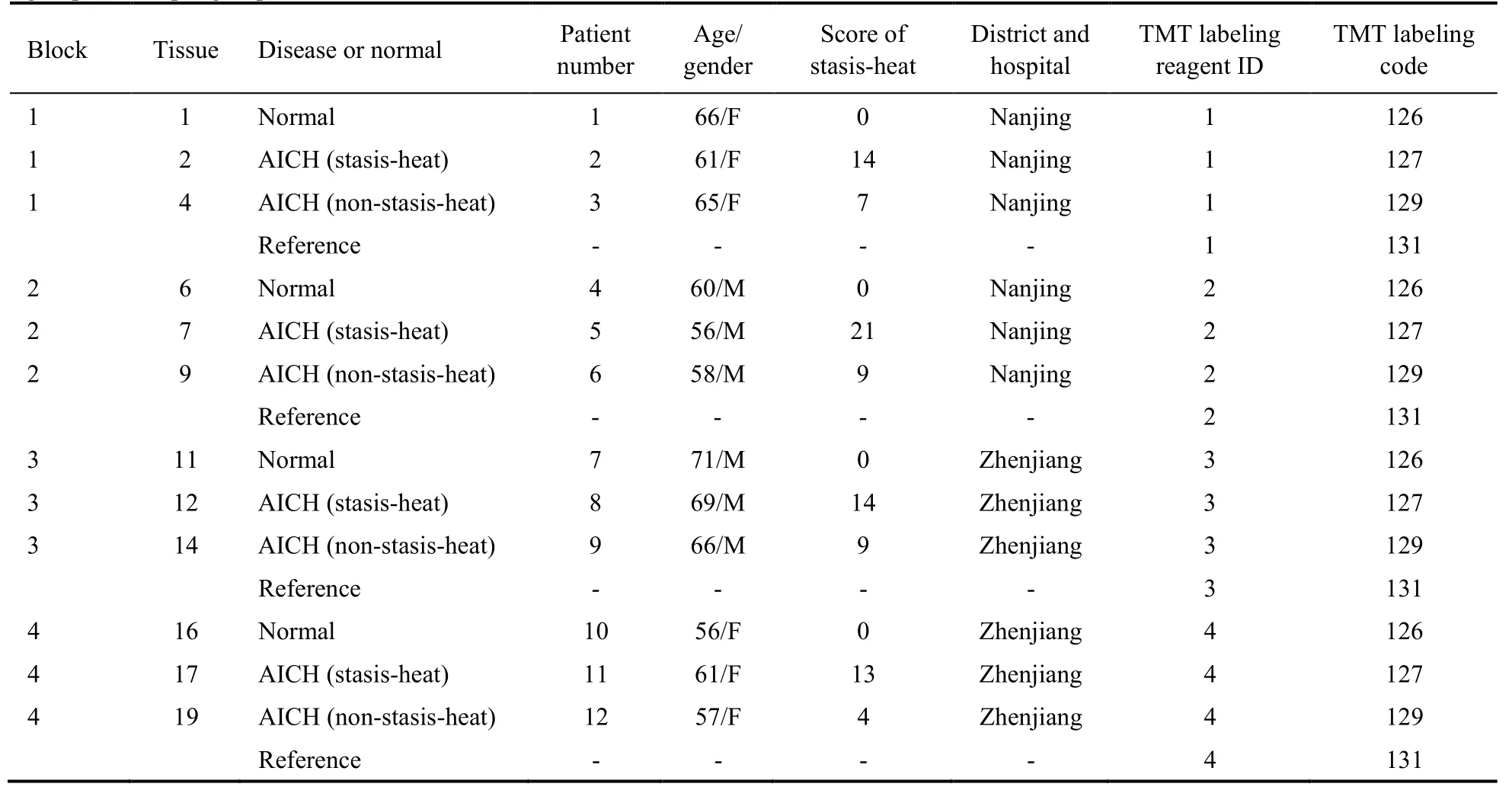
Table 1 Experimental protocol and basic characteristics in AICH patients with stasis-heat,AICH patients with non-stasis-heat,and health controls groups (n=4 per group)
3.4.Differential proteins between non-stasis-heat patients and health group
We found 11 DEPs in the nonSH compared to HC,which were mainly related to metabolism (Table 3,Supplementary 1 Tables S4 and S5).Overexpressed proteins FGA and FGB are involved in a pathway that launches plasminogen activating cascade,while underexpressed GSN is involved in FAS signaling pathway.The 23 canonical pathways and 1 significant protein interaction network were found from 11 differential proteins by IPA.Four proteins (APOA4,ORM1,LBP,GC) were in the negative LXR/RXR activation signaling pathway among the first five pathways:LXR/RXR activation (APOA4,ORM1,LBP,GC),FXR/RXR activation (APOA4,ORM1,GC),atherosclerosis signaling (APOA4,ORM1),IL-12 signaling and production in macrophages (APOA4,ORM1),acute phase response signaling (ORM1,LBP).There were 10 proteins in the network (APOA4,CA2,CD44,COL14A1,CUL3,GC,GSN,IGH,LBP,ORM1)regulated by p38 Mitogen-activated protein kinase(P38MAPK),transformation-related protein 53 (TP53),as well as an amyloid precursor protein (APP).The molecules in the network and the score of network clustering were reported in Supplementary 1 Table S6.
3.5.Differential proteins between stasis-heat patients and non-stasis-heat patients
To explore the impact of stasis-heat underlying in the AICH,we compared the SH with the nonSH and 7 DEPs were identified.All of the 7 proteins were almost overexpressed (Table 4) except CD44.The CD44,related to cell-surface glycoprotein involved in cell-cell interactions,cell adhesion,and migration,were underexpression in the SH (Supplementary 1 Tables S7 and S8).The 23 canonical pathways and 1 significant protein interaction network were found from 11 differential proteins by IPA.According to Top Canonical Pathways,the first five significant pathways were:leukocyte extravasation signaling (CD44,VCL,ACTN1),regulation of cellular mechanics by calpain protease (VCL,ACTN1),remodeling of epithelial adherens junctions (VCL,ACTN1),VEGF signaling(VCL,ACTN1),paxillin signaling (VCL,ACTN1).There were 7 proteins in the network (A1BG,ACTN1,CA1,CD44,CP,PRDX2,VCL) regulated by Tumor Necrosis Factor) (TNF) and Interferon-gamma (IFNG).In this network,CD44 was shown to be a key signaling molecule (Figure 2).The molecules in the network and the score of network clustering were reported in Supplementary 1 Table S9.
3.6.Overlap and differently expressed pattern among three subsets
We compared the three sets of differential proteins and the results were shown in Figure 2.There are three different patterns for proteins between SH and nonSH(Figure 2).Compared with the HC,the fold change of VCL,PRDX2,CA1,and ACTN1 in the SH was greater than that in the nonSH,whereas CD44 was the inverse of the proteins mentioned above (Figure 2B).Furthermore,the CP and A1BG had the opposite pattern of expression that the two proteins were up-regulated in the SH and down-regulated in the nonSH compared with the HC.
3.7.Validation of DEPs by Western blotting
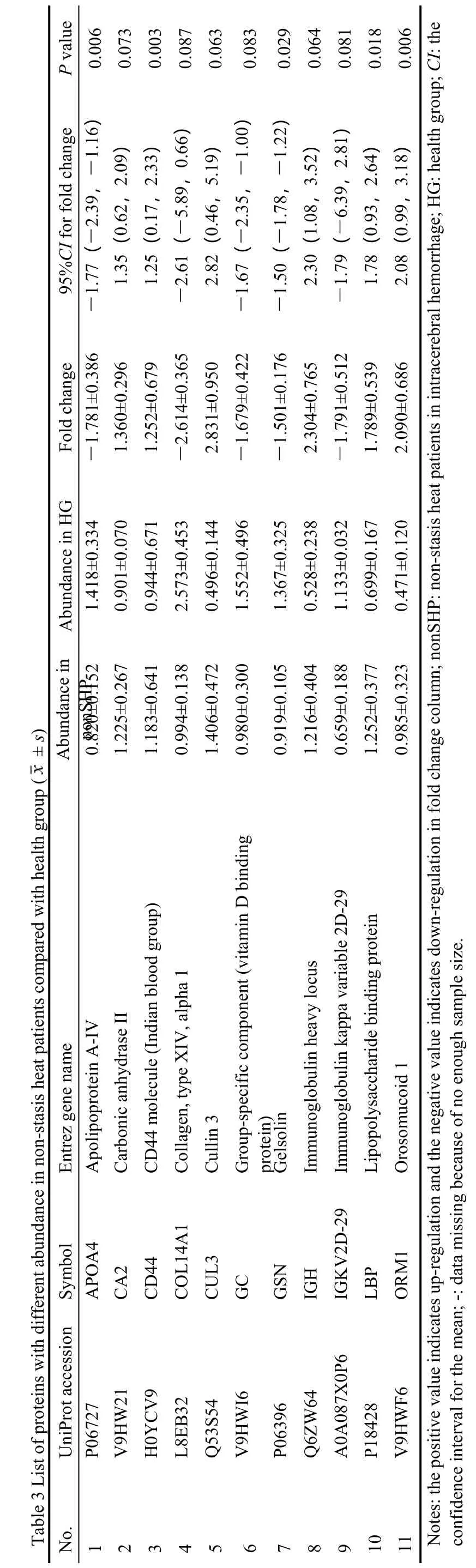
To reduce false-positive rates of differentially proteins between the SH and nonSH,the seven differential proteins (A1BG,ACTN1,CA1,CP,PRDX1,VCL1,and CD44) were verified by Western blotting.Con-sistent with iTRAQ prote-omics results,the intensity of bands of three proteins significantly changed in the plasma of AICH between subgroup (Figure 3,P<0.05).Although not all proteins have been verified,the SH seems to have a higher expression trend than the other groups in each protein.
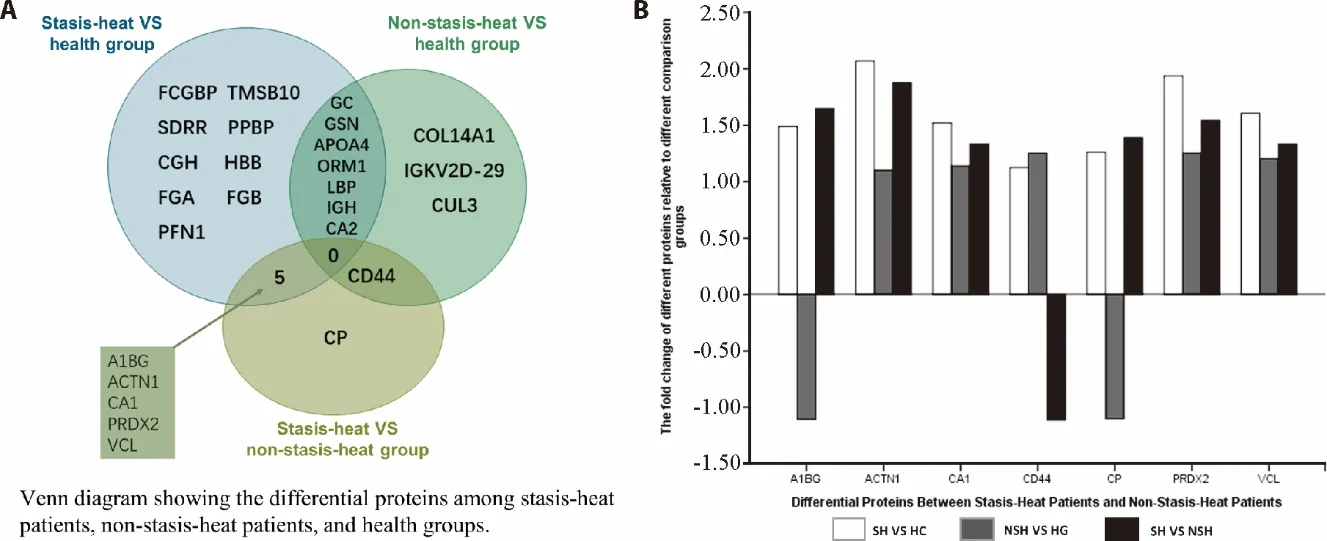
Figure 2 Comparison of differential proteins among stasis-heat patients,non-stasis-heat patients,and health groups
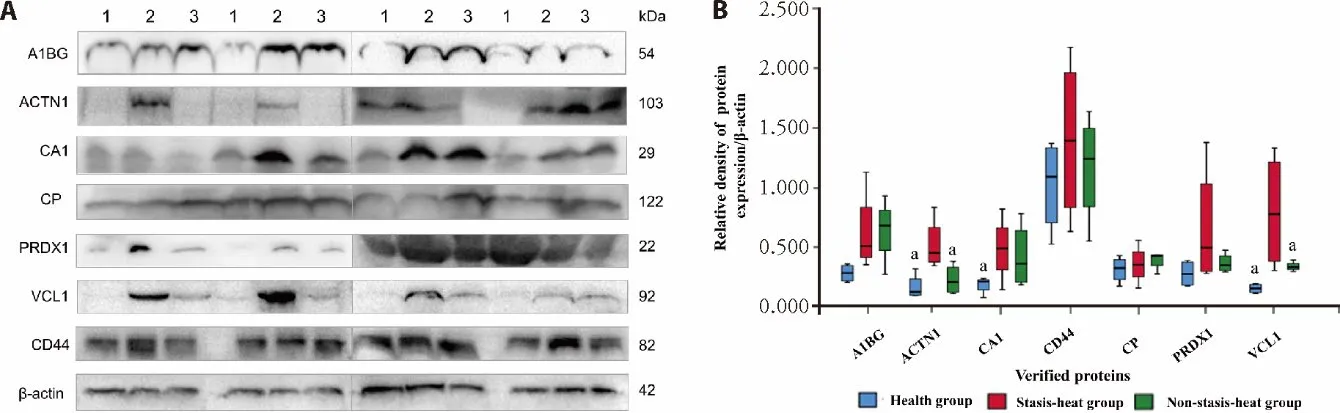
Figure 3 Western blotting analysis of A1BG,ACTN1,CA1,CP,PRDX1,VCL1,and CD44 from healthy controls,stasis-heat group,and nonstasis-heat group
4.DISCUSSION
This study revealed the potential biomarkers and therapeutic targets of stasisheat syndrome.We found that there are differences in the protein expression patterns between the stasisheat and non-statisis-heat syndrome.The A1BG and CP were up-regulated in the SH,but down-regulated in the nonSH.The expression abundance of ACTN1,CA1,PRDX2,and VCL is higher in the SH vs nonSH,while the CD44 is the opposite.The stasis-heat group indi-cated a higher expression trend than the other groups in each protein according to verification by Western Blotting.These differences maybe explain that stasisheat syndrome has more severe inflammatory dam-age.This provides a theor-etical basis for LTP through antiinflammatory and brain protection treatment.8-10
According to the important clinical contribution of Professor Zhou Zhongying,Stasis-heat syndrome is a key syndrome for the AICH and the severity of stasis-heat pathogenesis is posi-tively correlated with the severity of the disease.19,20Based on this,LTP can achieve promising therapeutic effects.8,9
For example,LTP can attenuate brain diseases like cerebral infarction and cerebral hemorrhage by antiinflammatory,10improving coagulation and anticoagulant and fibrinolytic system21and ameliorating brain edema.22LTP alleviated AICH partially by regulating the targets involved MAPK,calcium,apoptosis,and TNF signaling pathway and played antioxidation,anti-inflammatory,antiapoptosis and lowered blood pressure roles.10It can also decrease the brain edema and brain injury by inhibiting MMP-9 expression,up-regulating TIMP-1.22LTP can protect the injured brain by improving coagulation,anticoagulant and fibrinolytic system as well.21
The ‘stasis’ in TCM is a condition that there are blood clots within the vascular system and the blood flow was impeded.Also,‘stasis’ means blood clots or thrombus.The clinical symptoms of stasis include pain,fever,abnormal changes in the mucosa,tumors,and bleeding.The ‘heat’ is an etiology that causes diseases and pathogenesis in TCM.The clinical symptoms of heat involve hyperthermia,continued fever,remittent fever,low-grade fever,burning pain,dehydration,perspiration,and irritation.Stasis-heat is the depressed accumulated heat in the inner body,or the blood stasis transforming into heat,or vice versa.The common physiological and pathologic processes of stasis-heat include blood coagulation,vascular injury,inflammation,infection,and ischemia.In the review of Tianet al,13several biomarkers related to ‘heat’,‘stasis’ and ‘stasis-heat struggling and accumulation’ were illustrated.The most intuitive manifestation of heat syndrome is fever,as 91%of patients with cerebral hemorrhage have a fever,which is an important sign of the stasis-heat syndrome.23IL-1,IL-6,and TNF-α are common inflammatory factors and are also endogenous heat sources,which can cause fever.MMP-9 degrades and remodels the extracellular matrix of the brain basement membrane.24TIMP-1 is an inhibitor of MMP-9,which maintains the integrity of the cerebral vascular basement membrane.22Besides,the PGI2,NO,TXA2 and ET were correlated to the blood coagulation and fibrinolysis in ICH,which indicates the‘stasis’ condition.TNF-α,D-dimer,and IL-6 were related to the struggling and accumulation of ‘stasis-heat.
However,our study found that some new proteins are closely related to the syndrome of stasis-heat,and they reveal that the pathogenesis of heat-stasis has more severe acute-phase reactions and damage.They may be important biomarkers or therapeutic targets of the stasisheat syndrome.
Firstly,this study investigated the mechanisms of the proteins’ expression profiles induced by AICH.By comparing the proteomic profiles of 3 groups,several significant proteins are related to different subtypes of AICH CD44 promotes the remodeling of the endothelial pericellular matrix by associating with matrix metalloproteinases,and then promotes the formation of the endothelial tube.25The low expression of CD44 in the SH may imply the impairmentin vivoangiogenesis.ACTNs are actin-binding proteins.26VCL regulates both cell-cell and cell-matrix junctions and anchors adhesion complexes to the actin cytoskeleton through its interactions with the vinculin binding sites of ACTNs.
VCL binding to ACTN provides essential links for adhesion receptors with the actin cytoskeleton.27The overexpression of ACTNs and VCL in the SH were related to the migration of inflammatory cells and the hyperplasia of smooth muscles.28CA1 is a very early marker for erythroid differentiation and promotes retinal hemorrhage and erythrocyte lysis through prekallikrein activation.29It was highly expressed in the SH,which indicated more serious cell damage and bleeding. CP is a key protein involved in cell iron transport and also an acute phase reactant with several functions including ferroxidase activity,antioxidant functions and the prevention of the formation of free radicals,and inhibition of neutrophils myeloperoxidase.30Thrombin and iron are two major players in ICH-induced brain injury,and both can upregulate brain CP levels.31This protein showed different and opposite expression patterns in the two subtypes,which proved that the SH had more severe damage than the nonSH.A1BG is a member of the immunoglobulin superfamily.32BCL2L1 binding to A1BG may regulate the activities of A1BG through a signal transducer and activator of transcription 3 (STAT3).33STAT3 regulates the transcription of antiapoptotic proteins such as BCL-XL that control cell growth and survival.34,35This may imply that A1BG and BCL2L work together in the pathogenesis of the SH in AICH.PRDXs act as important regulators of redox signaling,but have also been implicated in inflammatory diseases.36PRDXs can induce the expression of inflammatory cytokines through the activation of tolllike receptors and are the key initiators of post-ischemic inflammation.37Furthermore,we investigated the pathways related to AICH with stasis-heat.Although in the analysis of Liet al,38several intriguing pathways correlated with ICH were provided,they did not examine the mechanisms of the sub-types of AICH,namely AICH with or without stasis-heat.Liver X receptor,retinoic acid X pathway,and FXR/RXR activation pathway are nuclear receptors signal transduction pathways associated with lipid metabolism,inflammatory regulation,and cholesterol metabolism.LXR and RXR might relate to lipid metabolism in the arterial wall.39FXR and RXR work together to keep the homeostasis of lipid. When the brain injury occurs,the cytokines were released into the blood flow by inflammatory cells,such as interleukins IL-1,IL-6,and TNF-α.The acute-phase response signal pathway is a key player in AICH,because it is involved in the production of cytokines and inflammatory mediators.40Atherosclerosis signaling includes inflammation responses leading to the release of cytokines,changes in vascular reactivity causing a release of endothelial cells,and control of vascular smooth cell proliferation via the release of growth factors and growth suppressor molecules.41Actin cytoskeleton signaling is connected with the shape,movement,division,secretion,and phagocytose of the cells.42This pathway involves vascular smooth muscle cells responding to extracellular signals.GTP-binding proteins,protein kinases,phosphoinositide kinases,and protein phosphatases invade cells through the signal transduction pathways that regulate actin polymerization.43This may indicate ‘stasis-heat’condition is associated with the activation of vascular smooth muscle cells and actin polymerization.The extrinsic prothrombin pathway is the shorter pathway of secondary hemostasis.Some thrombase was released when AICH occurs,then damages neuron and bloodbrain barrier through cytotoxicity,which is the primary reason for brain edema.44This pathway was only shown in the SH,which implies the hypercoagulability in this subtype.Leukocyte extravasation signaling,regulation of cellular mechanics by calpain protease,remodeling of epithelial adherent junctions,VEGF signaling,and paxillin signaling were closely linked to the differential proteins between the SH and nonSH.Leukocyte extravasation signaling is an inflammatory signaling pathway associated with the movement of leukocytes out of the circulatory system and towards the site of tissue damage or infection.Regulation of cellular mechanics by calpain protease is involved in the migration,movement,adhesion,differentiation,and apoptosis of cells,and many cardiovascular system diseases can be improved by inhibiting calpains.45Remodeling of epithelial adherent junctions participates in the reshaping of cell layers through the interactions of cadherins with the actin cytoskeleton,endocytosis,and recycling of cadherins.46
The VEGF signaling pathway involves in vascular development in the embryo and new blood vessel formation.The Paxillin signaling pathway participates in oxidative stress and inflammation.47These pathways all involve the upregulated proteins VCL and ACTN1,which means the stasis-heat is a complex disordered state that aggravates the disease,mainly involving cell movement,adhesion,inflammation,and coagulation disorders.
There are still some limitations in this study,which need to be further studied by increasing the sample size in the future.Other proteins like FCGBP,TMSB10,SDRR,PPBP,CGH,HBB,FGA,FGB,and PFN1 were shown in the SH compared with the HC group,which indicates they might be the biomarkers of AICH if the sample size is expanded.Proteins like GC,GSN,APOA4,ORM1,LBP,IGH,CA2 are shown in both the SH group and nonSH compared with the HC group,which implies they are the biomarkers of the disease for AICH.COL14A1,IGKV2D-29,and CUL3 are only associated with the nonSH,however,whether they are the biomarkers of AICH are still uncertain.We have verified three proteins(ACTN1,CA1 and VCL1),and other proteins show a certain trend of change.Maybe the sample size is not large enough,and further study are needed to verify these proteins in the future
In conclusion,our results demonstrated that these proteins are related to multiple pathways and biological processes,but in the inflammatory response and coagulation pathway for the most part.CD44,CP,ACTN1,CA1,VCL,PRDX2,and A1BG were found to have significant changes and were in key locations.
CD44,CP,ACTN1,CA1,VCL,PRDX2,and A1BG could be considered relevant as potential biomarkers and pattern of the stasis-heat subtype.Our study has some limitations.Although the sample size was small,which may reduce the statistical power,our study had discovered some interesting proteins that deeply disclose the pathogenesis of stasis-heat.Further studies still need to investigate the roles of these proteins in the pathogenesis of AICH and the relationship between these proteins and the efficacy of the LTP.
5.REFERENCES
1.Zhou M,Wang H,Zeng X,et al.Mortality,morbidity,and risk factors in china and its provinces,1990-2017:a systematic analysis for the global burden of disease study 2017.Lancet 2019;394:1145-58.
2.Wang Y,Li Z,Gu H,et al.Report on stroke prevention and treatment in China.Zhong Guo Zu Zhong Za Zhi 2020;15:1037-43.
3.Ma H,Campbell BCV,Parsons MW,et al.Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke.N Engl J Med 2019;380:1795-803.
4.O'Collins VE,Macleod MR,Donnan GA,Horky LL,van der Worp BH,Howells DW.1026 experimental treatments in acute stroke.Ann Neurol 2006;59:467-77.
5.Liu D,Liang J,Kang X,Ma S,Ding M.Molecule prescription:New generative point of science of medicine.Zi Ran Za Zhi 2006;2006:337-40.
6.Casas AI,Hassan AA,Larsen SJ,et al.From single drug targets to synergistic network pharmacology in ischemic stroke.Proc Natl Acad Sci USA 2019;116:7129-36.
7.Jiang C,Yang X,Dong J,Li G.Systematic review and Metaanalysis of randomized controlled trials of liangxue tongyu formula on patients with acute intracerebral hemorrhage.Front Pharmacol 2020;11:437.
8.Li X,Huang X,Tang Y,et al.Assessing the pharmacological and therapeutic efficacy of traditional chinese medicine liangxue tongyu prescription for intracerebral hemorrhagic stroke in neurological disease models.Front Pharmacol 2018;9:1169.
9.Guo Q,Yang S,Yang D,et al.Differential mrna expression combined with network pharmacology reveals network effects of liangxue tongyu prescription for acute intracerebral hemorrhagic rats.J Ethnopharmacol 2020;246:112231.
10.Chen Y,Dong J,Yang D,et al.Synergistic network pharmacology for traditional chinese medicine liangxue tongyu formula in acute intracerebral hemorrhagic stroke.Neural Plast 2021;2021:8874296.
11.Huang X,Li GC,Yin L,Zhang ZH,Liang YX,Chen HB.The effective parts of Liangxue Tongyu prescription on cooling-blood and activating-blood and analysis of chemical constituents by hplc-ms and gc-ms.Yao Xue Xue Bao 2015;50:86-93.
12.Guo W,Zhang L,Wu M,et al.Liangxue Tongyu Fang for treating acuter-phase cerebral hemorrhage in 168 cases.Beijing Zhong Yi Yao Da Xue Xue Bao.2012;35:603-6+19.
13.Tian T,Li G,Guo W.Research of biomarkers in the Yu-Re pathogenic unit of acute cerebral hemorrhage.Shi Zhen Guo Yi Guo Yao 2016;27:183-6.
14.Tian T,Guo W,Li G.Research on clinical manifestation of heat stagnation pathogenesis in acute cerebral hemorrhage.Zhong Yi Za Zhi 2016;57:838-42.
15.Xu X,Chen X,Zhang J,Xu B.Study on the accuracy and reliability of the abc/2 formula for volume assessment of intracerebral he⁃ matoma.Zhong Guo Shen Jing Jing Shen Ji Bing Za Zhi 2015;41:87-91.
16.Li G,Zhou X,Wang J,et al.Research on measuring scale for pathogen unit of hemorrhagic stroke due to heat-stasis.Nanjing Zhong Yi Yao Da Xue Xue Bao 2015;31:428-32.
17.Li GC,Zhang L,Yu M,et al.Identification of novel biomarker and therapeutic target candidates for acute intracerebral hemorrhage by quantitative plasma proteomics.Clin Proteomics 2017;14:14.
18.Mi H,Muruganujan A,Thomas PD.Panther in 2013:Modeling the evolution of gene function,and other gene attributes,in the context of phylogenetic trees.Nucleic Acids Res 2013;41:377-86.
19.Zhou Z.Research on the syndrome and treatment of intracerebral hemorrhage (haemostasis and heat syndrome).Zhong Yi Yao Xue Kan 2002;2002:709-11+23.
20.Zhou Z.Theory of stasis-heat.Nanjing Zhong Yi Yao Da Xue Xue Bao 2006;2006:273-6+331.
21.Jiang B,Tian L,Xu L.Effects of Liangxue Tongyu on hematological system and brain injury after cerebral ischemia in rats.Zhong Guo Lao Nian Xue Za Zhi 2015;35:134-6.
22.He C,Huang J,Wang W,et al.Effects of Liangxue Tongyu formula on brain edema and expressions of matrix metalloprooteinase-9 and tissue inhibitor of metalloproteinase-1 in rats with intracerebral hemorrhage.Zhong Xi Yi Jie He Xue Bao 2010;8:347-51.
23.Schwarz S,Hafner K,Aschoff A,Schwab S.Incidence and prognostic significance of fever following intracerebral hemorrhage.Neurology 2000;54:354-61.
24.Rosell A,Cuadrado E,Ortega-Aznar A,Hernandez-Guillamon M,Lo EH,Montaner J.Mmp-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type iv collagen degradation during hemorrhagic transformation after human ischemic stroke.Stroke 2008;39:1121-6.
25.Cao G,Savani RC,Fehrenbach M,et al.Involvement of endothelial cd44 duringin vivoangiogenesis.Am J Pathol 2006;169:325-36.
26.Winder SJ,Ayscough KR.Actin-binding proteins.J Cell Sci 2005;118:651-4.
27.Bois PR,O'Hara BP,Nietlispach D,Kirkpatrick J,Izard T.The vinculin binding sites of talin and alpha-actinin are sufficient to activate vinculin.J Biol Chem 2006;281:7228-36.
28.Shah DI,Singh M.Involvement of rho-kinase in experimental vascular endothelial dysfunction.Mol Cell Biochem 2006;283:191-9.
29.Liu X,Lu D,Bowser R,Liu J.Expression of carbonic anhydrase i in motor neurons and alterations in als.Int J Mol Sci 2016;17:1820.
30.Yang G,Hu R,Zhang C,et al.A combination of serum iron,ferritin and transferrin predicts outcome in patients with intracerebral hemorrhage.Sci Rep 2016;6:21970.
31.Liu H,Hua Y,Keep RF,Xi G.Brain ceruloplasmin expression after experimental intracerebral hemorrhage and protection against iron-induced brain injury.Transl Stroke Res 2019;10:112-9.
32.Tian M,Cui YZ,Song GH,et al.Proteomic analysis identifies MMP-9,DJ-1 and A1BG as overexpressed proteins in pancreatic juice from pancreatic ductal adenocarcinoma patients.BMC Cancer 2008;8:241.
33.Bai S,Liu S,Guo X,et al.Proteome analysis of biomarkers in the cerebrospinal fluid of neuromyelitis optica patients.Mol Vis 2009;15:1638-48.
34.Sevilla L,Zaldumbide A,Pognonec P,Boulukos KE.Transcriptional regulation of the Bcl-X gene encoding the antiapoptotic Bcl-XL protein by ets,rel/nfkappab,stat and ap1 transcription factor families.Histol Histopathol 2001;16:595-601.
35.Xi S,Gooding WE,Grandis JR.In vivoantitumor efficacy of STAT3 blockade using a transcription factor decoy approach:implications for cancer therapy.Oncogene 2005;24:970-9.
36.Liu J,Su G,Gao J,Tian Y,Liu X,Zhang Z.Effects of peroxiredoxin 2 in neurological disorders:a review of its molecular mechanisms.Neurochem Res 2020;45:720-30.
37.Frantz S,Kobzik L,Kim YD,et al.Toll4 (tlr4) expression in cardiac myocytes in normal and failing myocardium.J Clin Invest 1999;104:271-80.
38.Zhao F,Huang Y,Li G.Influence of Liangxue Tongyu Fang on proliferation,secretion and related signaling pathway proteins expression in vec.Nanjing Zhong Yi Yao Da Xue Xue Bao 2018;34:510-2.
39.Norata GD,Ongari M,Uboldi P,Pellegatta F,Catapano AL.Liver x receptor and retinoic x receptor agonists modulate the expression of genes involved in lipid metabolism in human endothelial cells.Int J Mol Med 2005;16:717-22.
40.Gruys E,Toussaint MJ,Niewold TA,Koopmans SJ.Acute phase reaction and acute phase proteins.J Zhejiang Univ Sci B 2005;6:1045-56.
41.Hajjar DP,Pomerantz KB.Signal transduction in atherosclerosis:Integration of cytokines and the eicosanoid network.FASEB J 1992;6:2933-41.
42.Schmidt A,Hall MN.Signaling to the actin cytoskeleton.Annu Rev Cell Dev Biol 1998;14:305-38.
43.Carpenter CL.Actin cytoskeleton and cell signaling.Crit Care Med 2000;4 Suppl(28):N94-9.
44.Suzuki M,Ogawa A,Sakurai Y,et al.Thrombin activity in cerebrospinal fluid after subarachnoid hemorrhage.Stroke 1992;23:1181-2.
45.Sorimachi H,Ono Y.Regulation and physiological roles of the calpain system in muscular disorders.Cardiovasc Res 2012;96:11-22.
46.Nishimura T,Takeichi M.Remodeling of the adherens junctions during morphogenesis.Curr Top Dev Biol 2009;89:33-54.
47.Lopez-Colome AM,Lee-Rivera I,Benavides-Hidalgo R,Lopez E.Paxillin:a crossroad in pathological cell migration.J Hematol Oncol 2017;10:50.
 Journal of Traditional Chinese Medicine2022年4期
Journal of Traditional Chinese Medicine2022年4期
- Journal of Traditional Chinese Medicine的其它文章
- Effectiveness of redcore lotion in patients with vulvovaginal candidiasis:a systematic review and Meta-analysis
- Efficacy and safety of external application of Chinese herbal medicine for psoriasis vulgaris:a systematic review of randomized controlled trials
- Effectiveness and safety of electroacupuncture for the treatment of pain after laparoscopic surgery:a systematic review
- Effect of astragaloside IV on the immunoregulatory function of adipose-derived mesenchymal stem cells from patients with psoriasis vulgaris
- Shenqihuatan formula (参七化痰方) reduces inflammation by inhibiting transforming growth factor-beta-stimulated signaling pathway in airway smooth muscle cells
- Drug response biomarkers of Pien Tze Huang (片仔癀) treatment for hepatic fibrosis induced by carbon tetrachloride
