Preparation of Alizarin Dye Dispersion Solution and Dyeing of Cotton Fabric
TAN Youzhi(谭由之), WANG Yunhong(王运红), XU Yajing(徐雅婧), GUO Lamei (郭腊梅), 2*
1 College of Textiles, Donghua University, Shanghai 201620, China2 Key Laboratory of Textile Science & Technology, Ministry of Education, Donghua University, Shanghai 201620, China
Abstract: Alizarin, extracted from rubia cordifolia, is a green natural dye. However, its application has been limited by its poor water solubility and pH sensitivity. In this study, linear sulfonic anionic surfactant (LAS) had been studied as a new dispersant, which promoted the dyeing of alizarin on fabrics under mild conditions. Ultraviolet visible (UV-vis) spectra and Fourier transform infrared (FTIR) spectra confirmed appropriate bonding between alizarin and LAS, and the nano particle size analysis showed that LAS could promote the dispersion of alizarin in aqueous solution. Under the optimized condition of alizarin 4.8% on mass of fabric (omf) and LAS solution 2×10-2 mol/L, the fabrics were dyed with alizarin at room temperature and pH neutral conditions. The dyed fabrics had good washing fastness and ironing fastness.
Key words: alizarin dye; linear sulfonic anionic surfactant (LAS); disperse dye; fastness property
Introduction
Alizarin is one of the oldest plant dyes, which exists widely in the root of rubia cordifolia. It has many advantages, such as wide source, low cost, high absorption coefficient, environmental friendliness and rapid natural degradation[1]. As a natural dye, alizarin has the advantages of non-toxic, pollution-free and high biodegradation rate. However, it also has some disadvantages, such as poor color fastness, difficult dyeing, and poor reproducibility. All these problems limit its application in textiles[2].

Based on the above research, linear sulfonic anionic surfactant (LAS) was used as the main dispersant to make alizarin dye cotton fabrics under mild conditions, and the results of this dyeing method were analyzed.
1 Experiments
1.1 Test materials and instruments
All test materials were obtained from commercial vendors and used as received unless otherwise noted.
The 14.6 tex×14.6 tex, 133 pieces/(10 cm)×100 pieces/(10 cm) pure cotton woven plain white cloth was purchased from Dongguan Fengying Clothing Co., Ltd., China. The multi-fiber interlining fabrics (including wool fiber, acrylic fiber, polyester fiber, nylon fiber, cotton fiber, and acetate fiber) was purchased from Shanghai Luozhong Technology Development Co., Ltd., China. Methanol was purchased from Shanghai Titan Chemical Co., Ltd., China. LAS was purchased from Sinopharm Chemical Reagent Co., Ltd., China, and it is C12-LAS. Alizarin was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd., China.
All the masses were weighed by the Sartorius electronic balance purchased from Beijing Saiduolis Balance Co., Ltd., China. All stirring operations were performed with an S10-3 constant temperature magnetic stirrer purchased from Shanghai Sile Instrument Co., Ltd., China. All drying processes were accomplished through DGG-9030B oven brought from Shanghai Senxin Experimental Instrument Co., Ltd., China. The reaction temperature was controlled by a DKB-501A super constant temperature water tank made by Shanghai Senxin Experimental Instrument Co., Ltd., China. The rubbing fastness experiments were tested by a Y571N dyeing rubbing fastness machine purchased from Nantong Hongda Instrument Co., Ltd., China. The washing fastness experiments were tested by an SW-8A washing fastness testing machine produced by Nantong Hongda Experimental Instrument Co., Ltd., China. TheK/Svalue was measured by a Datacolor 850 colorimeter purchased from Datacolor Company, USA. The particle sizes were measured by a Nanorac Wave II nanoparticle size and Zeta potential analyzer purchased from Malvern Company, Britain. The UV-vis spectra were determined by the Lambda 35 ultraviolet visible near infrared spectrophotometer purchased from Perkineimer Company, USA. The FTIR spectra were determined by the Nexus-670 Fourier infrared spectrometer purchased from Nicolet Company, USA.
1.2 Preparation and dyeing of alizarin disperse dye
1.2.1Preparationofdyeingsolution
Firstly, the raw white fabrics were weighted and the certain amount of the water was obtained according to the bath ratio of 1∶20. Secondly, a certain proportion of alizarin and LAS were added into the water to prepare the dyeing solution. Finally, the solution was stirred in a constant temperature magnetic stirrer for 10 min. According to the above steps, the alizarin dispersion solution (ADS) was prepared.
1.2.2Dyeing
A certain amount of ADS was prepared followed by the method in section 1.2.1. The fabrics were put into the ADS and stirred for a period of time for dyeing at constant temperature. The obtained dyed fabrics (ODFs) were taken out after dyeing and were washed under running clean water to remove the floating color. The clean ODFs were dried at 120 ℃. Using alizarin dosage, LAS dosage, dyeing temperature, and dyeing time as influence factors, a four-factor three-level orthogonal experiment was designed for dyeing the fabrics.
1.3 Test methods
1.3.1Characterizationtests
The chemical structure of the dye was characterized by FTIR spectrometer. The absorbance of the ADS was characterized by UV-vis spectrophotometer. Firstly, 2×10-3mol/L ADS was prepared according to the method in section 1.2.1, and 2×10-3mol/L alizarin aqueous solution (AAS), 2×10-3mol/L LAS solution (LASS), 8×10-5mol/L alizarin methanol solution (AMS) were prepared in the same way. Then, insoluble matter was filtered with 0.45 μm polyvinylidene fluoride membrane and clear solutions were prepared. Finally, the clear solutions were tested by UV-vis spectrophotometer, and the absorbance of the ADS was compared with AAS, LASS and AMS. The dispersing effect of LAS on alizarin was verified.
1.3.2Determinationofsolubilityanddyeparticlesizeindyeingsolution
Alizarin was dissolved in LASS to calculate the solubility. Firstly, 0.1 g alizarin was added into the 5 g/L LASS so that alizarin can dissolve in the solution through stirring. Then, the 0.45 μm polyvinylidene fluoride membrane was used to filtrate insoluble solute. The filtered filter cup and filter paper were dried in the oven and weighed after cooling. Finally, the solubility of alizarin in LASS was estimated shown as
(1)
wheresis the solubility (g/L),msis the mass of solute (g), andVlis the volume of liquids (L).
Both 2×10-2mol/L ADS and 2×10-2mol/L AAS were prepared according to the method in section 1.2.1. The particle size and distribution of the ADS was tested by a nano particle sizer under normal temperature condition, and the results were taken the average value after three measurements. The particle size distribution of AAS was used as control.
1.3.3Testsofdyeingeffect
Firstly, the ODFs were folded into many layers in order to prevent light transmission. Secondly, two points from ODFs were taken in the longitude and latitude directions respectively by Datacolor 850 colorimeter and the tests were conducted a total of 4 times. Finally, the results such asK/Svalue and color characteristic value were taken the average value as the final test results.
1.3.4Testsofdyeingfastness
Firstly, in the washing fastness test, the ODFs and the standard interlining fabrics should be stitched together to check the fading of the dyed fabrics and the staining of the interlining fabrics. The 100 mm × 40 mm size of ODFs were sewn with 100 mm×40 mm size of interlining fabrics together along the short side. Soaping solution with the concentration of 5 g/L was prepared and stirred for 10 min which could make the soap dissolve completely. The sewn fabrics were added soaping solution at the bath ratio of 1∶50, and then washed by washing fastness testing machine for 30 min at a constant temperature of 40 ℃. Secondly, in the test of rubbing fastness, the fabrics should be rubbed in dry and wet environment respectively so that the dry rubbing fastness and wet rubbing fastness were obtained. The ODFs were fixed on the dyeing rubbing fastness machine, while the dry lining fabric and wet lining fabric were fixed on the friction head for rubbing. The liquid carrying rate of wet lining fabric is 100%. Thirdly, in the test of ironing fastness, the fabrics should be ironed in dry and wet environment respectively so that the dry pressing fastness and wet pressing fastness were obtained. The ODFs were pressed by iron for 15 s, and a wet lining fabric sewn on ODFs were pressed. The liquid carrying rate of wet lining fabric is 100%. Finally, all treated dyed fabrics and interlining fabrics were evaluated with standard colorimetric gray card under D65 light source[12-14].
2 Results and Discussion
2.1 Characterization of alizarin disperse dye
2.1.1Infraredspectrumanalysisofdyes
The structural formula of alizarin is shown in Fig.1. Alizarin has relatively stable hydrophobicity due to its anthraquinone structure. With a very low solubility in water at room temperature, alizarin cannot be directly used for dyeing, and can only be dissolved in water at high temperature[15]. The results of infrared characterization are shown in Fig.2. Alizarin has a characteristic peak at 1 620 cm-1. However, the solid sample from the mixture of ADS shows a characteristic peak at 1 560 cm-1. In order to eliminate the influence of LAS, the infrared characterization of LAS was carried out. It can be seen that LAS also has a characteristic peak at 1 620 cm-1, but there is no characteristic peak at 1 560 cm-1. This shows that the ADS has red-shift in FTIR absorption spectra and the conjugation about ADS is increasing It means that there may be a π-π effect between alizarin and LAS in solution as shown in Fig.1, which enables alizarin to dissolve and disperse in water at room temperature so that it can form a stable dye disperse solution. In Fig.1, R means alkyl group.

Fig.1 Structure of alizarin: (a) structural formula of alizarin; (b) possible π-π effect between alizarin and LAS

Fig.2 FTIR spectra of dye
2.1.2AnalysisofdyesolutionbyUV-visspectrum
Due to the phenolic hydroxyl structure of alizarin, the solubility of alizarin is significantly affected by pH[16-17]. The hydroxyl group of alizarin is hardly ionized in the neutral aqueous solution environment, which makes the solubility very low. As shown in Fig.3, the filtered AAS is nearly colorless, while the conjugation of LAS to alizarin significantly increases the solubility of alizarin in aqueous solution. The ADS is atropurpureus after being filtered, which even redder than the filtered AMS. Then all solutions are diluted to determine the UV-vis spectra. The results about UV-vis spectra analysis can also prove the function about the LAS dispersant. As shown in Fig.3, AAS and LASS have no obvious absorption in the range of visible light. Considering that the solubility of alizarin in water was too low, for further comparison, the absorbance of alizarin dissolved in methanol was tested. The results show that the absorbed peaks of AMS are at 290 nm and 425 nm, while the main absorption peaks of ADS are at 330 nm and 520 nm. ADS has a significant red shift in UV-vis spectra compared with the AMS, which was similar to the alizarin solution when pH = 12[18]. But the pH of ADS was actually measured to be 7. It shows that the addition of LAS not only increases the dispersion and stability of alizarin in water, but also changes the aggregation structure of alizarin, resulting in color shift.
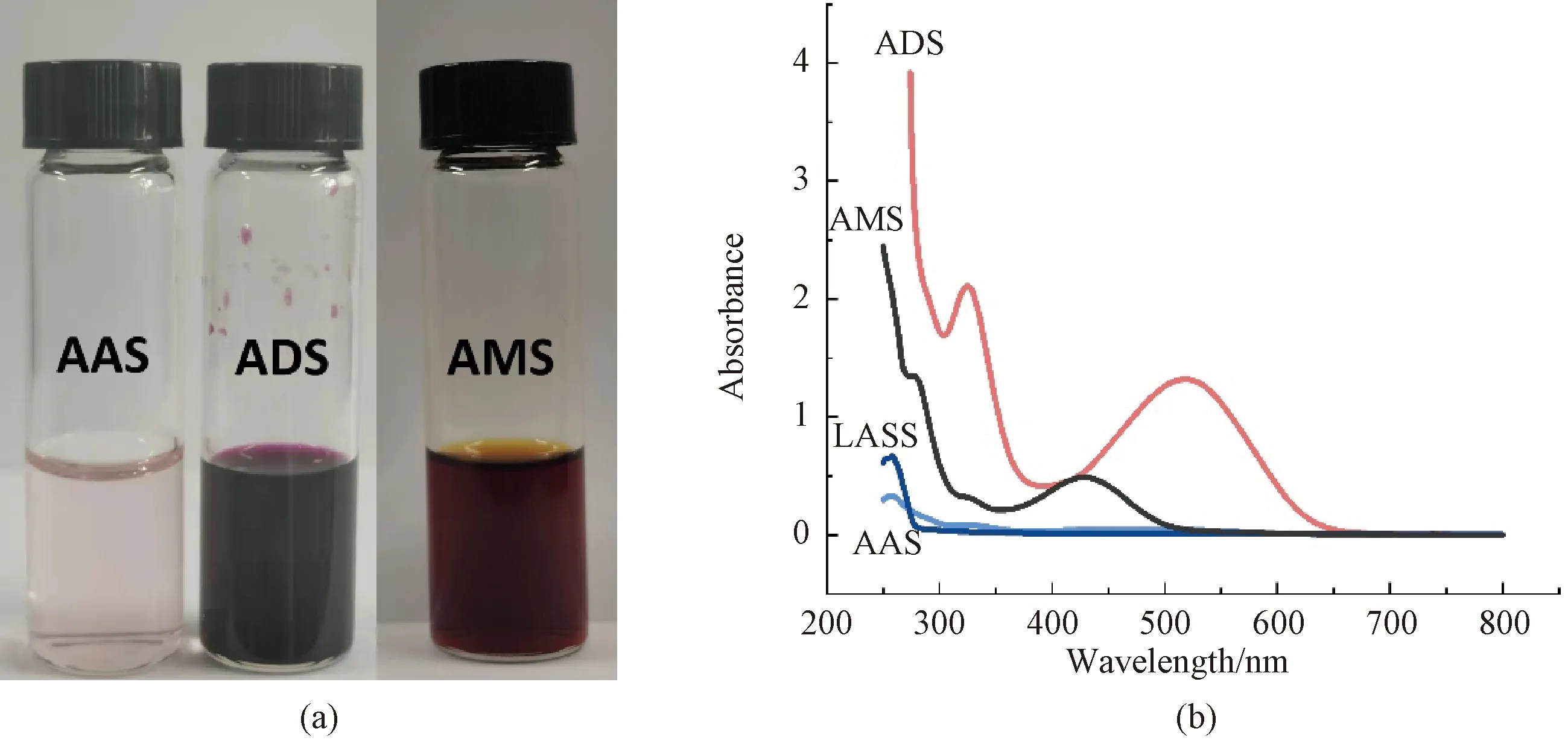
Fig.3 Color and spectra of dye solution: (a) physical pictures of dye solution AAS, ADS, and AMS; (b) UV-vis spectra of dye solution
2.1.3Solubilityandparticlesizeanalysisofdispersedyeingsolution
According to section 1.3.2 and Eq. (1), the solubility of alizarin in LASS is 0.713 g/L after being estimated when the temperature is 18 ℃, while the solubility of alizarin in H2O is 5.04×10-4g/L[19]. And as shown in Fig.4, the particle size of AAS is mainly distributed in the range of 900-2 500 nm, and the average particle size is 1 993 nm. However, due to the limitation of the maximum particle size range of the instrument, the actual particle size in the solution is larger than 10 000 nm. The ADS has a particle size distribution in the range of 100-2 000 nm, and the average particle size is 848.3 nm. The particle size distribution index (PDI) is 0.203 4, which means the solution has good dispersion. Although the ADS has not been especially grinded or treated, the particle size of ADS is more uniform compared with AAS. The results show that LAS not only increases the solubility of alizarin in aqueous solution but also makes the dye particles smaller and the particle size distribution uniform, which can further improve the dyeing effect of ADS.
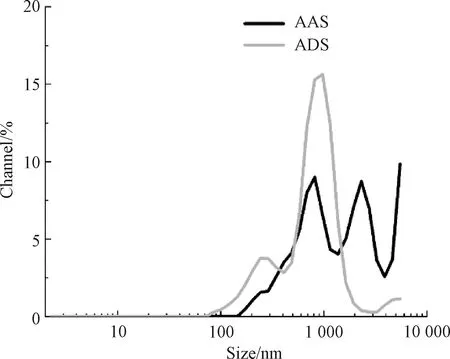
Fig.4 Particle size of alizarin disperse dye
2.2 Dyeing cotton fabric with alizarin dye
Taking the dosage of alizarin, LAS, dyeing temperature and dyeing time as the main influence factors, an orthogonal experiment which has four factors and three levels shown in Table 1 was designed to optimize the dyeing effect of alizarin dye on cotton fabrics. The optimization conditions were carried out in 20 mL of dye solution and the results and analysis are shown in Tables 2 and 3.K/Svalue represents the dyeing depth of dyed fabrics. While theK/Svalue is higher, the color of ODFs is darker and the dyeing effect is better. The importance of factors affecting dyeing result can be concluded by the analysis of Table 3 withK/Svalue as index. As shown in Table 3, the maximal range of the four factors is the dosage of alizarin, in which the range (r) is 0.728. And the minimal range of the four factors is the time of dyeing, in whichris 0.121. The most important factor affecting the dyeing effect of fabrics is the dosage of alizarin. It can be concluded from Table 1 that when the amount of alizarin is 4.8% (omf), the fabrics have a good dyeing effect. In Table 3,kj=∑(K/S)j/3, wherejis the level, andkjis the mean of the average about theK/Svalue underjlevel conditions shown in Table 2.

Table 1 Factors and levels of the orthogonal experiment

Table 2 Results of the orthogonal experiment
When the amount of alizarin was fixed and the LAS solution was 10 mmol/L, the fabrics were dyed for 20 min with the dyeing temperature changing. The dyeing effect is shown in Table 4, whereLrepresents the brightness of the dyed fabric,ais the range of dyed fabric from red to green, andbis the range of dyed fabric from yellow to blue. As shown in Table 4, the influence of temperature on ODFs is relatively limited. TheK/Svalue of the fabric dyed at 25 ℃ is 2.532, while theK/Svalue of the fabric dyed at 100 ℃ is only 2.710. And as the temperature rises,L,a, andbvalues of ODFs have no obvious changes. It means that even with the temperature rising, the brightness and chromaticity of ODFs have not changed obviously.
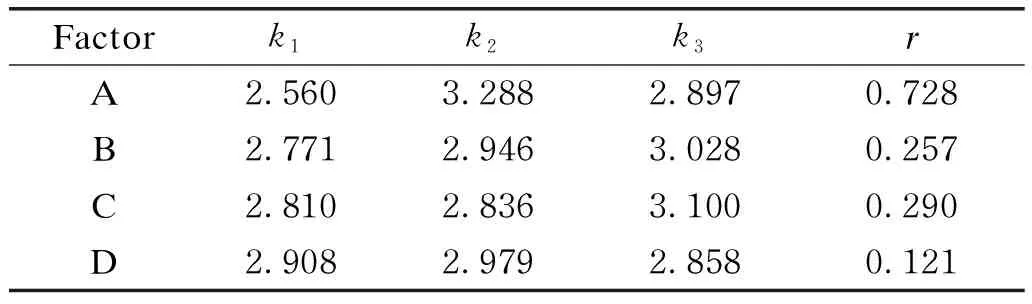
Table 3 Analysis of orthogonal experiment

Table 4 Influence of temperature on dyeing effect
When the amount of alizarin was fixed and the dyeing temperature was 25 ℃, the fabrics were dyed for 20 min with the dosage of LAS changing. The amount of alizarin was fixed at 4.8% (omf), and then the LAS dosage influence experiments were designed according to a different molar ratio of LAS and alizarin dosage. The dyeing effect is shown in Fig.5. When the molar ratio of alizarin to LAS was 3∶1, theK/Svalue of ODFs was 1.132. However, when the molar ratio of alizarin to LAS was 1∶1, theK/Svalue of ODFs was 2.754. It can be concluded that theK/Svalue of the fabric increases gradually with the decrease of the ratio of alizarin to LAS when the amount of alizarin is constant. When the dosage of LAS is gradually excessive, theK/Svalue of the fabric increases slowly, which means that when the dispersing effect of LAS as a dispersant has reached a threshold, even if the amount of dispersant is further increased, the dyeing effect of fabric cannot be improved.

Fig.5 Effect of LAS dosage on dyeing effect
When the amount of alizarin was fixed and the LAS solution was 20 mmol/L, the fabrics were dyed at 25 ℃ with the dyeing time changing. The dyeing effect is shown in Table 5. As shown in Table 5, theK/Svalue of the fabric dyed for 20 min is 2.589, while theK/Svalue of the fabric dyed for 80 min is only 2.766. The influence of time on ODFs is very small. So the dyeing time of the fabric is chosen as 20 min in consideration of cost.

Table 5 Influence of time on dyeing effect
The optimum dyeing process is obtained by designing orthogonal experiment, dyeing temperature influence experiment, dyeing time influence experiment, and LAS dosage influence experiment. When the amount of alizarin is fixed at 4.8% (omf) and the LAS solution is 20 mmol/L, the fabrics dyed for 20 min at 25 ℃ have a betterK/Svalue and dyeing effect.
2.3 Color fastness of fabrics
The fabrics were dyed according to the optimum dyeing process in section 2.2 and treated after dyed according to the preparation method in section 1.2.2. The ODFs were tested according to the test method in section 1.3.4, and the obtained color fastnesses are shown in Tables 6 and 7. It can be seen from Tables 6 and 7 that the color fastnesses of the fabrics obtained by ADS are 3-4 grades, which indicate the ODFs dyed by ADS have certain fastnesses about washing, friction and sublimation. All color fastnesses obtained by ODFs meet the garment standard. It can be seen from Table 6 that raw cloth fading fastness is only 3-4 grades and color change or fading of ODFs can be observed in the cleaning process. However, the result of staining test about dyed fabrics and interlining fabrics are good. The dyed cotton fabrics hardly stained with acrylic, polyester and cotton fabrics, but easily stained with wool, nylon and acetate fabrics. The dyeing fastness to ironing in Table 7 is relatively low in wet pressing, which indicates that high temperature resistance of the dyed cotton fabrics is slightly low,but it still meets the requirements of wearability. The new disperse dyeing method is feasible.
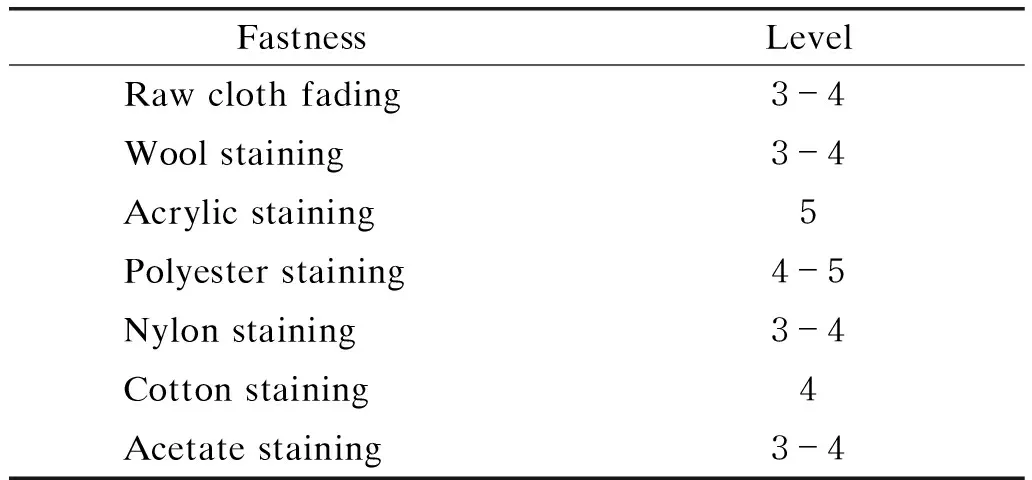
Table 6 Washing fastness of dyed fabrics

Table 7 Rubbing and ironing fastness of dyed fabrics
3 Conclusions
Through characterization analysis and particle size measurement, it can be seen that LAS and alizarin have a conjugation effect, which enables alizarin to be dispersed stably in water under neutral pH and room temperature conditions to form an alizarin dispersion dye solution. By designing orthogonal experiment, dyeing temperature influence experiment, dyeing time influence experiment and LAS dosage influence experiment, the optional dyeing process about 4.8% (omf) alizarin and 20 mmol/L LAS solution is confirmed. The obtained dyed fabrics have good color fastness to washing, rubbing and a certain color fastness to ironing. It lays a good foundation for the preparation of natural plant dye ink at neutral pH.
 Journal of Donghua University(English Edition)2022年6期
Journal of Donghua University(English Edition)2022年6期
- Journal of Donghua University(English Edition)的其它文章
- Conductive Polyacrylonitrile Fiber Prepared by Copper Plating with L-Ascorbic Acid as Reducing Agent
- Fabrication and Characterization of Yarn-Based Temperature Sensor for Respiratory Monitoring
- Anti-wrinkle Finishing of Cotton Fabrics with Pyromellitic Acid Enhanced by Polyol Extenders
- Preparation and Characterization of Sepiolite Microfibers with High Aspect Ratio
- Solution Blowing of Palygorskite-Based Nanofibers for Methylene Blue Adsorption
- Active Absorption of Perforated Plate Based on Airflow
