姜状三七根茎的皂苷类化学成分研究
李晓波,张高菊,张广辉,杨生超,姜薇薇,c*
(云南农业大学,a.云南省药用植物生物学重点实验室; b.西南中药材种质创新与利用国家地方联合工程研究中心; c.理学院,昆明 650201)
姜状三七(Panax zingiberensis)为五加科(Araliaceae)人参属植物,根茎入药,主要用于治疗跌打损伤、劳虚咳嗽、外伤出血及贫血等症,也作三七代用品。国外主要分布于尼泊尔中部、不丹高海拔地区、缅甸东枝、掸邦等地[1–4],国内分布于云南东南部的马关、砚山等地。野生资源分布狭窄及过度采挖,导致该物种于1997年被国际自然保护联盟列为濒危种。由于材料的难以采集导致关于该植物的研究较少。目前只从该植物中分离鉴定了三萜皂苷类化合物不到10 个,有人参二醇型、人参三醇型和齐墩果烷型,且其生物活性的研究也鲜见报道[5–7]。本课题组在前期研究[8]的基础上对其化学成分进行了系统研究,从中分离鉴定了9 个皂苷类化合物,为寻找活性物质提供了化学基础,也为姜状三七植物资源的合理开发和利用奠定理论基础。
1 材料和方法
1.1 材料
材料购自云南省普洱市镇沅县九甲镇三台村林下经济种植示范基地,经云南农业大学张广辉教授鉴定为五加科人参属植物姜状三七(Panax zingiberensis),标本存放于云南农业大学西南中药材种质创新与利用国家地方联合工程研究中心。
1.2 仪器和试剂
API Qstar Pulsa LC/TOF 质谱仪,Bruker AM-400、DRX-500 和AVANCE III-600 MHz 超导核磁共振仪(瑞士Bruker 公司),TMS 为内标,化学位移δ用ppm 表示,耦合常数J用Hz 表示;中药高速粉碎机(浙江永历制药机械有限公司);旋转蒸发仪(东京理化仪器有限公司);超声波清洗机(上海声源超声波仪器有限责任公司);柱层析硅胶和硅胶G薄层板(青岛海洋化工厂);AB-8 (大孔树脂天津波鸿树脂科技有限公司);反相硅胶板和柱层析用RP-18 (德国Merk 公司);Sephadex LH-20 (Amer- sham Pharmacia Biotech 公司);显色剂(5%硫酸乙醇溶液),甲醇、氯仿、丙酮和乙酸乙酯为工业级重蒸后使用,正丁醇、冰乙酸为分析纯,乙腈为色谱纯,水为哇哈哈纯净水。
1.3 提取和分离
姜状三七根茎粗粉2.8 kg,加8 倍量70%甲醇溶液常温浸泡3 d,超声提取3 次,提取时间分别为2、1.5和1.5 h,合并提取液回收甲醇至无醇味,药液分次过已预处理好的AB-8 大孔树脂柱(2 kg),以蒸馏水洗脱至Molish 反应为阴性,再以80%甲醇(共8 L)洗脱,收集甲醇洗脱液,回收甲醇,浓缩干燥得姜状三七总皂苷(440 g)。
将总皂苷吸附于1.0 kg 硅胶上进行柱层析,流动相以氯仿-甲醇-水(10∶1∶0~0∶1∶1)梯度洗脱,收集洗脱液,每份0.5 L,共收集292 份,薄层鉴别合并洗脱液得Fr1~Fr8 组分。组分Fr 4 (150 g)上硅胶柱色谱,用氯仿-甲醇-水溶剂系统进行梯度洗脱,得到3 个馏分Fr 4.1~Fr 4.3。Fr 4.1 (20 g)经硅胶柱色谱,以氯仿-甲醇-水(9∶1∶0.2~7∶3∶0.5)梯度洗脱,再以Sephadex LH-20 (甲醇)纯化,得到化合物1 (2 mg)、2 (8 mg)和3 (246 mg)。Fr 4.2 (50 g)经反复硅胶柱层析(氯仿-乙酸乙酯-甲醇-水;氯仿-甲醇-水)梯度洗脱,得到化合物4 (6 mg)、5 (10 mg)、6 (10 mg)和7 (2 g)。Fr 4.3 (30 g)过Rp-18 柱色谱,以水-甲醇(9∶1~0∶1)梯度洗脱,再以Sephadex LH- 20 (甲醇)纯化,得到化合物8 (6 mg)和9 (10 mg) (图1)。
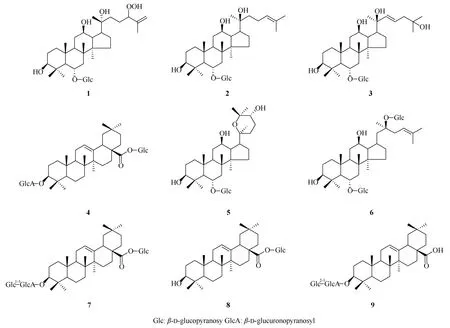
图1 化合物1~9 的结构Fig.1 Structures of compounds 1-9
1.4 结构鉴定
化合物1白色结晶,ESI-MSm/z: 693 [M +Na]+;1H NMR (400 MHz,CD3OD):δ4.34 (1H,d,J=7.8 Hz,H′-6),1.76,1.32,1.12,1.09,1.00,0.99,0.94(each 3H,s,CH3×7);13C NMR (125 MHz,CD3OD):δ40.5 (C-1),27.5 (C-2),79.1 (C-3),40.4 (C-4),61.8(C-5),79.9 (C-6),45.3 (C-7),41.8 (C-8),50.9 (C-9),40.2 (C-10),32.0 (C-11),71.7 (C-12),49.5 (C-13),52.5 (C-14),31.9 (C-15),27.4 (C-16),52.5 (C-17),17.9 (C-18),17.5 (C-19),74.2 (C-20),22.6 (C-21),40.4 (C-22),26.4 (C-23),91.2 (C-24),146.2 (C-25),113.9 (C-26),17.8 (C-27),31.4 (C-28),16.8 (C-29),17.3 (C-30),105.5 (C-1′),75.5 (C-2′),80.9 (C-3′),72.0 (C-4′),77.7 (C-5′),62.9 (C-6′)。以上数据与文献[9]报道基本一致,故鉴定为人参皂苷SL1。
化合物2白色粉末,ESI-MSm/z: 661 [M +Na]+;1H NMR (400 MHz,CD3OD):δ4.38 (1H,d,J=7.8 Hz,H-1′),1.70,1.64,1.34,1.17,1.11,1.02,1.01,0.96 (each 3H,s,CH3×8);13C NMR (100 MHz,CD3OD):δ40.2 (C-1),27.6 (C-2),79.9 (C-3),40.5(C-4),61.8 (C-5),77.6 (C-6),45.3 (C-7),41.8 (C-8),50.9 (C-9),40.4 (C-10),31.9 (C-11),71.7 (C-12),48.5(C-13),52.5 (C-14),31.9 (C-15),27.4 (C-16),55.1(C-17),17.8 (C-18),17.9 (C-19),74.5 (C-20),26.6(C-21),36.3 (C-22),23.3 (C-23),126.2 (C-24),132.1(C-25),26.0 (C-26),17.6 (C-27),31.4 (C-28),16.2(C-29),17.1 (C-30),105.5 (C-1′),75.5 (C-2′),81.8 (C-3′),71.7 (C-4′),79.0 (C-5′),62.9 (C-6′)。以上数据与文献[10]报道基本一致,故鉴定为人参皂苷Rh1。
化合物3白色粉末,ESI-MSm/z: 653 [M -H]–;1H NMR (400 MHz,C5D5N):δ5.07 (1H,d,J=7.8 Hz,H-1′),2.06,1.63,1.56,1.55,1.39,1.26,1.06,0.80 (each 3H,s,CH3×8);13C NMR (100 MHz,C5D5N):δ39.7 (C-1),28.0 (C-2),78.6 (C-3),40.1(C-4),61.5 (C-5),80.1 (C-6),45.2 (C-7),41.1 (C-8),50.2 (C-9),39.4 (C-10),32.2 (C-11),71.1 (C-12),48.5(C-13),51.7 (C-14),31.2 (C-15),26.8 (C-16),54.2(C-17),17.4 (C-18),17.8 (C-19),73.3 (C-20),27.7(C-21),137.6 (C-22),127.4 (C-23),40.4 (C-24),81.3(C-25),25.2 (C-26),25.3 (C-27),31.8 (C-28),16.4(C-29),16.8 (C-30),106.0 (C-1′),75.5 (C-2′),79.7(C-3′),71.8 (C-4′),78.3 (C-5′),63.1 (C-6′)。以上数据与文献[11]报道基本一致,故鉴定为三七皂苷R8。
化合物4白色粉末,ESI-MSm/z: 793 [M -H]–;1H NMR (400 MHz,CD3OD):δ6.53 (1H,d,J=7.6 Hz,H-1′),4.82 (1H,d,J= 7.2 Hz,H-1″),1.15,1.04,0.94,0.93,0.90,0.83,0.796,0.80 (each 3H,s,CH3×7);13C NMR (100 MHz,CD3OD):δ40.2 (C-1),26.4 (C-2),90.8 (C-3),40.0 (C-4),57.0 (C-5),19.5(C-6),33.5 (C-7),40.8 (C-8),49.0 (C-9),37.8 (C-10),24.7 (C-11),123.8 (C-12),144.8 (C-13),43.0 (C-14),28.7 (C-15),24.1 (C-16),48.0 (C-17),42.9 (C-18),47.4 (C-19),31.6 (C-20),35.0 (C-21),34.1 (C-22),28.6 (C-23),17.0 (C-24),16.1 (C-25),17.8 (C-26),26.4 (C-27),178.0 (C-28),33.5 (C-29),24.0 (C-30),106.7 (3-Glc,C-1′),75.5 (C-2′),78.0 (C-3′),73.7 (C-4′),75.5 (C-5′),176.6 (C-6′),95.7 (28-Glc,C-1″),73.9(C-2″),78.6 (C-3″),71.1 (C-4″),78.7 (C-5″),62.5(C-6″)。以上数据与文献[12]报道基本一致,故鉴定为竹节参皂苷IVa。
化合物5白色粉末,ESI-MSm/z: 653 [M -H]–;1H NMR (400 MHz,C5D5N):δ5.03 (1H,d,J=7.8 Hz,H-1′),2.06,1.68,1.60,1.52,1.31,1.19,1.03,0.80 (each 3H,s,CH3×7);13C NMR (100 MHz,C5D5N):δ39.5 (C-1),28.1 (C-2),78.7 (C-3),40.5(C-4),61.6 (C-5),78.3 (C-6),45.1 (C-7),41.2 (C-8),50.2 (C-9),39.7 (C-10),32.2 (C-11),70.7 (C-12),48.9(C-13),52.4 (C-14),31.8 (C-15),28.0 (C-16),52.1(C-17),17.6 (C-18),17.9 (C-19),78.1 (C-20),26.4(C-21),27.8 (C-22),25.9 (C-23),74.5 (C-24),78.7(C-25),23.3 (C-26),30.1 (C-27),31.8 (C-28),16.4(C-29),17.3 (C-30),106.1 (3-Glc,C-1′),75.7 (C-2′),80.3 (C-3′),71.9 (C-4′),79.7 (C-5′),63.1 (C-6′)。以上数据与文献[13]报道基本一致,故鉴定为越南人参皂苷R10。
化合物6白色粉末,ESI-MSm/z: 799 [M -H]–;1H NMR (500 MHz,CD3OD):δ5.15 (1H,d,J=7.8 Hz,H-1″),4.98 (1H,d,J= 7.8 Hz,H-1′),2.03,1.58,1.56,1.56,1.55,1.11,0.98,0.75 (each 3H,s,CH3×8);13C NMR (125 MHz,CD3OD):δ39.5 (C-1),28.0(C-2),78.7 (C-3),40.5 (C-4),61.5 (C-5),78.3 (C-6),45.2 (C-7),41.2 (C-8),50.1 (C-9),39.7 (C-10),31.0(C-11),70.4 (C-12),49.2 (C-13),51.5 (C-14),30.8(C-15),26.7 (C-16),51.7 (C-17),17.7 (C-18),17.7(C-19),83.4 (C-20),22.5 (C-21),36.1 (C-22),23.4(C-23),126.0 (C-24),131.1 (C-25),17.9 (C-26),25.9(C-27),31.9 (C-28),17.2 (C-29),16.2 (C-30),106.1(3-Glc,C-1′),75.5 (C-2′),80.3 (C-3′),71.8 (C-4′),80.0 (C-5′),63.1 (C-6′),98.3 (20-Glc,C-1″),75.2(C-2″),79.3 (C-3″),71.6 (C-4″),78.4 (C-5″),62.8 (C-6″)。以上数据与文献[14]报道基本一致,鉴定为人参皂苷Rg1。
化合物7白色粉末,ESI-MSm/z: 955 [M -H]–;1H NMR (400 MHz,CD3OD):δ5.37 (1H,d,J=8.0 Hz,H-12),1.15,1.04,0.94,0.93,0.90,0.83,0.79(each 3H,s,CH3×7);13C NMR (100 MHz,CD3OD):δ39.8 (C-1),27.0 (C-2),90.8 (C-3),40.3 (C-4),57.0(C-5),19.0 (C-6),34.0 (C-7),40.7 (C-8),48.4 (C-9),37.5 (C-10),23.9 (C-11),123.8 (C-12),144.8 (C-13),41.9 (C-14),28.9 (C-15),23.5 (C-16),47.5 (C-17),42.2 (C-18),46.4 (C-19),32.0 (C-20),33.5 (C-21),33.5 (C-22),28.5 (C-23),17.7 (C-24),16.0 (C-25),17.7 (C-26),26.3 (C-27),178.1 (C-28),33.5 (C-29),24.0 (C-30),109.4 (3-Glc A,C-1′),73.9 (C-2′),86.4(C-3′),71.1 (C-4′),76.7 (C-5′),176.2 (C-6′),106.8(20-Glc,C-1″),75.4 (C-2″),76.6 (C-3″),76.5 (C-4″),78.7 (C-5″),63.2 (C-6″),95.7 (28-Glc,C-1‴),73.9 (C-2‴),82.9 (C-3‴),71.1 (C-4‴),78.7 (C-5‴),62.4 (C-6‴)。以上数据与文献[12,15]报道基本一致,鉴定为菠棱皂苷A 28-O-葡萄糖苷。
化合物8白色粉末,ESI-MSm/z: 617 [M -H]–;1H NMR (400 MHz,C5D5N):δ6.32 (1H,d,J=6.5 Hz,H-1′),1.23,1.22,1.12,1.02,0.90,0.89,0.85(each 3H,s,CH3×7);13C NMR (100 MHz,C5D5N):δ39.1 (C-1),28.4 (C-2),78.3 (C-3),40.4 (C-4),55.9(C-5),18.9 (C-6),33.3 (C-7),39.5 (C-8),48.2 (C-9),39.7 (C-10),23.9 (C-11),123.0 (C-12),144.3 (C-13),41.9 (C-14),28.9 (C-15),23.5 (C-16),47.1 (C-17),42.2 (C-18),46.3 (C-19),30.9 (C-20),34.1 (C-21),32.6 (C-22),30.9 (C-23),15.7 (C-24),16.7 (C-25),17.6 (C-26),26.2 (C-27),176.7 (C-28),23.8 (C-29),33.2 (C-30),95.9 (28-Glc,C-1′),79.5 (C-2′),79.0 (C-3′),71.2 (C-4′),74.2 (C-5′),62.3 (C-6′)。以上数据与文献[16]报道数据一致,故鉴定为齐墩果酸28-O-β-D-葡萄糖苷。
化合物9白色粉末,ESI-MSm/z: 793 [M -H]–;1H NMR (500 MHz,C5D5N):δ5.33 (1H,d,J=7.7 Hz,H-1″),4.91 (1H,d,J= 7.3 Hz,H-1′),1.24,1.22,1.03,0.95,0.92,0.91,0.66 (each 3H,s,CH3×7);13C NMR (125 MHz,C5D5N):δ38.6 (C-1),26.6 (C-2),89.3 (C-3),39.7 (C-4),55.8 (C-5),18.5 (C-6),33.4(C-7),39.6 (C-8),48.0 (C-9),36.9 (C-10),23.7 (C-11),122.6 (C-12),144.9 (C-13),42.0 (C-14),28.3(C-15),23.8 (C-16),46.7 (C-17),42.1 (C-18),46.5 (C-19),31.0 (C-20),34.2 (C-21),33.2 (C-22),28.2 (C-23),15.5 (C-24),16.8 (C-25),17.4 (C-26),26.2 (C-27),180.3 (C-28),23.8 (C-29),33.2 (C-30),106.0 (3-GluUA C-1′),82.8 (C-2′),78.5 (C-3′),73.7 (C-4′),77.6 (C-5′),174.9 (C-6′),105.3 (C-1″),77.9 (C-2″),78.5 (C-3″),71.6 (C-4″),77.2 (C-5″),62.6 (C-6″)。以上数据与文献[5]报道一致,故鉴定为姜状三七苷R1。
2 结果和讨论
对姜状三七根茎中的化学成分进行了研究,从70%甲醇提取物中分离鉴定了9 个皂苷类化合物,分别是人参皂苷SL1(1)、人参皂苷Rh1(2)、三七皂苷R8(3)、竹节参皂苷IVa (4)、越南人参皂苷R10(5)、人参皂苷Rg1(6)、菠棱皂苷A 28-O-葡萄糖苷 (7)、齐墩果酸28-O-β-D-葡萄糖苷 (8)和姜状三七苷R1(9)。化合物1、3、5、7 和8 为首次从姜状三七中分离得到。化合物1~3 和6 为原人参三醇型皂苷,化合物4、7~9 为齐墩果烷型皂苷,化合物5 为奥克梯隆醇型人参皂苷,此类型皂苷为首次从姜状三七中得到。
人参皂苷Rg1(6)是中药人参、三七等人参属药材的主要活性成分之一,可通过抗氧化作用维持细胞内Ca2+的稳态达到保护心肌细胞的作用[17]; 对多种动物中枢神经系统疾病模型具有治疗作用,主要是通过NMDA 受体、MAPK、MEK- ERK1/2-PI3K、Ca2+-CaM-CaMK II 等信号通路进行调控,作用于皮层、海马、纹状体、下丘脑,影响神经递质的合成与释放,促进神经细胞生长、神经突触的发生,保护神经元,促进神经干细胞分化等[18]; 还可明显抑制人白血病TF-1 细胞的增殖并促进其凋亡,辅助T细胞免疫活性增加,对小鼠免疫性肝损伤也有一定的保护作用[19]。此外,人参皂苷Rg1还有抗肿瘤,降血糖、降血脂,抗骨质疏松和抗炎等作用[20]。
竹节参苷Ⅳa (4)也具有多种药理活性,尤其是其卓越的心脑血管保护作用,降糖、降脂作用,抗炎、抗凝血作用以及特异性的肿瘤细胞增殖抑制作用等,相关作用机制及靶点主要包括激活SIRT1/ERK1/2/Homer1a 途径,增强机体清除氧自由基的能力、降低心肌细胞膜脂质过氧化的程度,上调PTEN、下调NF-κB 的表达,调节Ca2+、K+、Na+等离子的跨膜转运,促进GSK-3β的磷酸化,抑制PKC的磷酸化,抑制Wnt/β-catenin 信号通路,抑制炎症因子表达,抑制血小板聚集,诱导肿瘤细胞周期停滞及凋亡,导致病毒直接失活或抑制子代病毒释放[21]。越南人参皂苷R10(5)为奥克梯隆醇型皂苷,是一类侧链具有呋喃环的四环三萜类皂苷,主要存在于人参属植物西洋参(P.quinquefolius)、竹节参(P.japonicus)、狭叶竹节参(P.bipinnatifidusvar.angustifolius)、珠子参(P.japonicusvar.major)、喜马拉雅假人参(P.pseudo-ginsengsubsp.himalaicus)中,葫芦科(Cucurbitaceae)和桦木属(Betula)植物中也发现了多种奥克梯隆型皂苷。奥克梯隆型皂苷在这些植物中虽然含量甚微,但具有较强的生物活性,包括保护心肌损伤、增强神经元活性、抗肿瘤等[22–23]。

