Peptide Transporter OsNPF8.1 Contributes to Sustainable Growth under Salt and Drought Stresses,and Grain Yield under Nitrogen Deficiency in Rice
QIU Diyang ,HU Rui ,LI Ji,LI YingDING JierongXIA Kuaifei,ZHONG XuhuaFANG Zhongming,ZHANG Mingyong
(1Key Laboratory of South China Agricultural Plant Molecular Analysis and Genetic Improvement,Guangdong Provincial Key Laboratory of Applied Botany,South China Botanical Garden,Chinese Academy of Sciences,Guangzhou 510650,China;2Institute of Fruit Tree Research,Guangdong Academy of Agricultural Sciences,Guangzhou 510640,China;3Rice Research Institute of Guangdong Academy of Agricultural Sciences,Guangzhou 510640,China;4University of Chinese Academy of Sciences,Beijing 100049,China;5College of Agricultural Sciences,Guizhou University,Guiyang 550025,China;6Center of Economic Botany,Core Botanical Gardens,Chinese Academy of Sciences,Guangzhou 510650,China;#These authors contributed equally to this work)
Abstract: Peptide transport is important for plant tissues where rapid proteolysis occurs,especially during germination and senescence,to enhance redistribution of organic nitrogen (N).However,the biological role of peptide transporters is poorly investigated in rice.We characterized the function of the peptide transporter OsNPF8.1 of rice nitrate transporter 1/peptide transporter family (NPF).Ectopic expression of OsNPF8.1 in yeast revealed that OsNPF8.1 encoded a high-affinity di-/tri-peptide transporter,and the osnpf8.1 mutants had a lower uptake rate of the fluorescent-labelled dipeptide c in leaves of rice seedlings.Histochemical assays showed that OsNPF8.1 was highly expressed in mesophyll cells and vascular parenchyma cells,but not detected in root hairs and epidermises.Expression of OsNPF8.1 was induced by N deficiency,drought,NaCl and abscisic acid,and kept at a high level in senescing leaves.Under N deficiency conditions,compared with the wild type Zhonghua 11,the osnpf8.1 mutants grew slower at the seedling stage,and had lower grain yield and lower N content in the grains.In contrast,OsNPF8.1-over-expressing rice (OsNPF8.1-OE) grew faster at the seedling stage and had a higher grain yield.The osnpf8.1 seedlings were less tolerant to salt and drought stresses.These results suggested that stress-induced organic N transportation mediated by OsNPF8.1 might contribute to balance plant growth and tolerate to salt/drought stress and N-deficiency.
Key words: abiotic stress;nitrogen;peptide transporter;rice
Nitrogen (N) is the most important nutrient for plant growth and crop productivity,and is mainly absorbed by roots from the soil as inorganic or organic N(Rakotoson et al,2021).Plants also recycle stored inorganic and organic N to sustain growth or survival during leaf senescence,seed germination,and development of new generations from tubers,as well as under N deficiency and other stresses (Okumoto and Pilot,2011).In cereals,N reutilization from senescing leaves contributes to 50%-90% of the grain N content (Masclaux et al,2001).For rice,approximately 80% of N in panicles has been remobilized from senescing leaves (Mae and Ohira,1981;Wu et al,2018).Organic N remobilization is tightly regulated by the activities of transporters for amino acids,ureides and peptides to control the distribution of N within and between cells over short and long distances(Okumoto and Pilot,2011).Most of proteins in old leaves can be degraded and recycled through proteolysis and autophagy (Masclaux-Daubresse et al,2010;Li et al,2015).
Transport of small peptides across the plasma membrane (PM) has been reported in different species(Fei et al,1998),and peptides can be hydrolyzed to amino acids for reusing in protein synthesis or as sources for carbon and N.Peptide transport is important in plant tissues where rapid proteolysis occurs and translocation of peptides can enhance reallocation of organic N (Higgins and Payne,1978;Granell et al,1998).The uptake of peptides by germinating barley embryos is generally more efficient than that of free amino acids (Higgins and Payne,1978).Scutella of germinating grains of four cereals (barley,wheat,rice and maize) participate in the uptake of peptides(Salmenkallio and Sopanen,1989).The mesophyll tissues of broad bean (Vicia faba) leaves can also take up di-/tri-peptides through a single proton-dependent transporter (Jamai et al,1994).These indicate the ubiquitous existence of peptide transport activities in various plant tissues at different life stages.Plant peptide transporters generally belong to three different gene families.The nitrate transporter 1 (NRT1)/peptide transporter (PTR) family (NPF) can transport di-/tripeptides,the oligopeptide transporter (OPT) family can transport tetra-and penta-peptide oligopeptides,and the ATP-binding cassette transporter (ABC) family can transport peptide conjugates (Stacey et al,2002;Rentsch et al,2007).
The NPF has enigmatic specificity to diverse substrates including di-/tri-peptides,nitrate,plant hormones and glucosinolates (Léran et al,2014),and its members play multi-functions in plant growth and response to stress.Lotus japonicas LjNPF3.1andMedicago truncatula MtNPF7.6play a role in the root nodule symbiosis (Wang et al,2020;Vittozzi et al,2021).ArabidopsisAtNPF7.3 can transport nitrate and indole-3-butyric acid,and then is involved in root gravitropism and nitration mobilization (Chen et al,2012;Watanabe et al,2020).Rice has at least 89 members of NPF,and theOsNPF8subfamily has 20 members (Zhao et al,2010;Léran et al,2014).In theOsNPF8subfamily,OsNPF8.9/OsNRT1is a nitrate transporter and plays a role in inorganic N utilization efficiency for rice (Lin et al,2000;Hu et al,2015),and over-expression ofOsNPF8.20/OsPTR9improves N utilization efficiency,growth and grain yield,but its transport substrate is unknown (Fang et al,2013).
The discovery of the yeast peptide transporter mutantptr2has prompted the identification of peptide transporters of the PTR subfamily of the NPF family from other species (Island et al,1991).The peptide transporter AtPTR1/AtNPF8.1 is localized on PM,and is a putative long-distance transporter of peptides to play a role in the uptake of peptides by roots (Dietrich et al,2004;Komarova et al,2008).The PM-located AtPTR2/AtNPF8.3 transports a wide spectrum of di-/tri-peptides (Steiner et al,1994;Song et al,1997;Chiang et al,2004),and antisense expression ofAtPTR2-BdelaysArabidopsisflowering and inhibits seed development (Song et al,1997).The woundinduced peptide transporter geneAtPTR3/AtNPF5.3may protect plants against biotic and abiotic stresses(Karim et al,2007).The PM-located AtPTR5/AtNPF8.2 facilitates peptide transport into germinating pollen and possibly into mature pollen,ovules and seeds(Dietrich et al,2004;Komarova et al,2008).Barley HvPTR1 (West et al,1998),faba bean VfPTR1(Miranda et al,2003),andHakea actitesHaPTR4(Paungfoo-Lonhienne et al,2009) are also functional peptide transporters.The barleytransporter HvPTR1 might play a central role in the uptake of peptides in the germinating grain (West et al,1998).OsPTR6/OsNPF7.3 is involved in peptide transportation by complementation of theptr2yeast mutant (Ouyang et al,2010),and its over-expression can enhance N utilization efficiency (Fan et al,2014;Fang et al,2017).Rice grain yield is highly related to the physiology of N nutrition (Makino,2011).The proteolytic activity increases remarkably in senescing rice leaves,and rapid organic N remobilization occurs in these organs(Mae and Hoshino,1985).However,the physiologic functions of rice peptide transporters and the effects of peptide transportation on growth and grain yield under limitation of N and abiotic stresses are little known in rice.Here,OsNPF8.1 was identified as a high affinity peptide transporter of rice,and peptide transportation mediated by OsNPF8.1 may contribute to sustain growth under salt and drought stresses,and to grain yield under N limitation.
RESULTS
Rice OsNPF8.1 is a high-affinity peptide transporter
OsNPF8.1/OsPTR7 is highly homologous to the yeast peptide transporters Ptr2p and HvPTR1 (West et al,1998;Ouyang et al,2010).Does OsNPF8.1 function the same as a peptide transporter? We investigated the possible existence of a substrate for OsNPF8.1 using a yeastptr2mutant,the ABC738 strain (Bourbouloux et al,2000).We found that theptr2yeast transformed with an empty vector could not grow on a synthetic medium with the di-peptide Pro-Leu as the sole N source,indicating that theptr2yeast mutant was unable to absorb di-peptides from the media (Fig.1-A).When introduced intoptr2yeast,OsNPF8.1andPTR2could rescue yeast growth on this synthetic medium (Fig.1-A).However,OsNPF8.2andOsNPF8.5could not.These results showed that OsNPF8.1 can mediate the uptake of Pro-Leu into yeast.
The 20 common amino acids can form 400 di-peptides and 8 000 tri-peptides,and peptide transporters in many species exhibit broad specificities (Jones et al,2003;Jensen et al,2012;Solcan et al,2012).To verify the transport affinity of OsNPF8.1 to other small peptides,fluorescent-labelled di-peptide β-Ala-Lys-N-7-amino-4-methylcoumarin-3-acetic acid (AMCA) was used as a tracer substrate to conduct a competition assay according to Dieck et al (1999).OsNPF8.1 could mediate the uptake of β-Ala-Lys-AMCA into yeast cells in a time-dependent manner,but yeastptr2cells could not absorb β-Ala-Lys-AMCA in the time-course uptake assay (Fig.S1-A).Importantly,a kinetic assay showed that the transport of β-Ala-Lys-AMCA was concentration dependent and saturable(Fig.1-B).Then,50 μmol/L physiological di-/tri-peptides were used as competitive substrates to compete with the tracer substrate β-Ala-Lys-AMCA at 50 μmol/L(Fig.1-C).Since the competitive substrates and tracer substrate shared the same binding site,peptides with higher affinity would impair the accumulation of β-Ala-Lys-AMCA (Ito et al,2013).Moreover,an isotope-labeled di-peptide,[15N]Ala-Trp,could be transported by OsNPF8.1 into yeast cells at a rate depending on the substrate concentration (Fig.1-D),indicating that some di-/tri-peptides could compete the uptake of β-Ala-Lys-AMCA,and OsNPF8.1 can take up different di-/tri-peptides into yeast cells.The Michaelis constant (KM) was 139 μmol/L and the maximum transport rate (Vmax) was 918 nmol/(g·h) in yeast,indicating that OsNPF8.1 worked as a highaffinity transporter for physiological di-/tri-peptides as the yeast PTR2 (Cai et al,2006).In addition,many NPF transporters are low-affinity nitrate transporters(Léran et al,2014),and glutathione (GSH) is a nonribosomal tri-peptide abundant in plants.However,nitrate and GSH could barely compete with β-Ala-Lys-AMCA (Fig.S1-B),indicating that OsNPF8.1 might not transport nitrate and GSH.
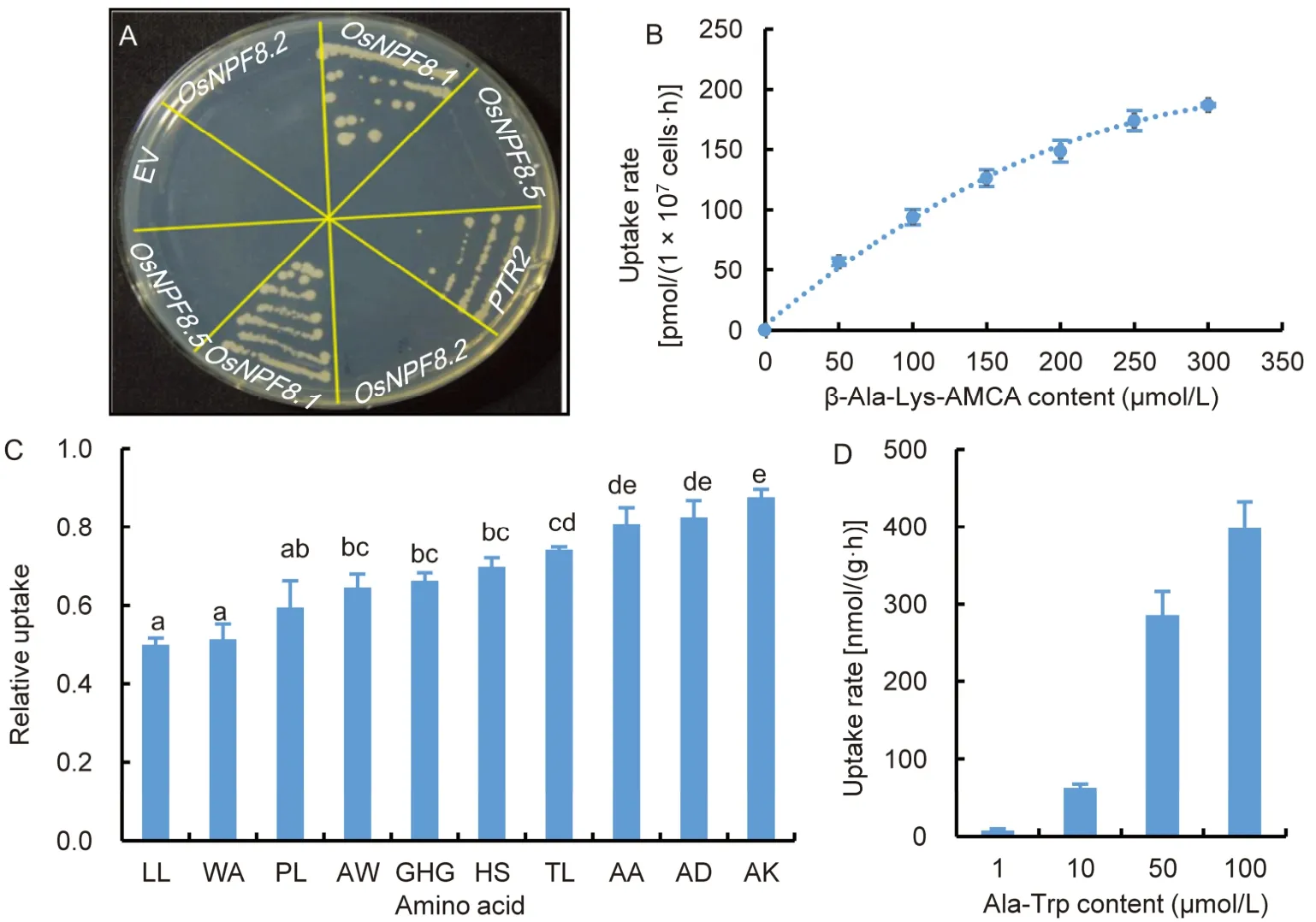
Fig.1.OsNPF8.1 mediates di-/tri-peptide transport.
To investigate the roles ofOsNPF8.1,its mutants(osnpf8.1),generated using the CRISPR/Cas9 genome editing system (Fig.S2-A and -B),andOsNPF8.1-over-expressing rice (OsNPF8.1-OE) were developed(Fig.S2-C).To test whetherOsNPF8.1affects absorption of di-peptide in rice,the uptake rates of β-Ala-Lys-AMCA in roots of the germinating seeds were measured under free N conditions (Fig.2-A).When compared with wild type Zhonghua 11 (ZH11),the uptake rate ofosnpf8.1root for β-Ala-Lys-AMCA showed no difference.This may suggest that OsNPF8.1 did not affect peptide uptake of roots.Then,the uptake rate of the peptide β-Ala-Lys-AMCA mediated by OsNPF8.1 in rice seedling leaves was further examined (Fig.2-B to -C).The uptake rates ofosnpf8.1leaves were lower,and the uptake rates ofOsNPF8.1-OEleaves were higher than that of ZH11 leaves,indicating thatOsNPF8.1could affect the peptide uptake in leaves of rice seedling,rather than in roots.
Expression profile of OsNPF8.1
The expression ofOsNPF8.1in various organs of rice is induced by drought,salt and dimethylarsenate(DMA) (Ouyang et al,2010;Tang et al,2017).β-glucuronidase (GUS) staining confirmed thatOsNPF8.1was expressed in almost all organs (Fig.3-A to -H).GUS staining of transgenic rice bearingPOsNPF8.1::GUSshowed thatOsNPF8.1was not expressed in young lateral roots,root hairs,and root epidermises (Fig.3-E to -H),but was highly expressed in mesophyll cells and vascular parenchyma cells of leaves (Fig.3-C),and in pericycle and cortex of roots(Fig.3-G).qRT-PCR showed thatOsNPF8.1was highly expressed in older leaves at the seedling and heading stages (Fig.3-I) under normal growth conditions.In addition,expression ofOsNPF8.1may be very high in rice according to the high Cy3 signal intensity ofOsNPF8.1(Fig.S3).
To analyze the expression response ofOsNPF8.1to N deficiency and abiotic stresses in shoots and roots,OsNPF8.1expression levels under low N,NaCl,drought and ABA treatments were investigated (Fig.4).OsNPF8.1expression was induced in both shoots and roots by N deficiency and treatment with NaCl,ABA,and water stress.However,under NaCl treatment,OsNPF8.1expression in roots and shoots peaked at 4 and 8 h,respectively,and then decreased;conversely,OsNPF8.1expression remained stable after 6 h of low N treatment.Re-watering after drought also caused a decrease inOsNPF8.1expression.In addition,OsNPF8.1expression was not induced by phosphate(P) and potassium (K) deficiencies (Fig.S4-A).

Fig.2.OsNPF8.1 mediates uptake of β-Ala-Lys-N-7-amino-4-methylcoumarin-3-acetic acid (AMCA) in rice leaves.
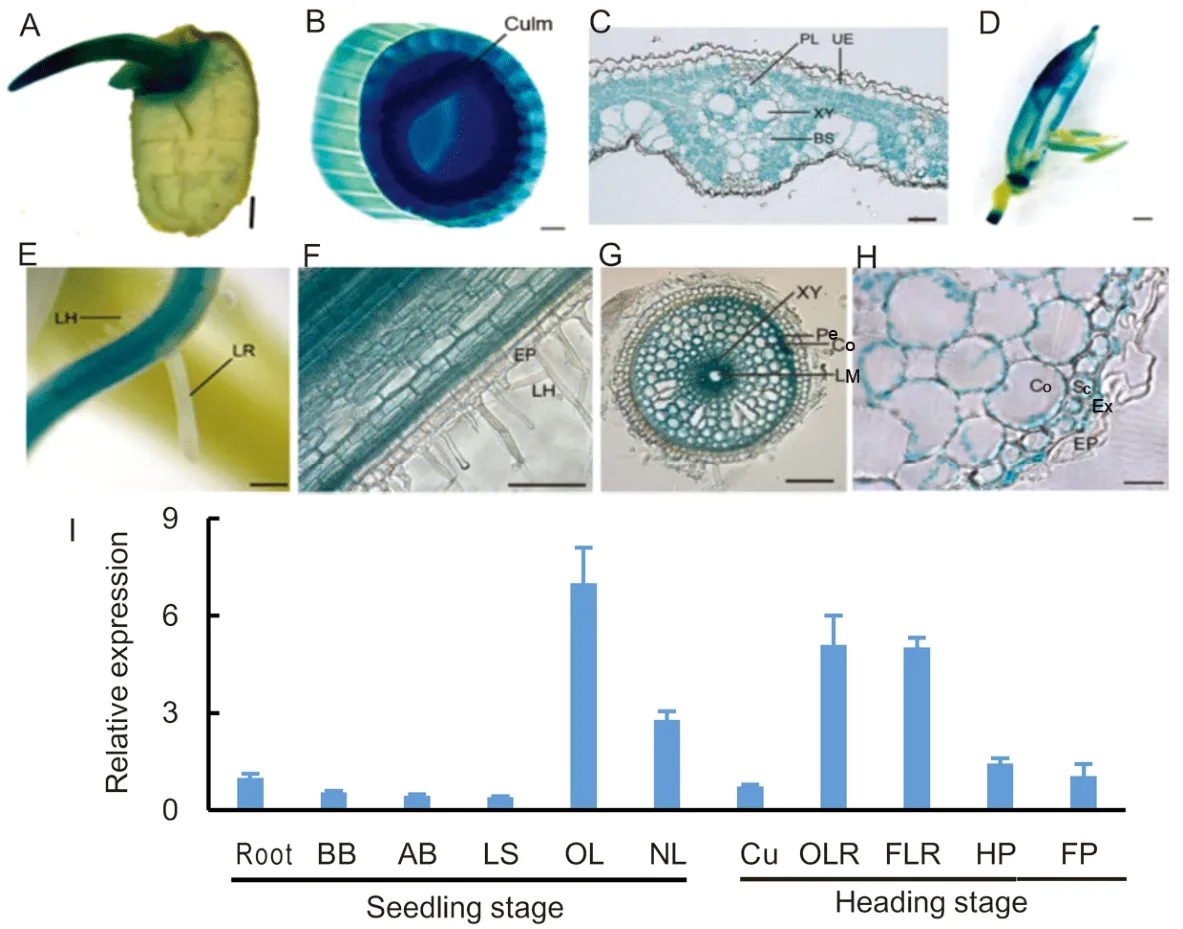
Fig.3.Expression of OsNPF8.1 in wild type rice Zhonghua 11 (ZH11) plants under normal growth conditions.

Fig.4.Induced expression of OsNPF8.1 by low N and abiotic stresses.
To investigate the expression ofOsNPF8.1in functional and senescing leaves at the reproductive stage,OsNPF8.1expression levels in flag leaf blades(FLB) and penultimate leaf blades (PLB) were determined at the grain filling and maturity stages.The expression ofOsNPF8.1did not differ between stages in either FLB or PLB,but was slightly higher in PLB than in FLB (Fig.S4-B).However,OsSGR,a senescence marker gene (Mao et al,2017),was up-regulated in these leaves at the maturity stage (Fig.S4-C),indicating that FLB and PLB undergo senescence at the maturity stage.The senescenceassociated glutamine synthetase-encoding geneOsGS1;1(Kamachi et al,1991) showed a similar expression pattern to that ofOsNPF8.1(Fig.S4-D).These results indicated that expression ofOsNPF8.1remained constant in FLB and PLB during leaf senescence.
OsNPF8.1 affects rice yield under low N conditions and seedling growth under drought and salt stresses
When rice plants were grown under normal N conditions,the biomass and grain yield,N contents ofosnpf8.1andOsNPF8.1-OEplants were not significantly different from those of ZH11 (Figs.5-A,-B and S5-A to -E).However,the biomass ofosnpf8.1seedlings was lower under low N conditions (Fig.5-C to -F),and that ofOsNPF8.1-OEseedlings was higher under very low N conditions (Fig.5-E and -F),when compared with that of ZH11 seedlings.When plants were grown in a paddy field without N fertilization,the number of tillers per plant,number of filled grains per plant,1000-grain weight,and grain yield per plant ofosnpf8.1were lower,and those ofOsNPF8.1-OEwere higher than those of ZH11 plants (Fig.6),but the seed-setting rate showed no significant difference (Fig.6-C).These results indicated thatOsNPF8.1affected growth and grain yield of rice under low N conditions.
Roles ofOsNPF8.1in tolerance to drought and salt stresses were investigated (Fig.7).The survival rates ofosnpf8.1seedlings were lower than that of ZH11 after water stress treatment and re-watering (Fig.7-A to -C).Similarly,under 150 mmol/L NaCl treatment,the survival rates ofosnpf8.1seedlings were lower than that of ZH11 (Fig.7-D to -F).The leaf water potential ofosnpf8.1decreased faster and their proline content in leaves was lower than that of ZH11 after drought treatment (Fig.S7).However,the survival rate,leaf water potential and proline content ofOsNPF8.1-OEseedlings showed no significant difference with those of ZH11 (Figs.7 and S7).These results showed that knock-out ofOsNPF8.1led to a diminished tolerance of rice to salt and drought stresses.
OsNPF8.1 may play a role in organic N mobilization
To investigate whetherOsNPF8.1affects the transport of organic N in different organs,the free amino acids and soluble proteins were measured in FLB,PLB,culms,and grains ofosnpf8.1and ZH11 plants.The free amino acid content in FLB ofosnpf8.1plants did not differ from that in FLB of ZH11 plants (Fig.8-A).Nevertheless,the free amino acid content of PLB was higher inosnpf8.1than in ZH11 plants (Fig.8-B).Moreover,the culms and grains ofosnpf8.1plants contained fewer free amino acids than those of ZH11 plants (Fig.8-C and-D).On the other hand,the soluble protein contents of these organs were unaltered inosnpf8.1plants (Fig.S6).These results suggested that the impairment of OsNPF8.1-mediated peptide transport might lead to the retention of organic N in the source organs (PLB) and limit its transport to sinks(culms and grains).In a paddy field without N fertilization,the total N contents in the filled grains and in a whole plant ofosnpf8.1were lower than those of ZH11,but no difference in the straw.However,there was no difference in the N content among them in a paddy field with N fertilization (Fig.8-E to -G).This may indicate that less N content was transported to the grains ofosnpf8.1under low N conditions.

Fig.5.Low N affects growth of OsNPF8.1 transgenic rice seedlings.
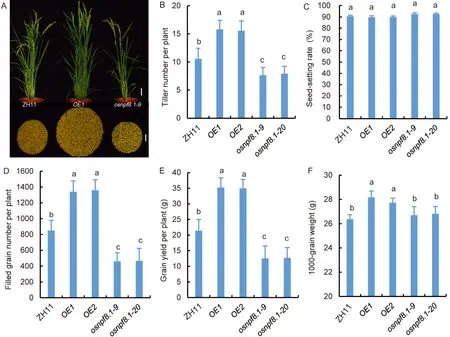
Fig.6.Low N affects grain yield of OsNPF8.1 transgenic rice.

Fig.7.Knock-out of OsNPF8.1 decreases tolerance of rice to drought and NaCl stresses.

Fig.8.Free amino acid contents (A-D) and N contents (E-G) in various organs of osnpf8.1 mutants and ZH11 plants.
DISCUSSION
Improving N use efficiency (NUE) is the key to increase crop yields while reducing demand for N fertilizer and environmental pollution.N remobilization is an important component of NUE.Plants have evolved several strategies to optimize N remobilization.Enhancing source-to-sink nitrate remobilization in plants,for instance by inducing the expression of the nitrate transporter geneNRT1.7/OsNPF2.5in rice,has been demonstrated to enhance NUE and crop production (Chen et al,2020).Senescence-associated protein degradation mediated by autophagy and proteases contributes to organic N remobilization,NUE and grain filling,and produces many amino acids and peptides (Roberts et al,2012;Yu et al,2019;Fan et al,2020).Here,we demonstrated that OsNPF8.1-mediated peptide transportation may contribute to organic N mobilization and affect rice growth and grain productivity under N deficiency and abiotic stresses.
Because of its size and diversity,the NPF transporter family includes transporters of diverse substrates,such as nitrate,peptides,plant hormones,and glucosinolates,which play multiple roles in plant growth and response to stress (Léran et al,2014;Corratgé-Faillie and Lacombe,2017;Watanabe et al,2020).In this study,we found thatOsNPF8.1can functionally complement the yeastptr2mutant and enhance the accumulation of a fluorescent dipeptide inptr2yeast cells with an apparentKMof 139 μmol/L andVmaxof 918 nmol/(g·h) (Figs.1 and S1).A series of physiological di-/tri-peptides can compete with β-Ala-Lys-AMCA for uptake by OsNPF8.1 (Fig.1-C),and showed different efficiencies of competition with β-Ala-Lys-AMCA,indicating different affinities of OsNPF8.1 for various di-/tri-peptides.Tang et al(2017) indicated that OsNPF8.1/OsPTR7 is localized on the PM and transport the toxic dimethyarsinate.Besides,OsNPF8.1 can’t rescue the growth ofptr2yeast fed with four kinds of di-/tri-peptides containing glycine (Gly-Lys,Gly-His,Gly-Gly-Lys,and Gly-His-Gly)(Ouyang et al,2010).However,in this study,GHG slightly competed with β-Ala-Lys-AMCA for uptake by OsNPF8.1 (Fig.1-C),indicating that OsNPF8.1 might have a selectivity for the transport of di-/tripeptides containing glycine,such as Gly-His-Gly.In this study,the leaf uptake rate of fluorescent β-Ala-Lys-AMCA was lower inosnpf8.1seedlings than in ZH11 seedlings(Fig.2),further confirming that OsNPF8.1 mediated peptide transportation in rice.Therefore,we concluded that OsNPF8.1 is a high affinity PM-localized peptide transporter with selectivity for amino acid residue.
N is one of the most re-mobilizable elements during the vegetative and reproductive stages of rice plants(Mae and Ohira,1981).FourArabidopsisNPF nitrate transporters (NRT1.7/NPF2.13,NPF2.12,NPF4.6 and NPF7.1) mediate leaf N export and are necessary for plant growth under inadequate soil N availability (Fan et al,2009;Babst et al,2019).Over-expression of the peptide transporter geneOsNPF7.3can increase growth and grain yield of rice,and possibly contribute to N allocation (Fan et al,2014;Fang et al,2017).This study showed thatOsNPF8.1was widely expressed in all rice organs,with the highest expression in old leaves (Figs.3 and S3).At the grain filling and maturity stages,OsNPF8.1remained constant expression level in FLB and PLB,similar to the senescence marker geneOsSGR(Fig.S4).OsNPF8.1was highly expressed in mesophyll cells and vascular parenchyma cells of leaves and roots (Fig.3-C and -G).Therefore,OsNPF8.1 may be involved in the intercellular transport of peptides within leaves and in longdistance vascular transportation of peptides,especially from old leaves.However,OsNPF8.1 was not expressed in the root epidermises and root hairs (Fig.3-E to -H),and mutation ofOsNPF8.1did not decrease the root uptake rate for β-Ala-Lys-AMCA (Fig.2-A),indicating that OsNPF8.1 may be not involved in peptide uptake by roots from the soil.Organic N remobilization occurs intensively during N deficiency and senescence(Mae and Ohira,1984).Approximately 80% of N in rice panicles has been remobilized from senescing leaves (Mae and Ohira,1981;Wu et al,2018).During the reproductive stage,leaf proteins are rapidly degraded to amino acids and small peptides that are transported to grains (Masclaux-Daubresse et al,2008).Peptides can be hydrolyzed and the resulting amino acids are reused for protein synthesis or as sources for carbon and nitrogen.In theosnpf8.1plants,the free amino acid content of PLB was higher (Fig.8-B),while those of culms and grains were lower than that of the same organs in ZH11 seedlings (Fig.8-C to-D).This indirectly suggested that knock-out ofOsNPF8.1might impair organic N transport from senescing PLB to grains and culms.Knock-out ofOsNPF8.1also impaired seedling growth (Fig.5),grain yield (Fig.6),and N content in the grains (Fig.8)under low N conditions.However,under normal N conditions,seedling growth,grain yield and N content ofosnpf8.1plants did not differ from those of ZH11 plants (Figs.5,8 and S4),consistent with the results of Tang et al (2017).Therefore,OsNPF8.1-mediated organic N transportation from old leaves to filling grains may play a role in rice growth and grain yield under N deficiency.It may be used for molecular breeding by enhancing organic N recycling from old leaves to grains.
The hydrolysis of storage proteins in the starchy endosperm of rice and maize results in a large amount of peptides and only a small amount of free amino acids (Salmenkallio and Sopanen,1989),because of the limited carboxypeptidase activity in the endosperm of maize and rice,when compared with barley and wheat (Winspear et al,1984).In this study,OsNPF8.1was highly expressed in the buds of germinating seeds(Fig.3-A),suggesting that OsNPF8.1played a role in the transfer of N stored in the seeds for seedling construction during the germination of rice seeds.Furthermore,the uptake of peptides might play an even more important role in the mobilization of storage proteins in maize and rice than in barley and wheat (Salmenkallio and Sopanen,1989).This is substantiated by the observation of millimolar concentrations of small peptides in the endosperm of germinating cereal grains (Higgins and Payne,1981).Peptide and amino acid transport has been demonstrated in the scutella of barley,wheat,rice and maize(Salmenkallio and Sopanen,1989),which plays an essential role in the mobilization of reserve proteins during the germination (Sopanen,1979).However,there were no differences in germination rates between ZH11 andosnpf8.1seeds (data not shown).This may be due to the fact that other transporters assist in the transport of organic N during seed germination.
The expression ofOsNPF8.1was induced by abiotic stresses,such as N deficiency,salt,drought,ABA (Fig.4),and DMA treatments (Tang et al,2017).Consistently,theosnpf8.1plants showed phenotypic differences with respect to ZH11 plants under N deficiency and abiotic stresses of salt and drought(Figs.5-7).In addition,osnpf8.1seedlings were less tolerant to NaCl and drought treatments (Fig.7).After drought treatment,osnpf8.1leaves showed faster decrease of water potential and lower proline content than those of ZH11 (Fig.S7).Mutation ofOsNPF8.1may also affected organic remobilize from old organs to new organs,resulting in amino acids accumulated in old leaves (Fig.8).These may cause less-tolerance ofosnpf8.1to abiotic stress.But over-expression ofOsNPF8.1does not enhance tolerance to drought and NaCl stresses (Fig.7).This may be caused by the change of the water potential and proline content ofosnpf8.1andOsNPF8.1-OEcompared with those of ZH11 (Fig.S7).AtNPF5.2/AtPTR3 is a peptide transporter encoded by a defense-related gene that protectsArabidopsisagainst biotic and abiotic stresses(Karim et al,2007).Stress induced nitrate allocation mediated by the nitrate transporter OsNPF7.9 to roots retains nitrate in roots and rebalances plant growth and stress tolerance (Guan et al,2022).AtNPF8.3/AtPTR2is negatively regulated by ABI4 and plays a key role in water uptake by seeds,ensuring that imbibed seeds proceed to germination (Choi et al,2020),and AtNPF4.6 and AtNPF5.1 transport ABA to control leaf stomatal aperture to response to drought (Shimizu et al,2021).Similar,the stress-induced organic N transportation mediated by the peptide transporter OsNPF8.1 may rebalance plant growth and tolerance of salt,drought and low N stresses,since OsNPF8.1 may participate in redistribution of organic N when stress comes,and the remobilization of organic N may cause water potential and proline change to tolerate stress.
METHODS
Rice materials and growth conditions
Oryza sativasubsp.japonicacv.ZZH11 was used for the experiments and served as the wild type for transgenic rice.For hydroponic experiments,rice seedlings were grown in boxes with Yoshida solution (Yoshida et al,1976),containing 1.43 mmol/L NH4NO3,0.32 mmol/L NaH2PO4,0.51 mmol/L K2SO4,1.00 mmol/L CaCl2,1.65 mmol/L MgSO4,8.9 μmol/L MnSO4,0.5 μmol/L Na2MoO4,18.4 μmol/L H3BO3,0.14 μmol/L ZnSO4,0.16 μmol/L CuSO4and 40 μmol/L FeSO4.The growth chamber was under a 14 h light (30 °C)/10 h dark (25 °C)photoperiod with about 350 μmol/(m2·s) photon density and 70% humidity.For field experiments,rice was transplanted at 30 d after germination to the controlled paddy fields under normal cultivation practices (120 kg/hm2N) or without fertilization in Guangzhou and Guiyang,China.
Analysis of di-/tri-peptide uptake in yeast
The yeast expression vector pYES260 was modified by replacing theGAL1promoter with a truncatedADH1promoter from pGBKT7 (Melcher,2000).The resulting pYES260ADH was used as the backbone of the yeast expression vector.The coding DNA sequences of yeastPTR2,OsNPF8.1,OsNPF8.2,andOsNPF8.5were cloned into pYES260ADHvia theBamH I-XbaI,HindIII-EcoR I,HindIII-EcoR I,andHindIII-XhoI sites,respectively.TheSaccharomyces cerevisiaeptr2mutant strain ABC738 (MATa ura3-52 leu2-Δ1 lys2-801 his3-Δ200 trp-Δ63 ade2-101 ptr2Δ::KanMX2) was transformed with the expression vector and empty vector using the lithium acetate-mediated method (Bourbouloux et al,2000).For growth complementation of the yeastptr2mutant,the positive transformants were streaked on synthetic defined (SD) agar plates with 100 μmol/L Pro-Leu as the sole leucine source and cultured at 28oC for 72 h.For the fluorescent dipeptide uptake assay,the procedure was performed as described by Ito et al(2013).Briefly,pYES260ADH-OsNPF8.1transformants were cultured in synthetic drop-out (DO) medium without uracil until reaching an OD660of 0.6.Then,the yeast cells were washed in uptake buffer (150 mmol/L NaCl and 50 mmol/L Na-phosphate buffer,pH 6.0) and resuspended in uptake buffer to OD660=15.The fluorescent dipeptide β-Ala-Lys-AMCA was added at a concentration of 50 μmol/L for the time-course uptake assay.For the kinetic assay,various concentrations of β-Ala-Lys-AMCA were added and cells were incubated at 28 °C for 2 h.After incubation,the yeast cells were washed three times in uptake buffer and resuspended in uptake buffer to OD660=15.β-Ala-Lys-AMCA uptake was quantified by whole-cell fluorescence (excitation at 355 nm and emission at 460 nm) using an LS55 fluorescence spectrometer (PerkinElmer,UK).For the competition assay,competitive peptides were added to uptake buffer at a final concentration of 50 μmol/L,while NaNO3and GSH were added at a final concentration of 500 μmol/L,together with 50 μmol/L β-Ala-Lys-AMCA.The uptake was measured at 28 °C for 1 h and quantified as described above.For the15N-labeled dipeptide uptake assay,a similar procedure to that of the fluorescent dipeptide uptake assay was followed.The amine group of the Ala residue of the dipeptide Ala-Trp was labeled with15N (GL Biochem,China).[15N]Ala-Trp uptake was measured at 28 °C for 1 h and quantified by accumulation of15N in yeast cells with a vario ISOTOPE cube elemental analyzer in tandem with an IsoPrime 100 isotope ratio mass spectrometer (Elementar,Germany).TheKMandVmaxwere determined using the Michaelis-Menten equation afterk-mean clustering.
Fluorescent dipeptide uptake in rice
For leaf uptake,the third leaf of the seedlings under normal and free N conditions was laid on a flat plate and covered with filter paper.The filter paper was moistened with 200 μL of 100 μmol/L β-Ala-Lys-AMCA dissolved in 0.1% Tween 20.Then,the seedlings were cultured in the dark,and 100 μL ddH2O was added every 2 h to keep the filter paper moist.After 4-8 h of absorption,each third leaf covered with filter paper was cut off,washed twice with water,frozen,and stored for detection.For root uptake,5 d germinating seeds under water condition (free N) was put into 500 μL of 100 μmol/L β-Ala-Lys-AMCA for 24 h,and then the roots were cut off for detection.β-Ala-Lys-AMCA uptake was quantified by whole-cell fluorescence(excitation at 355 nm and emission at 460 nm) using an LS55 fluorescence spectrometer.
Vector construction and rice transformation
The activity of theOsNPF8.1promoter was detected by recordingOsNPF8.1promoter-driven GUS activity in transgenic rice.ThePOsNPF8.1::GUSvector was constructed by inserting a 3.5-kb promoter DNA sequence into pCAMBIA 1301 via theXbaI-NcoI site.osnpf8.1rice mutants were generated using the CRISPR/Cas9 genome editing system (Ma et al,2015).The anchor sequences were 5'-CCAACTGGTCGGGGACTTGCTA-3'driven by the U6a promoter,and 5'-CAATGTCACCAACTG GTCGGGG-3'driven by the U3 promoter.Both anchor sequences were targeted to the second exon ofOsNPF8.1.Genomic DNA templates of the transformed rice plants were extracted.The editing site was amplified by PCR using a pair of flanking primers.The PCR products were sequenced and analyzed using DSDecode (Ma et al,2015) to confirmOSNPF8.1knock-out.To further confirm the edited sequences ofosnpf8.1cDNA,RT-PCR products fromosnpf8.1seedlings were sequenced again.To constructOsNPF8.1-over-expressing plants,a 1743-bpOsNPF8.1cDNA fragment was inserted downstream of the 35S promoter in pCAMBIA 1306 usingKpnI andXbaI to produce the plasmid p35S-OsNPF8.1.These vectors were introduced into theAgrobacterium tumefaciensstrain EHA105,and then ZH11 was transformed byAgrobacterium-mediated transformation,and 50 mg/L hygromycin was used to select transgenic calli (Hiei et al,1997).The primers are listed in Table S1.
Promoter activity analysis
GUS activity inPOsNPF8.1::GUStransgenic rice was detected by GUS staining to visualize the histological distribution ofOsNPF8.1promoter activity.The GUS staining buffer was composed of 0.5 mg/L 5-bromo-4-chloro-3-indolyl-β-Dglucuronide (X-Gluc,a chromogenic substrate for GUS),3 mmol/L K4Fe(CN)6,3 mmol/L K3Fe(CN)6,0.05% Triton X-100,and 100 mmol/L Na2HPO4-NaH2PO4,pH 7.0.The staining was terminated with 70% ethanol.The stained plants were subjected to fresh sectioning with a VT1200 vibrating blade microtome(Leica,Wetzlar,Germany) or fixed sectioning in Spurr resin(Sigma-Aldrich,Germany).
RNA extraction and qRT-PCR
Total RNA was extracted using a RNAiso Plus Kit (TaKaRa,Beijing,China).First-strand cDNA was synthesized using M-MLV reverse transcriptase (TaKaRa,Beijing,China) with oligo(dT) primers.qPCR was conducted with SYBR®Premix ExTaqGC (TaKaRa,Beijing,China) in a LightCycler 480 system (Roche,Basel-Stadt,Switzerland).UBC(LOC_Os02g42314.2) transcripts were served as a housekeeping reference (Jain et al,2006).
Treatments of N,ABA,salt and drought
Rice seeds were sterilized with 5% sodium hypochlorite solution for 30 min and immersed in pure water for germination for 3 d.The germinated seeds were transferred to a box with whole-nutrient Yoshida solution for 2 weeks or into a controlled paddy.
To determine gene expression,the 2-week old seedlings were used.For N treatment,the seedlings were transferred to base Yoshida solution with 0.25,1.00 or 2.50 mmol/L NH4NO3.For NaCl or ABA treatment,the seedlings were transferred to Yoshida solution supplied with 150 mmol/L NaCl or with 0.5 μmol/L ABA for different times,respectively.For drought treatment,the seedlings were taken out of the solution for different time and then transferred again to Yoshida solution.All the solution was renewed every 2 d.
To test the effects of low N on seedling growth,the germinated seeds were grown in Yoshida solution with 2.5 mmol/L NH4NO3,1.0 mmol/L NH4NO3,or 0.25 mmol/L NH4NO3for 2 weeks,and fresh biomass of the seedlings were measured.To examine the effects of salt on seedling growth,2-week old seedlings were transferred to Yoshida solution with 150 mmol/L NaCl and allowed to grow for 7 d,then recovered with Yoshida solution for one week,and the surviving seedlings were counted.For drought tolerance test,the 2-week old rice seedlings were taken out of Yoshida solution until the leaves wilted for 12 h,then recovered with Yoshida solution for one week,and the surviving seedlings were counted.
For measurements of main agronomic traits,the plants were grown in a controlled paddy field in Guiyang,China.For the senescence experiment,FLB and PLB were harvested at the grain filling and maturity stages in a paddy field under normal N fertilization.
Analysis of free amino acids and soluble proteins
The plants were grown in a controlled paddy field under normal fertilization.At the filling [approximately 25 d after flowering (DAF)] and maturity (approximately 35 DAF) stages,FLB,PLB,culms and grains were harvested and immediately frozen in liquid N2.Free amino acids were extracted from frozen samples using sodium acetate buffer (pH 5.4).The free amino acid content was determined using a ninhydrin assay(Moore,1968).Soluble proteins were extracted from frozen samples with extraction buffer (137 mmol/L NaCl,2.7 mmol/L KCl,10 mmol/L Na2HPO4,1.8 mmol/L KH2PO4,0.5% SDS,and 0.5% Triton X-100,pH 7.4).The soluble protein content was determined using a NanoOrange assay (Jones et al,2003).
Measurement of leaf water potential and proline content under drought stress
The 45 d seedlings grown in soiled boxes were poured out from water for 3 d drought,and then rehydrated for 2 d.The leaf water potential value was measured at 15:00 pm every day,and the leaf samples were taken to determine the proline content at 17:00 pm every day.The water potential of rice leaves was measured by a pressure chamber (Corvallis,OR,USA).Leaf proline content was determined according to Bates et al (1973).
ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Foundation of China (Grant No.31772384) and Science and Technology Project of Zhanjiang,Guangdong Province,China(Grant No.2021A05030).
SUPPLEMENTAL DATA
The following materials are available in the online version of this article at http://www.sciencedirect.com/journal/rice-science;http://www.ricescience.org.
Fig.S1.β-Ala-Lys-N-7-amino-4-methylcoumarin-3-acetic acid(AMCA) uptake and competition assay of OsNPF8.1 in yeast.
Fig.S2.Molecular analysis ofOsNPF8.1transgenic rice plants.
Fig.S3.Spatio-temporal expression ofOsNPF8.1in different organs through entire growth in the field.
Fig.S4.OsNPF8.1expression under deficiency of N,P and K and expression of senescence-related genes during the maturity stage.
Fig.S5.Absence of significant differences in growth and N content betweenosnpf8.1mutants and Zhonghua 11 (ZH11)plants under normal N conditions.
Fig.S6.Soluble protein contents in various organs ofosnpf8.1mutants and their wild type Zhonghua 11 (ZH11).
Fig.S7.Leaf water potential change and proline content of
OsNPF8.1transgenic rice under drought stress.Table S1.List of primers.
- Rice Science的其它文章
- Recombinase Polymerase Amplification Based Rapid Detection of Aroma Gene in Rice
- Transfer Learning-Based Image Recognition of Nitrogen and Potassium Nutrient Stress in Rice
- Novel Deletion in Exon 7 of Betaine Aldehyde Dehydrogenase 2(BADH2)
- A Pleiotropic Drug Resistance Family Protein Gene Is Required for Rice Growth,Seed Development and Zinc Homeostasis
- NaCl Facilitates Cell Wall Phosphorus Reutilization in Abscisic Acid Dependent Manner in Phosphorus Deficient Rice Root
- Antioxidant Activities and Characterization of Polyphenols from Selected Northern Thai Rice Husks: Relation with Seed Attributes

