Fuzheng Kang' ai decoction (扶正抗癌方) inhibits cell proliferation,migration and invasion by modulating mir-21-5p/human phosphatase and tensin homology deleted on chromosome ten in lung cancer cells
LI Jinhua,LIU Chunping,ZHAO Yueyang,WU Wanyin,SUN Pengtao,LI Longmei,YANG Xiaobing,ZHOU Yushu
LI Jinhua,SUN Pengtao,Department of Ultrasound,Guangdong Provincial Hospital of Chinese Medicine,the Second Affiliated Hospital of Guangzhou University of Chinese Medicine,Guangzhou 510120,China;Department of Ultrasound,Institute of Ultrasound in Musculoskeletal Sports Medicine,Guangdong Second Provincial General Hospital,Guangzhou 510317,China
LIU Chunping,State Key Laboratory of Dampness Syndrome of Chinese Medicine,Guangdong Provincial Hospital of Chinese Medicine,The Second Affiliated Hospital of Guangzhou University of Chinese Medicine,Guangzhou 510120,China
ZHAO Yueyang,Department of Hematology,Guangdong Provincial Hospital of Chinese Medicine,the Second Affiliated Hospital of Guangzhou University of Chinese Medicine,Guangzhou 510120,China
WU Wanyin,LI Longmei,YANG Xiaobing,ZHOU Yushu,Department of Oncology,Clinical and Basic Research Team of TCM Prevention and Treatment of NSCLC,Guangdong Provincial Hospital of Chinese Medicine,the Second Affiliated Hospital of Guangzhou University of Chinese Medicine,Guangzhou 510120,China
Abstract OBJECTIVE:To elucidate the potential molecular mechanism by which Fuzheng Kang’ai decoction (扶正抗癌方,FZKA) inhibits proliferation,migration,and invasion of lung cancer cells.METHODS:Varying FZKA concentrations were used to manage lung cancer cells (A549 and PC9).We employed:cell counting kit-8 (CCK-8) and plate clone formation assays to examine the cell viability;flow cytometry(FCM) to analyze the cycle arrest;transwell and woundhealing assays to assess the cell invasion and migration,respectively.Further,a quantitative real-time polymerase chain reaction (qRT-PCR) assay was adopted to evaluate the miR-21-5p expression.For protein expression analysis,we employed the Western blot technique.Recombinant miR-21-5p overexpression adenovirus vector harboring GFP was constructed and transfected into A549 and PC9,after which we explored the effect of FZKA on miR-21-5p overexpression.RESULTS:Notably,treatment with FZKA inhibited viability,clone-formation ability,invasion,and migration of lung cancer cells.Mechanistically,FZKA markedly suppressed miR-21-5p expression but elevated the human phosphatase and tensin homology deleted on chromosome ten (PTEN) protein level in both A549 and PC9 cells.Over-expression of miR-21-5p lowered PTEN protein expression.Besides,overexpressed miR-21-5p levels with adenovirus antagonized FZKA-upregulated PTEN protein expression.CONCLUSION:The present study demonstrates how FZKA modulates cell biological behaviors,for instance,it impedes the proliferation by upregulating PTEN expression with miR-21-5p as the target.These findings unveil the potential novel molecular mechanisms from the microRNA aspect by which FZKA suppresses the growth of human lung cancer cells.
Keywords:lung neoplasms;miR-21-5p;PTEN phosphohydrolase;Fuzheng Kang’ai decoction
1.INTRODUCTION
Based on the current understanding,lung cancer is the leading cause of malignant tumor mortality.With rapid population growth and aging globally,lung cancer remains the most prevalent malignant tumor with the top global incidence (11.6% of the total cancer cases) and mortality (18.46% of the total cancer-related deaths) in both sexes combined.1,2Most lung cancer patients are diagnosed at an advanced stage;there are challenges in early finding and diagnosis despite the ongoing exploration of comprehensive treatments.3Although proliferation and metastasis are the foremost causes of lung cancer progression,they can be inhibited by activating tumor suppressor genes.
MicroRNAs (miRNAs),small non-coding RNAs (17-22 nucleotides),are inextricably associated with the fundamental biological processes of malignant tumors.4,5The assembly of mature miRNAs into RNA-induced silencing complexes (RISC) regulates gene expressionviaa post-transcriptional control mechanism.6Posttranscriptional regulation of miRNAs is linked to several pivotal biologic processes,including cell proliferation,growth,invasion,metastasis,and cell death;this could offer new therapeutic targets for cancer management.7MiR-21,one of the early discovered miRNAs,is a conserved small RNA located on chromosome 17q21;it is associated with cell proliferation,growth,invasion,metastasis,and drug resistance in various cancers.8-10Reports have validated that MiR-21-5p,a mature form of miR-21,is over-expressed in most human cancer cell lines and carcinoma tissues.11-13In NSCLC,miR-21-5p is significantly upregulated,it is also correlated with tumor growth,metastasis,and poor survival in patients.12-15Based on the existing knowledge,human phosphatase and tensin homology deleted on chromosome 10 (PTEN) is a tumor suppressor gene located on human chromosome 10q23.3;it exerts a critical role in the negative regulation of cell proliferation,growth,adhesion,invasion,migration,and apoptosis.16However,PTEN expression is much lower in malignant tumor tissues such as lung cancer compared to normal tissues.17,18Besides,the sustained activation of phosphatidylinositol kinase 3-kinase/protein kinase B(PI3K/Akt) signaling pathway plays a highly oncogenic pathway role,whereas it is negatively regulated by PTEN.19-21Various reports have verified PTEN as one of the target genes for miR-21-5p.22-24Compelling evidence shows that miR-21-5p promotes cell proliferation by sustaining the activation of the PTEN/PI3K/Akt pathway in cancer cells.25Therefore,inhibiting miR-21-5p expression to suppress the activation of PI3K/Akt,with PTEN as the target is a popular approach for regulating proliferation and apoptosis of lung cancer cells.
As revealed previously,Fuzheng Kang’ai decoction (扶正抗癌方,FZKA) was prescribed by Dr.Wu Wanyin based on his experience (more than 20 years) to manage tumors,using a combination of Chinese traditional and Western medicine.26In our clinical researches,we verified that FZKA could prolong progression-free survival (PFS) and median survival time (MST),as well as elevate the disease control rate in lung cancer patients.27,28Besides,following our experimental studies,FZKA could impede lung cancer cell proliferation,induce apoptosis,and suppress the growth of NSCLC xenografts in nude mice via numerous classical pathways,among them,Vimentin/N-cadherin/MMP-9/p-STAT326and AMPKα/IGFBP1/FOXO3a.29More recently,FZKA was found to inhibit the growth of lung cancer cells via the mitochondrial pathway,28phosphatidylinositol-3-kinase (PI3-K)/protein kinase B (Akt)-mediated inhibition of Nuclear factor-κB (NF-κB) subunit p65,followed by reduced expression of cell surfaceassociated mucin-1 (MUC1),30and the DNA methyltransferase 1(DNMT1) and specificity protein 1(SP1)in vitroandin vivo.31In the present study,we highlight further evidences demonstrating the proapoptotic effects of FZKA in lung cancer cells on miRNAs area.
2.MATERIALS AND METHODS
2.1.FZKA
The FZKA decoction was purchased from Guangdong Kangmei pharmaceutical Company Ltd.(Puning,Guangdong,China).The primary composition of the FZKA and the amount of each herb followed previously reported findings.26,32Through high-performance liquid chromatography (HPLC) analysis,we obtained the batch to batch consistency study (the specific chromatograms of FZKA).29Ultra-high pressure liquid chromatography coupled with LTQ Orbitrap mass spectrometry was employed for chemical profiling analysis of major constituents in FZKA,reported previously.29,31FZKA preparation followed a previously described method.28Briefly,the whole herbs (310 g in all) were soaked in ultrapure water for 30 min,decocted for 2 h,after which the initial liquid was poured out.Finally,the concentration liquid was spray-dried into particles.The above protocol was conducted by the Yifang Pharmaceutical Co.,Ltd.(Foshan,Guangdong,China),meeting the requirements of Good Manufacturing Practice (GMP).Forinvitroexperiments,the granules were dissolved in Dulbecco's modified Eagle's medium(DMEM) medium to a final concentration of 20 mg/mL,then fragmentedviaultrasonication and a 10 min centrifugation at 14 000 rpm.The supernatant was collected and filtered through a 0.22 μm filter.Notably,the pH value of the cultured cells in media was adjusted to 7.2-7.4 following FZKA addition.The direct administration methodology of FZKA decoction directly has been proven to be reliable.28,30
2.2.Chemicals
Monoclonal antibodies specific to PTEN and GAPDH were purchased from Cell Signaling Technology Inc.(Beverly,MA,USA).The CCK-8 was purchased from Beyotime Institute of Biotechnology (Shanghai,China).miR-21-5p mimics and inhibitors,miRNA primers and Bulge-Loop miRNA qRT-PCR Starter Kits,ribo FECT™ CP Transfection Kit,the miR-mimic negative control,miR-21-5p mimic,miR-inhibitor negative control,miR-21-5p inhibitor were obtained from Ribo Biological Co.,Ltd.(Guangzhou,Guangdong,China).MiR-21-5p overexpression adenoviruses and adenoviruses negative control (AD-miR-21-5p mimic and AD-NC) were synthesized by Vigene Biosciences (Jinan,Shangdong,China).
2.3.Cell lines and cell culture
Human lung cancer cells (A549 and PC9) were obtained from The Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai,China),authenticated for mycoplasma detection,isozyme detection,drug response,DNA-fingerprinting,morphology,and cell vitality detection;both had been described previously.28We cultured the cells in a basic DMEM medium (Life Technologies,USA) containing 10% (v/v) fetal bovine serum (FBS) (Gibco,Grand Island,NY,USA),100 U/mL penicillin,and 100 U/mL streptomycin (Gibco,Grand Island,NY,USA),at 37 ℃in a humidified atmosphere containing 5% CO2.At 80%-90% confluence,cells were digested with 0.25% trypsin-EDTA for subsequent experiments.Countstar automated cell counter (Inno-Alliance Biotech,Wilmington,DE,USA) was employed for cell counting.
2.4.CCK-8 assay
Cell viability was assessed via the CCK-8 assay.Here,A549 and PC9 cells were seeded into 96-well plates(5 × 103cells/well) and incubated in a complete medium at 37 ℃ with 5% CO2for 24 h before treatment with increasing concentrations of FZKA for up to 72 h (0 mg/mL FZKA cultured cells served as the untreated control cells).Afterward,cell viability was assessed following the stipulated protocol for the CCK-8 assay.Lastly,we employed an automated microplate reader (Perkin Elmer,Waltham,MA,USA) to test absorbance at 450 nm.Each experiment was repeated thrice.Cell viability (%) was calculated as follows:(absorbance of test sample/absorbance of control) ×100%.The 50% inhibitory concentration (IC50 value)was calculated and compared to that of the control group.Growth inhibition curves of cells were investigated using the CCK-8 assay.
2.5.Flow cytometry analysis
In this experiment,we assessed the effect of FZKA on the cell cycle.In brief,cells were cultured in 6-well plates at 2 × 105cells/well and treated with increasing doses of FZKA for 24 h.Afterwards,the cells were harvested and resuspended in 1 mL of PBS,then incubated with 0.1%sodium citrate containing propidium iodide (PI) 0.05 mg and 50 μg RNase for 30 min at room temperature.Finally,the cell cycle analysis was detected by flow cytometry(Beckman,USA),and the proportion of cells within the G0/G1,S,and G2/M phases of the cell cycle were analyzed using the Multi Cycle AV DNA Analysis software.
2.6.Plate clone formation assay
Through plate clone formation assay,we analyzed the colony-forming ability of A549 and PC9 cells with miR-21-5p overexpression post FZKA treatment.Cells in the exponential growth phase were plated onto 6-well plates at 1000 cells/well,then incubated for 24 h to settle.Cells were treated with varying FZKA doses.After approximately 10 d,cell colonies were washed with PBS,fixed,and stained with Wright-Giemsa Stain Kit(Jiancheng,Nanjing,China) following the manufacturer's protocol.Cell colonies were counted using an inverted microscope,with the standard definition that a colony consists of ≥10 cells.The inhibition ratios (%) of colony formation were calculated as the ratio of the indicated treatment group to the control group as follows:% IR=100% × Nt/Nc,where IR denotes inhibition ratio;Nt denotes the number of the treatment group colonies;Nc denotes the number of control group colonies.
2.7.Transwell matrigel invasion assay
Transwell assay was employed to evaluate the invasive potential of A549 and PC9 cells.Before the experiment,cells were transfected with adenovirus with miRNC(AD-NC) or miR-21-5p mimic (AD-miR-21-5p).Matrigel (Bioscience,San Jose,CA,USA) was diluted 8-fold using DMEM,injected into the upper inserts of the transwell system (Corning Incorporation,Corning,NY,USA),and dried in the incubator.Afterward,500 μL cell culture medium with 20% FBS was introduced to the lower wells.Then,cells were diluted to 0.5 × 106/mL,pretreated with a culture medium supplemented with 2% FBS,FZKA (3 mg/mL).After 24 h incubation (37 ℃,5% CO2),non-invadedcells in the upper membrane were wiped out,whereas invaded-cells were fixed and stained with Wright-Giemsa Stain Kit.Images were captured under a 100×magnification microscope.Subsequently,200 µL 33%acetic acid was added to the chamber,and the eluent was moved into 96-well plates.Fluorescence measurement was taken at 570 nm using an enzyme-linked immuneosorbent assay reader (Perkin Elmer,Waltham,MA,USA).The absorbance of the control and the FZKA groups were calculated according to the formula:Absorbance (% of control group)=(experimental group/control group) × 100%.
2.8.Wound-healing assay
We performed a wound-healing assay to evaluate the migratory potential of A549 and PC9 cells.Cells were cultured in 6-well plates (4 × 105/well) and incubated to 90% confluence.Wounds were introduced in cell monolayers by scratching using a 200 μL pipette tip,then washed with PBS to remove detached cells.Thereafter,cells were treated with Basic DMEM comprising 2%FBS,FZKA (1,2,3 mg/mL),for 12 or 24 h.Later,on the 12th or 24th hour,the medium was replaced with PBS.The wound gap was assessed;an image was taken using an Olympus microscope fitted with a digital camera(Olympus,Tokyo,Japan).
2.9.Adenoviruses transfection experiment
Adenoviruses transfection experiment was employed to explore the effect of miR-21-5p overexpression on cell lines,A549 and PC9.NSCLC cells in the exponential growth phase were plated onto 6-well plates using a medium without antibiotics for 24 h.Afterward,adenoviruses harboring miR-21-5pmimic (AD-miR-21-5p) and its non-specific control (AD-NC) (Vigene Biosciences,Jinan,China) were transfected following the manufacturer’s instructions,awaiting subsequent experiments.
2.10.Quantitative real-time polymerase chain reaction(qRT-PCR)
QRT-PCR assay was prepared to examine the expression of miR-21-5p and PTEN transcripts.Cells were washed thrice in cold PBS before they could be harvested with TRIzol reagent (Ambion,Waltham,MA,USA).The concentration and purity of the total RNA were assessed using a NanoDrop Lite spectrophotometer (Thermo,Waltham,MA,USA).The QuantStudio™ 7 Flex Real-Time PCR System was employed for qRT-PCR.To detect miR-21-5p transcripts expression,both reverse transcription and PCR reaction were performed using a Bulge-Loop miRNA qRT-PCR Starter Kit (RiboBio Co.,Guangzhou,China) following protocol stipulated by the manufacturer.miR-21-5p expression was normalized to U6 snRNA.Primers were also purchased from RiboBio Co.RT-qPCR conditions were as follows:95 ℃ and 30 s for template denaturation,60 ℃ as the annealing temperature and 70 ℃ as the extension temperature for miRNA for 40 cycles.The purity of PCR products was confirmedviamelting curve analysis.Data were analyzed using the 2-ΔΔCtfor relative changes in gene expression.Primers used were designed as highlighted in (Supplementary Table S1).
2.11.Western blot analysis
We employed the Western blot assay to analyze the protein expression.After treatment with specified doses of FZKA,cells were harvested and lysed in 1×RIPA buffer (CST,Beverly,MA,USA) with Complete Protease Inhibitor Cocktail (Roche,Switzerland).Measurement of protein concentrations was taken using the Pierce BCA Protein Assay Kit (Thermo,Waltham,MA,USA).Equal amounts of protein (25 μg) from whole-cell lysates were solubilized in 3× loading buffer(CST,Beverly,MA,USA),separated by 10% sodium dodecyl sulfate-polyacrylamide gel electropheresis,then transferred onto polyvinylidene difluoride (PVDF)membranes (Millipore,Billerica,MA,USA).The PVDF membranes were incubated with antibodies against PTEN (cat.9188;CST,Beverly,MA,USA),and GAPDH (cat.2118;CSI,Beverly,MA,USA) at 4 ℃overnight.Subsequently,the membranes were washed and subjected to a one-hour incubation with a secondary antibody against rabbit IgG,HRP-linked Antibody (cat.5174;CST,Beverly,MA,USA) at room temperature.The membranes were washed again and transferred to a freshly prepared enhanced chemiluminescence solution(Millipore,Billerica,MA,USA).Signals were then evaluated and scanned under the Bio-Rad ChemiDoc Touch Chemiluminescence imaging system (Bio-Rad,Hercules,CA,USA).Results were analyzed using ImageJ software (version 1.48;National Institutes of Health).
2.12.Statistical analysis
All statistical data were analyzedviaSPSS GraphPad Prism software version 8.0 (GraphPad Software,Inc.La Jolla,CA,USA) statistical software and expressed as mean ± standard error of the mean (SEM) of three independent experiments.Data analysis was performed using Student’s t-test for comparisons between two groups;differences between groups were assessed by one-way analysis of variance followed by Tukey's multiple comparison test for multiple groups involved.Pvalues < 0.05 denoted statistical significance.
3.RESULTS
3.1.FZKA inhibited the proliferation of NSCLC cells
We explored the effect of FZKA on cell proliferation,cell growth,clonality,and cell cycle arrest.Compared to the control cells,the CCK-8 assay results demonstrated that the growth of A549 and PC9 cells treated with FZKA was significantly inhibited;notably,the halfmaximal inhibitory concentration(IC50)recorded at 24,48,and 72 h were 3.25,2.93 and 1.64 mg/mL in A549(Figure 1A);3.46,2.91 and 2.27 mg/mL in PC9(Supplementary Figure S1A,S1B),respectively.Upon examining the effect of FZKA on the clone formation ability of NSCL cells,the clonogenic ability of the cells was dramatically suppressed by FZKA with concentration at 2 mg/mL in both A549 (Figure 1C,1D)and PC9 (Supplementary Figure S1A,S1B).Furthermore,we assessed the cell cycle arrest induced by FZKA,whereby A549 cells treated with increasing concentrations of FZKA for 24 h elevated the proportion of cells at G0/G1 phases in A549 (Figure 1E,1F) cells and at the S phase in PC9 cells (Supplementary Figure S1C,S1D),as detectedviaflow cytometry.Concomitantly,the population of cells at the S phase in A549 and the G2/M phase in PC9 cells was significantly decreased following FZKA treatment.Based on these findings,it is implicated that FZKA inhibited cell proliferation at least by prolonging G0/G1 phases or S phase in NSCLC.

Figure 1 Effect of FZKA on cell proliferation
3.2.FZKA inhibited the invasion and migration of NSCLC cells
Using wound healing assay and transwell matrigel invasion assay,we assessed the effect of FZKA on cell migration and invasion.Of note,the wound healing assay showed that the migration was also significantly suppressed by FZKA with concentration at 2 mg/mL in A549 (Figure 2A,2B) and at 1 mg/mL in PC9(Supplementary Figure S1E,S1F).And the transwell assay demonstrated that FZKA significantly lowered the invasion ability at 3 mg/mL compared to the untreated control in A549 (Figure 2C,2D) and at 1 mg/mL in PC9(Supplementary Figure S1C,S1D).Meanwhile,the wound closure of the two lung cancer cells was markedly delayed following FZKA treatment in a dose-dependent manner.The aforementioned results depicted the inhibitory effects of FZKA on lung cancer cell invasion and migration.
3.3.FZKA suppressed the cell proliferation,invasion and migratory of NSCLC cells following miR-21-5p overexpression
miR-21-5p exert oncogene functions;it promotes cell proliferation,invasion,migration,metastasis,and antiapoptosis.Notably,miR-21-5p is overexpressed in numerous human cancer types.To establish whether the medical action of FZKA above-mentioned was associated with changes in miR-21-5p expression in NSCLC cells,we first assessed the expression of miR-21-5p post FZKA treatment using qRT-PCR.Results demonstrated that FZKA treatment significantly lowered miR-21-5p expression level in A549 (Figure 3A) at 2 mg/mL and PC9 at 3 mg/mL (Supplementary Figure S2A).Moreover,to evaluate the effect of FZKA on miR-21-5p overexpression-induced cell proliferation,cells were transfected with adenovirus with miR-NC (AD-NC)or miR-21-5p mimic (AD-miR-21-5p) for 24 h,then treated with FZKA.We reported elevated cell proliferation following overexpression of miR-21-5ped by transfecting NSCLC cells with miR-21-5p mimic adenoviruses;however,FZKA reversed this trend by decreasing the cell proliferation ability (Figure 3B,Supplementary Figure S2B).
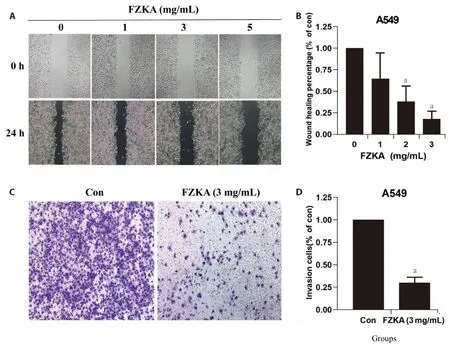
Figure 2 Effect of FZKA on cell migration and invasion
Based on the aforementioned results,we noted the potential inhibitory effects of FZKA on lung cancer growth and miR-21-5p expression level and antagonistic effect on the AD-miR-21-5p-induced cell growth.To further dissect the potential impact on miR-21-5p regulation by FZKA,we continually overexpressed miR-21-5p in A549 and PC9 cells with AD-miR-21-5p (cells with AD-NC served as a control),then evaluated the invasion and migration characteristics.Like it was revealed by the cell proliferation assay,cell invasion and migration increased following miR-21-5p overexpression in both A549 and PC9 cells;however,FZKA turned around the situation (Figure 3C-3F,Supplementary Figure S2C-S2F).Collectively,these findings demonstrated the role of miR-21-5p as an oncogene-like miRNA in NSCLC;also,FZKA could inhibit the invasion and migration of NSCLC cells by down-regulating miR-21-5p expression.
3.4.Effect of FZKA on protein expression of PTEN in NSCLC cells following miR-21-5p overexpression
PTEN,known as a tumor suppressor gene,located in human chromosome l0q23.3,exert a vital negative regulatory role in cell proliferation and tumorigenesis;however,the expression in lung cancer and other malignant tumors is lower than in normal tissues.Hence,in our first study,we assessed the effect of FZKA on PTEN expression in NSCLC cells,A549 and PC9.Results demonstrated remarkable FZKA-mediated upregulation of PTEN in a dose-dependent manner,in the two lung cancer cell lines with significant induction,respectively (Figure 4A,4B,Supplementary Figure SGH).Moreover,FZKA treatment elevated the expression levels of PTEN protein but decreased following transfection with overexpressed miR-21-5p levels (Figure 4CD,Supplementary Figure S2IJ).These findings implied a potential mechanism by which FZKA elevates PTEN protein expression by downregulating the level of miR-21-5p.
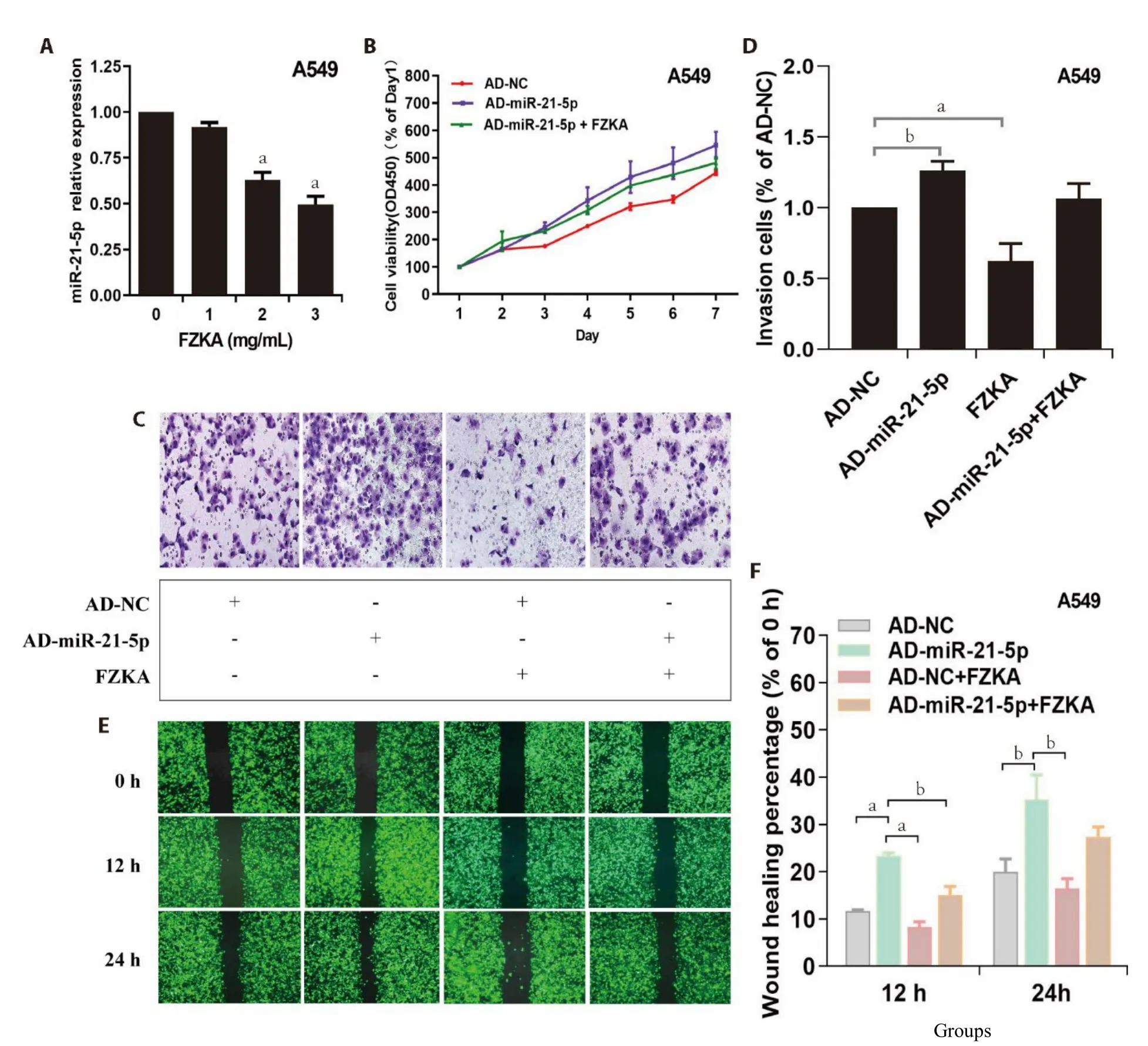
Figure 3 Effect of FZKA on miR-21-5p expression and its overexpression-induced cell proliferation,invasion and migratory
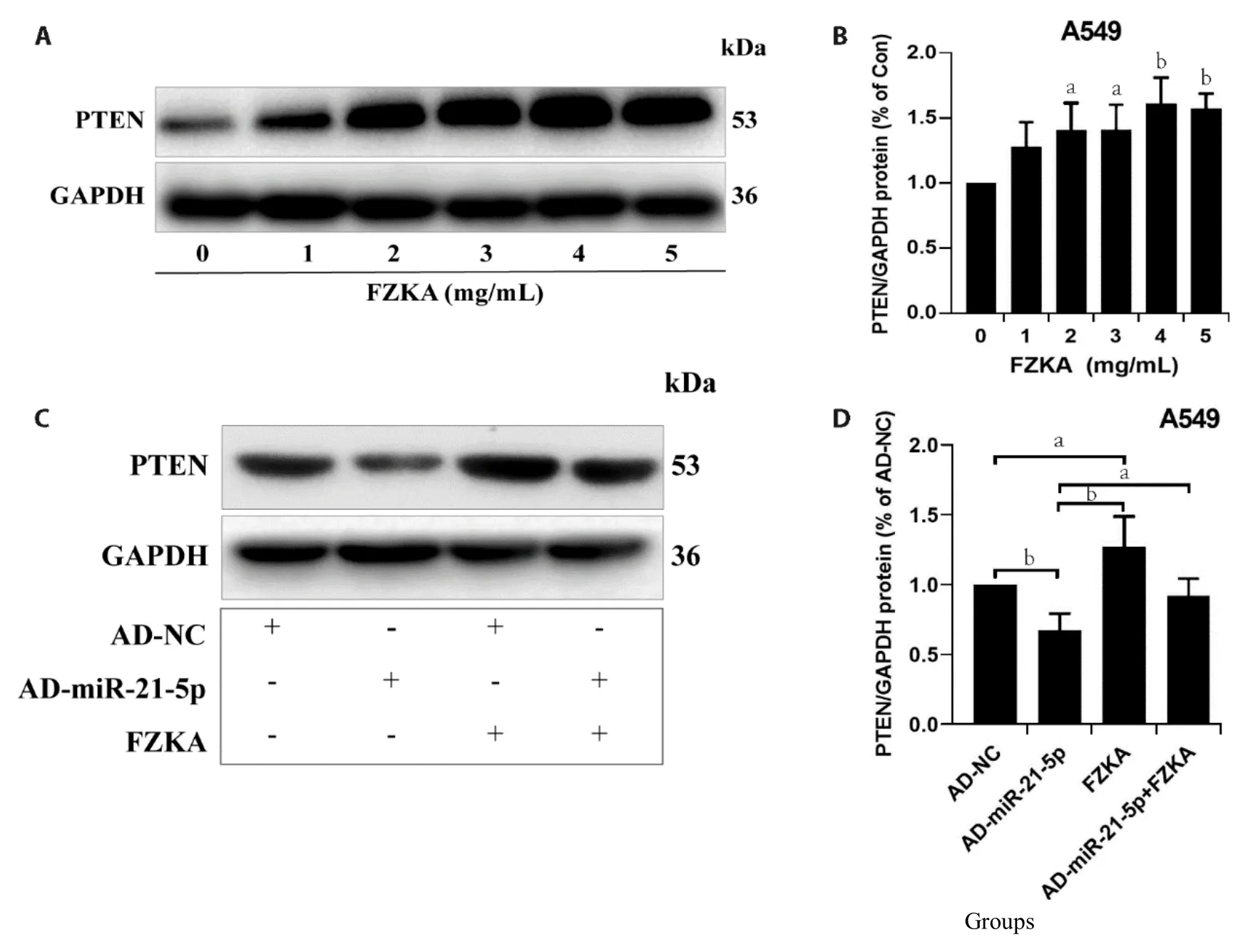
Figure 4 Effect of FZKA on the interaction between miR-21-5p and PTEN
4.DISCUSSION
Lung cancer,one of the most prevalent malignant tumors across the globe,is also the leading cause of tumorrelated deaths.The malignant biological behaviors of cells,including proliferation,anti-apoptosis,cell cycle perturbation,invasion,and metastasis,are the premises for lung cancer occurrence and development.Proliferation is among the crucial physiological functions of viable cells,and one of the essential life characteristics of organisms.33Tumor cells are,in most cases,prone to abnormally high proliferation.Of note,organisms exhibit an increase in the number of cells exponentially or replace those that die off from individual or multiple cellsviaproliferation.Clone formation ability depicts the potential of anchoragedependent cells to survive and form clones after plating;this reflects the growth of cells.34Notably,cells with clonality must be proliferative.The cell cycle is related
to the process from the completion of cell division to the end of the next cell division.As the cell cycle process gets uncontrollable,unlimited reproduction of cells occurs.35Invasion and migration,two significant features of malignant tumors different from normal tissues and benign tumors,potentially cause infiltration and distant metastasis of malignant tumors.36Consequently,the disease progresses,eventually causing death in NSCLC patients.36Cell proliferation and apoptosis processes are entirely regulated by multiple genes at multiple levels,in a dynamic equilibrium state.Any disorder in the proliferation and apoptosis would cause disease.Malignant tumors are precisely caused by the abnormal proliferation of cells and the anti-apoptotic properties in our bodies.Moreover,the biological cell characteristics are crucial indications on anti-tumor drugs including FZKA take effect,on the contrary.
Despite substantial achievements and advances in the treatments of human lung cancer,5-year survival remains low.1In the past decades,the demand for complementary and alternative medicine (CAM)services to help patients with malignant tumors has been on the rise.31Traditional Chinese Medicine,an important CAM category,possess the proven benefits,for example,helping cancer patients to avoid complications and reduce the side effects associated with conventional treatment.In our previous clinical research,we found that FZKA could prolong the PFS and MST in lung cancer patients.Through laboratory experiments,we demonstrated that FZKA could inhibit lung cancer growth bothin vivoandin vitroviasignaling pathways,including the Vimentin/N-cadherin/MMP-9/p-STAT3 pathway,DNMT1/SP1 pathway,and AMPKα/IGFBP1/FOXO3a pathway at mRNA and protein levels,orviatranscription factors.26,29,31Compared to commonly used drugs (such as gefitinib or erlotinib) for lung cancer,FZKA decoction exhibited a similar inhibitory effect;30,31there was no statistical significance between groups.Of note,the combination of FZKA decoction and gefitinib of erlotinib synergistically inhibited the growth of lung cancer cells.30,31However,whether FZKA impact miRNAs levels when administered to manage advanced lung malignancies remain poorly understood.Herein,we presented further evidence regarding cell proliferation,miRNA expression,and protein regulation to elucidate the critical role in mediating the anti-lung cancer effects of FZKA.Initially,we confirmed the cell growth inhibitory role of FZKA in lung cancer,a phenomenon that concurred with the findings from previous studies.28Subsequently,the present findings demonstrate that cell clonality,migration,and invasion of lung cancer were significantly inhibited by FZKA.Notably,the overall effects mentioned above suggest the substantial inhibitory effects of FZKA on lung cancer.
MiR-21-5p,a biomarker as well as an oncogene-like miRNA,is closely associated with cell proliferation,growth,development,invasion,metastasis,and drug resistance in various malignancies.10,14,22,37We have implicated miR-21-5p as a vital regulatory factor during the process of FZKA-induced inhibition of lung cancer cell growth.Based onqPCR assay results,we demonstrated that the relative miR-21-5p expression is inhibited following FZKA treatment.Also,PTEN,as a tumor-suppressor gene,can suppress proliferation,invasion,and metastasis of lung cancer cells,16by activating numerous canonical signaling pathways,for example,the PI3K/AKT pathway.According to previous reports,PTEN is one of the miR-21-5p targets;it modulates cell proliferation apoptosis and drug resistance in lung cancer.25Furthermore,other reports have found that the miR-21-5p/PTEN axis participates in a considerable quantification of cancer progression.22,38Therefore,we suggest the FZKA-induced inhibition of miR-21-5p expression as a crucial mechanism by which FZKA inhibits lung cancer cell proliferation.Moreover,PTEN expression was elevated following treatment with FZKA.Notably,in A549 and PC9 cells overexpressing miR-21-5p,the PTEN expression was slightly suppressed,but this trend was reversible with FZKA treatment.In a previous report,low or loss of PTEN expression contributed to cancer progression in human malignancies.19Hence,FZKA-associated inhibition of miR-21-5p may inhibit tumor progression.Herein,we reported similar results,suggesting that the miR-21-5p/PTEN axis may mediate the inhibitory effects of FZKA on lung cancer cell progression.
In conclusion,the present study explored the effects of FZKA on the biological behavior of lung cancer cells,including cell proliferation,growth,development,invasion,and metastasis.These findings validate that FZKA can significantly debilitate the proliferation and progression of lung cancer cells by inhibiting miR-21-5p expression,with PTEN as the target.Although it remains unclear whether FZKA directly or indirectly interacts with miR-21-5p,the present report has afforded a new potential mechanism by which it regulates human lung cancer cell growth.
5.REFERENCES
1.Siegel RL,Jemal A.Cancer statistics,2020.CA Cancer J Clin 2020;70:7-30.
2.Siegel RL,Miller KD,Jemal A.Cancer statistics,2018.CA Cancer J Clin 2018;68:7-30.
3.Yap S,Goldsbury D,Yap ML,et al.Patterns of care and emergency presentations for people with non-small cell lung cancer in New South Wales,Australia:a population-based study.Lung Cancer Amst Neth 2018;122:171-9.
4.Huang W.MicroRNAs:biomarkers,diagnostics,and therapeutics.Methods Mol Biol Clifton NJ 2017;1617:57-67.
5.Thomson DW,Dinger ME.Endogenous microRNA sponges:evidence and controversy.Nat Rev Genet 2016;17:272-83.
6.Hessam S,Sand M,Skrygan M,Bechara FG.The microRNA effector RNA-induced silencing complex in hidradenitis suppurativa:a significant dysregulation within active inflammatory lesions.Arch Dermatol Res 2017;309:557-65.
7.Faraji F,Hu Y,Yang HH,et al.Post-transcriptional control of tumor cell autonomous metastatic potential by CCR4-NOT deadenylase CNOT7.PLoS Genet 2016;12:e1005820.
8.Sahraei M,Chaube B,Liu Y,et al.Suppressing miR-21 activity in tumor-associated macrophages promotes an antitumor immune response.J Clin Invest 2019;129:5518-36.
9.Bose RJC,Uday Kumar S,Zeng Y,et al.Tumor cell-derived extracellular vesicle-coated nanocarriers:an efficient theranostic platform for the cancer-specific delivery of anti-miR-21 and imaging agents.ACS Nano 2018;12:10817-32.
10.Cao LQ,Yang XW,Chen YB,Zhang DW,Jiang XF,Xue P.Exosomal miR-21 regulates the TETs/PTENp1/PTEN pathway to promote hepatocellular carcinoma growth.Mol Cancer 2019;18:148.
11.Liao J,Shen J,Leng Q,Qin M,Zhan M,Jiang F.MicroRNAbased biomarkers for diagnosis of non-small cell lung cancer(NSCLC).Thorac Cancer 2020;11:762-8;
12.Li C,Yin Y,Liu X,Xi X,Xue W,Qu Y.Non-small cell lung cancer associated microRNA expression signature:integrated bioinformatics analysis,validation and clinical significance.Oncotarget 2017;8:24564-78.
13.Liang H,Jiao Z,Rong W,et al.3’-Terminal 2’-O-methylation of lung cancer miR-21-5p enhances its stability and association with Argonaute 2.Nucleic Acids Res 2020;48:7027-40.
14.Wang G,Zhou Y,Chen W,et al.miR-21-5p promotes lung adenocarcinoma cell proliferation,migration and invasion via targeting WWC2.Cancer Biomark Sect Dis Markers 2020;28:549-59.
15.Yang C,Sun C,Liang X,Xie S,Huang J,Li D.Integrative analysis of microRNA and mRNA expression profiles in non-small-cell lung cancer.Cancer Gene Ther 2016;23:90-7.
16.Lee Y-R,Chen M,Pandolfi PP.The functions and regulation of the PTEN tumour suppressor:new modes and prospects.Nat Rev Mol Cell Biol 2018;19:547-62.
17.Cai J,Li R,Xu X,et al.CK1α suppresses lung tumour growth by stabilizing PTEN and inducing autophagy.Nat Cell Biol 2018;20:465-78.
18.VanderLaan PA,Rangachari D,Mockus SM,et al.Mutations in TP53,PIK3CA,PTEN and other genes in EGFR mutated lung cancers:correlation with clinical outcomes.Lung Cancer Amst Neth 2017;106:17-21.
19.Tsai C-Y,Wu JCC,Fang C,Chang AYW.PTEN,a negative regulator of PI3K/Akt signaling,sustains brain stem cardiovascular regulation during mevinphos intoxication.Neuropharmacology 2017;123:175-85.
20.Álvarez-Garcia V,Tawil Y,Wise HM,Leslie NR.Mechanisms of PTEN loss in cancer:it’s all about diversity.Semin Cancer Biol 2019;59:66-79.
21.Juric D,Castel P,Griffith M,et al.Convergent loss of PTEN leads to clinical resistance to a PI(3)Kα inhibitor.Nature 2015;518:240-4.
22.Yan L,Wang LZ,Xiao R,et al.Inhibition of microRNA-21-5p reduces keloid fibroblast autophagy and migration by targeting PTEN after electron beam irradiation.Lab Investig J Tech Methods Pathol 2020;100:387-99.
23.Li N,Wang Z,Gao F,Lei Y,Li Z.Melatonin ameliorates renal fibroblast-myofibroblast transdifferentiation and renal fibrosis through miR-21-5p regulation.J Cell Mol Med 2020;24:5615-28.
24.Chen J,Zhou C,Li J,et al.miR-21-5p confers doxorubicin resistance in gastric cancer cells by targeting PTEN and TIMP3.Int J Mol Med 2018;41:1855-66.
25.Ren W,Hou J,Yang C,et al.Extracellular vesicles secreted by hypoxia pre-challenged mesenchymal stem cells promote nonsmall cell lung cancer cell growth and mobility as well as macrophage M2 polarizationviamiR-21-5p delivery.J Exp Clin Cancer Res CR 2019;38:62.
26.Li L,Wang S,Yang X,et al.Traditional Chinese Medicine,Fuzheng Kang-Ai decoction,inhibits metastasis of lung cancer cells through the STAT3/MMP9 pathway.Mol Med Rep 2017;16:2461-8.
27.Yang XB,Wu WY,Long SQ,et al.Fuzheng Kang’ai decoction combined with gefitinib in advanced non-small cell lung cancer patients with epidermal growth factor receptor mutations:study protocol for a randomized controlled trial.Trials 2015;16:146.
28.Wang S,Peng Z,Li W,Long S,Xiao S,Wu W.Fuzheng Kang’ai decoction enhances the effect of Gefitinib-induced cell apoptosis in lung cancer through mitochondrial pathway.Cancer Cell Int 2020;20:185.
29.Zheng F,Wu J,Li X,et al.Chinese herbal medicine Fuzheng Kang’ai decoction inhibited lung cancer cell growth through AMPKα-mediated induction and interplay of IGFBP1 and FOXO3a.Evid-Based Complement Altern Med ECAM 2016;2016:5060757.
30.Li L,Wang S,Zheng F,Wu W,Hann SS.Chinese herbal medicine Fuzheng Kang’ai decoction sensitized the effect of gefitinib on inhibition of human lung cancer cells through inactivating PI3-K/Akt-mediated suppressing MUC1 expression.J Ethnopharmacol 2016;194:918-29.
31.Zheng F,Zhao Y,Li X,et al.The repression and reciprocal interaction of DNA methyltrans.ferase 1 and specificity protein 1 contributes to the inhibition of MET expression by the combination of Chinese herbal medicine FZKA decoction and erlotinib.J Ethnopharmacol 2019;239:111928.
32.Li L,Wang S,Zheng F,Wu W,Hann SS.Chinese herbal medicine Fuzheng Kang’ai decoction sensitized the effect of gefitinib on inhibition of human lung cancer cells through inactivating PI3-K/Akt-mediated suppressing MUC1 expression.J Ethnopharmacol 2016;194:918-29.
33.Calcinotto A,Kohli J,Zagato E,Pellegrini L,Demaria M,Alimonti A.Cellular senescence:aging,cancer,and injury.Physiol Rev 2019;99:1047-78.
34.Kohsaka S,Petronczki M,Solca F,Maemondo M.Tumor clonality and resistance mechanisms in EGFR mutation-positive non-small-cell lung cancer:implications for therapeutic sequencing.Future Oncol Lond Engl 2019;15:637-52.
35.Li W,Zheng G,Xia J,et al.Cell cycle-related and expressionelevated protein in tumor overexpression is associated with proliferation behaviors and poor prognosis in non-small-cell lung cancer.Cancer Sci 2018;109:1012-23.
36.Appert-Collin A,Hubert P,Crémel G,Bennasroune A.Role of ErbB receptors in cancer cell migration and invasion.Front Pharmacol 2015;6:283.
37.Chen JC,Hsieh YY,Lo HL,Li A,Chou CJ,Yang PM.In vitroand in silico mechanistic insights into mir-21-5p-mediated topoisomerase drug resistance in human colorectal cancer cells.Biomolecules 2019;9.
38.Tang C,Gu Y,Wang H,et al.Targeting of microRNA-21-5p protects against seizure damage in a kainic acid-induced status epilepticus modelviaPTEN-mTOR.Epilepsy Res 2018;144:34-42.
 Journal of Traditional Chinese Medicine2022年3期
Journal of Traditional Chinese Medicine2022年3期
- Journal of Traditional Chinese Medicine的其它文章
- Efficacy of meridian massage for motor function after a stroke:a systematic review and Meta-analysis
- Antiviral Activity of Medicinal Plants against Human Coronavirus:a systematic scoping review of in vitro and in vivo experimentations
- Correlation between slow transit constipation and spleen Qi deficiency,and gut microbiota:a pilot study
- Efficacy of Kushen decoction (苦参汤) on high-fat-diet-induced hyperlipidemia in rats
- Hyperpolarization-activated cyclic nucleotide-gated 2 contributes to electroacupuncture analgesia on lumbar disc herniation-induced radicular pain through activation of microglia in spinal dorsal horn
- Electroacupuncture preconditioning alleviates myocardial ischemiareperfusion injury through the hypothalamic paraventricular nucleusinterposed nucleus nerve pathway
