Endoscopic ultrasound radiofrequency ablation of pancreatic insulinoma in elderly patients: Three case reports
Gemma Rossi, Maria Chiara Petrone, Gabriele Capurso, Stefano Partelli,Massimo Falconi, Paolo GiorgioArcidiacono
Abstract
Key Words: Endoscopic ultrasound; Radiofrequency ablation; Insulinomas; Neuroendocrine neoplasms; Ablative therapies; Case report
lNTRODUCTlON
The incidence of pancreatic neuroendocrine neoplasms (p-NENs) has increased over the last decades due to advances in imaging methods[1]. Non-functional p-NENs that were typically diagnosed at advanced stages when the volume of the lesions determined symptoms, are now often incidentally diagnosed as small (< 2 cm) lesions and whether any treatment should be pursued is debatable[2].
Functional p-NENs (F-pNENs) are usually recognized at early stages, due to the presence of a specific syndrome[3]. Surgery is always indicated in symptomatic cases as the gold standard. However, given the high morbidity and mortality of pancreatic surgery, alternative treatments such as endoscopic ultrasound (EUS)-guided radiofrequency ablation (RFA) can be considered in order to obtain resolution of the syndrome in elderly patients with comorbidities and high surgical risks. The current literature[4] is scarce regarding data on EUS-RFA treatment of F-pNENs. Therefore, safety concerns remain and long-term data on the efficacy of this treatment are needed[5]. Moreover, specific RFA settings (particularly in terms of ablation power) are not standardized.
CASE PRESENTATlON
Chief complaints
This is a case series presenting data on the feasibility, safety and clinical efficacy of EUS-guided RFA to induce relief of the clinical syndrome in 3 elderly patients with symptomatic pancreatic insulinomas at high surgical risk.
History of present illness
Three elderly patients with symptomatic pancreatic insulinomas underwent a total of 4 EUS-RFA procedures performed after failure or limited control with medical treatments.
Case 1:An 84-year-old male patient had repeated episodes of syncope for 3 years, associated with blood glucose < 20 mg/dL and neuroglycopenic symptoms with prompt relief of symptoms following the administration of glucose. The diagnosis of insulinoma was supported by a preoperative fasting test.
Case 2:An 82-year-old male patient, with 2 previous episodes of syncope and marked hypoglycemia (glucose = 38 and 32 mg/dL) was referred to our center. A fasting test confirmed the diagnosis of insulinoma with glucose and C-peptide levels (glucose 40 mg/dL, C-peptide 0.7 ng/mL).
Case 3:An 84-year-old female patient was referred to our center after two years of symptomatic hypoglycemic episodes (glucose < 30 mg/dL). A fasting test was suggestive of pancreatic insulinoma, with neuroglycopenic symptoms after fasting associated with levels of glucose 30 mg/dL and C-peptide 0.9 ng/mL (normal values: 1.1-4.4 ng/mL).
History of past illness
Case 1:The patient had chronic renal failure and severe ischemic heart disease.
Case 2 and case 3:These 2 patients were affected by severe chronic obstructive pneumopathy disease.
Personal and family history
No family history of NENs was present in these cases.
Physical examination
Case 1 had moderate obesity. The other two patients did not present specific signs at physical examination.
Laboratory examinations
All three patients had consistent and constant neuroglycopenic symptoms and diagnosis was supported by elevated insulin, C-peptide and proinsulin blood levels at the preoperative fasting test. The same plasma markers were monitored after EUS-guided RFA to support the relief of hypoglycemic symptoms and clinical syndrome.
Imaging examinations
All the patients underwent magnetic resonance imaging (MRI) with administration of contrast medium and the lesions were diagnosed as likely p-NENs ranging in size from 9 to 14 mm.
MULTlDlSClPLlNARY EXPERT CONSULTATlON
The patients were referred to our multidisciplinary neuroendocrine tumor board, and due to their age and comorbidities it was decided to treat the lesions with EUS-RFA at the Hospital’s Endosonography Unit.
FlNAL DlAGNOSlS
A cytological diagnosis of insulinoma was obtained with EUS-FNA in case 2. In case 1 and case 3 the clinical, biochemical and radiological findings were considered typical for insulinoma and multidisciplinary evaluation considered biopsies unnecessary as cited in international guidelines[2,6].
TREATMENT
During the endoscopic procedure the patients underwent deep sedation and were placed in the left lateral position. In each case, RFA was delivered by a 19-gauge needle (EUSRA; STARmed Co., Ltd., Goyang, Korea), with a 5 mm-active monopolar electrode on the distal part of the probe (delivering the ablation). The needle was inserted in the operative channel of a therapeutic EUS-scope (Pentax EG-3870UTK or Pentax 38J10UT), connected to an ultrasound platform (Hitachi Arietta 750 or Hitachi Arietta 850). The needle was also connected to a RFA generator (VIVA; STARmed Co., Ltd., Goyang, Korea) delivering the thermal energy to ablate the lesions and was also connected to a peristaltic pump infusing cold saline solution (at 0 °C, to avoid tissue charring around the probe, maximizing the lesion ablation volume). Figure 1 describes the RFA system. The generator was set at 30 W of power in all procedures and treatment was applied for different times depending on tissue impedance (system was stopped at impedance > 500 Ω, resulting in an ineffective treatment), until a complete “cloud effect” was obtained in the lesion area (multiple RFA applications were performed during the same endoscopic session). Each patient underwent a computed tomography (CT) scan 24-72 h after the RFA procedure, in order to assess the size of the necrotic area inside the lesions and exclude complications.
Case 1
One single endoscopic session was conducted with 4 subsequent EUS-RFA applications at 3 W for 12-16-12-10 s each and stopped when the impedance increased.
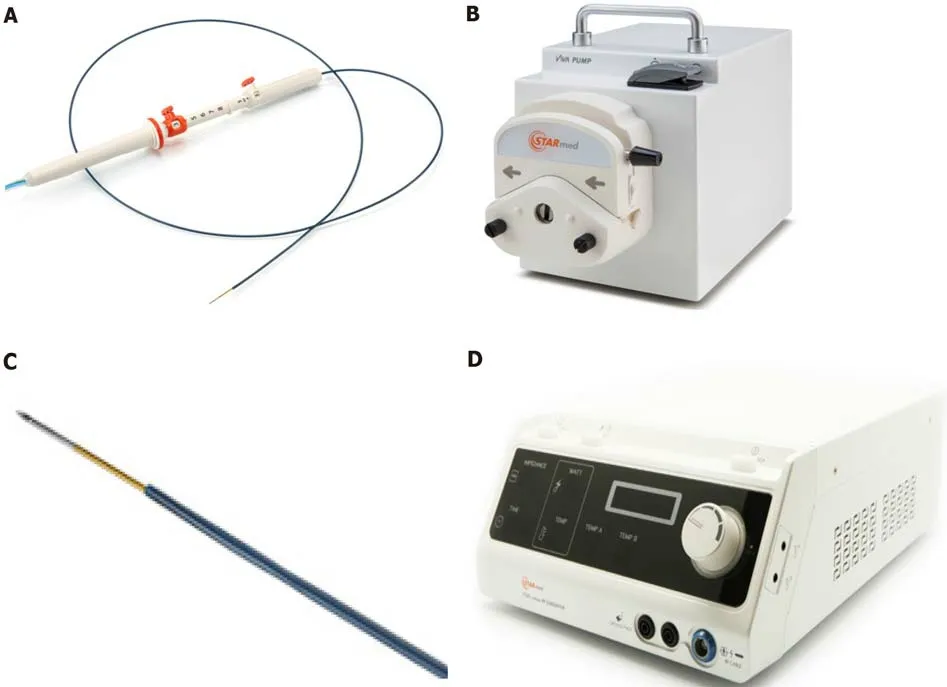
Figure 1 Radiofrequency ablation system. A: Needle, similar to an endoscopic ultrasound fine needle aspiration or biopsy needle; B: Peristaltic pump which can infuse during the ablation, the electrode with chilled solution, maximizing volume ablation; C: Electrode on the distal needle tip, delivering radiofrequency ablation; D: Radiofrequency generator, with the possibility to monitor ablation parameters: Power, time, impedance. Citation: Rossi G, Petrone MC; Capurso G, Albarello L, Testoni SGG, Archibugi L, Lena MS, Doglioni C, Arcidiacono PG. Standardization of a Radiofrequency Ablation Tool in an Ex-Vivo Porcine Liver Model. Gastrointest Disord 2020; 2: 300-309. Copyright© The Authors 2020. Published by MDPI. No special permission is required to reuse all or part of article published by MDPI, including figures and tables, see https://www.mdpi.com/openaccess#Permissions. The authors have obtained the permission for figure using from Rossi G (Supplementary material).
Case 2
A first RFA procedure was performed with 3 applications lasting 20, 15 and 15 s each at a power of 30 W. Complete relief of symptoms was not obtained, while a 72-h CT scan showed a 7 mm hypodense necrotic area. Blood tests were consistent with ablation failure. A second EUS-RFA session was performed after 1 mo. Four RFA applications were carried out for 10, 8, 6, and 8 s, respectively, until complete covering of the pancreatic insulinoma by a hyperechoic cloud was observed. Possibly due to the proximity between the lesion and gastroduodenal artery (Figure 2A), immediate post-procedural bleeding was endoscopically evidenced with a submucosal hematoma located at the superior duodenal genus, due to a side-branch artery injury. Bleeding was immediately treated by mechanical (metallic clip) and injective (adrenalin dilution: 1:10000) hemostatic therapy with success (Figure 2B).
Case 3
The procedure was performed by 3 applications lasting 6 s each at the standard power of 30 W.
OUTCOME AND FOLLOW-UP
Case 1
No immediate or late complications occurred and immediate clinical success with syndrome relief was obtained. A CT scan performed 48-h after the procedure showed a 14 mm hypodense necrotic area in the pancreatic tail, as the outcome of the procedure. A subsequent diagnostic EUS performed after 3 mo, showed a total non-vascularized 12 mm area on contrast enhancement (Sonovue®, Bracco) at the site of the previous RFA (Video). The patient is still asymptomatic (with mild hyperglycemia), after 27-mo of clinical follow-up. No further radiological examinations were performed due to chronic renal failure and related-risks of CT or MRI-contrast medium administration.
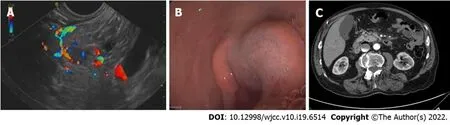
Figure 2 Case 2 imaging. A: A hyper-vascularized lesion compatible with an insulinoma, extremely close to the gastroduodenal artery is visible; B: Submucosal bleeding after radiofrequency ablation, treated by endoscopic hemostasis; C: Computed tomography scan 72 h after radiofrequency ablation: An 8 mm hypodense necrotic area at the previous lesion location, without signs of bleeding.
Case 2
The patient showed relief of hypoglycemic symptoms immediately after the second procedure with normalization of glucose blood levels. A CT scan performed 72 h after RFA revealed an 8-mm hypodense necrotic area at the site of the lesion, without evidence of bleeding (Figure 2C). The patient refused further radiological follow-up and complete symptom relief persists at 24 mo with normalization of biochemical tests.
Case 3
A CT scan with contrast enhancement was performed 72 h after RFA and confirmed the presence of a 13-mm necrotic area inside the lesion, and complete relief from hypoglycemic symptoms was obtained. After 15 mo the patient remains asymptomatic without the need for treatment. A contrast-enhanced MRI performed 14 mo after the procedure confirmed the complete disappearance of the treated lesion in the pancreatic body.
DlSCUSSlON
EUS-RFA represents a potentially useful and safe option to treat insulinomas and related symptoms in patients at high surgical risk, especially in cases of pancreatic head/neck lesions, requiring a Whipple resection. EUS-RFA is relatively safe, although specific care needs to be paid to the bleeding risk of such hypervascularized lesions. Usually RFA-related complications can be endoscopically treated by a highly experienced endoscopist. In the present series, EUS-RFA led to symptom relief during a relatively long follow-up, with a single endoscopic session in 2 patients and 2 endoscopic sessions in the remaining patient. Notably, while most of the published case series on this topic did not present specific and standardized ablation settings[7-10], in the present study we standardized the setting of the ablation power in line with previousex-vivoanimal[11] and human studies (unpublished data), with the application of 30 W and stopping energy delivery when tissue impedance increased. All 3 patients are symptom-free after more than 12 mo of clinical and biochemical follow-up and the lesion is no longer visible after 14 mo in one of the patients who underwent radiological examination.
CONCLUSlON
Larger multicenter studies with a longer and standardized follow-up are needed in order to confirm the safety and long-term clinical success of EUS-RFA in patients with p-NENs. The results of a large ongoing multicenter study endorsed by the European Neuroendocrine Tumour Society are eagerly awaited (ClinicalTrials.gov Identifier: NCT03834701).
FOOTNOTES
Author contributions:Rossi G, Petrone MC, Capurso G, Arcidiacono PG made substantial contributions to conception and design of the study, acquisition of data, or analysis and interpretation of data; Rossi G, Petrone MC, Capurso G, Partelli S, Falconi M, Arcidiacono PG drafted the article or made critical revisions related to important intellectual content of the manuscript; and all authors provided final approval of the version of the article to be published.
lnformed consent statement:All three patients included in the present case series gave their consent prior to study inclusion.
Conflict-of-interest statement:The authors have note conflicts of interest to declare.
CARE Checklist (2016) statement:The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Italy
ORClD number:Gemma Rossi 0000-0003-4071-8247; Maria Chiara Petrone 0000-0002-1045-209X; Gabriele Capurso 0000-0002-0019-8753; Stefano Partelli 0000-0001-8938-6170; Massimo Falconi 0000-0001-9654-7243; Paolo Giorgio Arcidiacono 0000-0001-6692-7720.
S-Editor:Wang JJ
L-Editor:Webster JR
P-Editor:Wang JJ
 World Journal of Clinical Cases2022年19期
World Journal of Clinical Cases2022年19期
- World Journal of Clinical Cases的其它文章
- Current guidelines for Helicobacter pylori treatment in East Asia 2022: Differences among China, Japan, and South Korea
- Review of epidermal growth factor receptor-tyrosine kinase inhibitors administration to non-small-cell lung cancer patients undergoing hemodialysis
- Arteriovenous thrombotic events in a patient with advanced lung cancer following bevacizumab plus chemotherapy: A case report
- Acute choroidal involvement in lupus nephritis: A case report and review of literature
- Choroidal thickening with serous retinal detachment in BRAF/MEK inhibitor-induced uveitis: A case report
- Esophageal granular cell tumor: A case report
