Effects of π-conjugation-substitution on ESIPT process for oxazoline-substituted hydroxyfluorenes
Di Wang(汪迪) Qiao Zhou(周悄) Qiang Wei(魏强) and Peng Song(宋朋)
1Department of Physics,Liaoning University,Shenyang 110036,China
2College of Mathematics and Information Engineering,Chongqing University of Education,Chongqing 400065,China
3School of Science,Chongqing University of Technology,Chongqing 400050,China
Keywords: density functional theory(DFT)and time-dependent DFT(TDDFT),excited-state proton transfer,intramolecular hydrogen bonding,π-conjugation-substitution
1.Introduction
Excited-state intramolecular proton transfer (ESIPT)molecules are extended applied in laser dyes, molecular switches, and fluorescent probes,[1-5]because of their distinguished characteristics like dual emission behavior and large Stokes shifts.[6,7]Since the ESIPT reaction in salicylate derivatives was reported by Weller,[8]the ESIPT reaction has attracted a lot of attention.Their investigative results show that ESIPT usually undergo a reversible four-step cyclic reaction process,that is to say,the transformation from normal to tautomer form.[9-11]According to this transformation, the chemical and physical properties of the molecule will change substantially, particularly the spectroscopic behavior.In recently years, lots of investigators have made numerous researches on the ESIPT reaction processes for developing and designing the innovative ESIPT molecules.[12-16]Therein, the introduction of substituent can make the ESIPTbased molecules have different properties.Ravikanthet al.showed that the substituent groups on tetraphenylethylene at the ortho position to benzothiazole have a great influence on the electronic structure.[17]Xuet al.found that substituted group could block the normal-tautomer tautomerism occurring through ES charge transfer.[18]Hasserodtet al.advised amino substituent could make the emission wavelengths shift to the deep red region.[19]
Recently, Nachtsheim’s group successfully synthesized a series of ESIPT-based fluorenes oxazoline-substituted hydroxyfluorenes and hydroxylated benzoxazole, which could vary the emission colors.[20]Through altering the acceptor unit, they diversified the fluorescence recognition functions.However, their photophysical properties were blurry.Here,oxazoline-substituted hydroxyfluorenes (Oxa-OH), hydroxylated benzoxazole (BO-OH), and naphthoxazole (NO-OH)were selected.To elucidate the influence of substituent on ESIPT and photophysical properties, the density functional theory (DFT) and time-dependent density functional theory(TDDFT)are advised.By analyzing the mainly structural parameters of Oxa-OH,BO-OH,NO-OH and frequency analysis,the effects of under different substitution on the hydrogen bond strength were studied.In addition, the frontier molecular orbitals (FMOs), reduction density gradient (RDG) isosurfaces,and homologous potential energy surfaces(PESs)of these configurations were provided and analyzed, which can proclaim the direct information for the ESIPT process.
2.Calculation details
In this paper, the DFT and TD-DFT theories were advised to study the effect of substitution.All electronic structure calculations were provided by the 6-31+G(d) basis set,and B3LYP functional in Gaussian 09 program suite.[21-23]In addition,the integral equation formalism variant of the polarizable continuum model (PCM) (IEFPCM) was selected.[25]Simultaneously, chloroform was taken into account, because the experiments were implemented in chloroform.In addition,the infrared(IR)vibrational frequencies of these stable geometries were carried out, and its results imply that there is no imaginary frequency,which penetrates that the computational geometries were at the local minimum point.[26,27]Then the deducted spectra of Oxa-OH,BO-OH,and NO-OH were calculated in view of the sustainable geometric configuration,and the calculated absorption and fluorescence spectrum is consistent with the experimental results.Furthermore, the FMOs and RDG were used to analyze the variation of intermolecular hydrogen bond strength by Multiwfn program suite.[28,29]Finally, their PESs were scanned to reveal transfer paths of hydrogen protons in different substitutions.
3.Results and discussion
3.1.Optimized geometric structures
Figure 1 show their optimized enol and keto configurations.Meanwhile, vibrational frequency analysis of these geometries are provided, which could assure the validity of these stable points.To clearly describe the important parameters of the hydrogen bonds, we numerically marked the atoms, and the related structure parameters are listed in Table 1.Interestingly, the calculative results uncover that the bond lengths of O1-H2of these configurations are 0.999 °A,0.991 °A, and 0.992 °A, while they change to be 1.016 °A,0.997 °A, and 0.994 °A in the S1state, respectively.Thus, it can draw a conclusion that shortening of the O-H distance could be found with addingπ-conjugation.In addition, the computational bond lengths of H2-N3were 1.746 °A,1.799 °A,and 1.796 °A in the S0state.When the molecules transfer to the S1state,the bond lengths vary to be 1.677 °A,1.769 °A,and 1.786 °A.Clearly,the H2-N3distance was shortened,which indicates that the intramolecular hydrogen bonds(O1-H2···N3)are strengthened in the S1state.In addition, the bond angleδ(O-H-N)varies from 146.86°,145.66°,and 145.55°in the S0state to 149.27°, 147.42°, and 146.96°in the S1state for these structures.That is to say, the intramolecular hydrogen bonds in these configurations can be strengthened in excited electronic state.
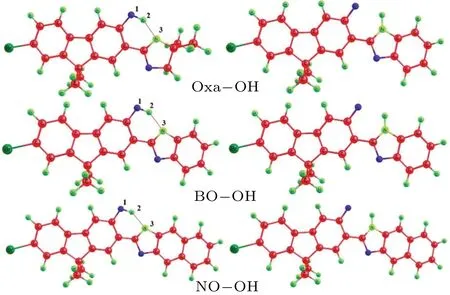
Fig.1.Optimized geometries of NO-OH,BO-OH and Oxa-OH in the enol and keto-forms.
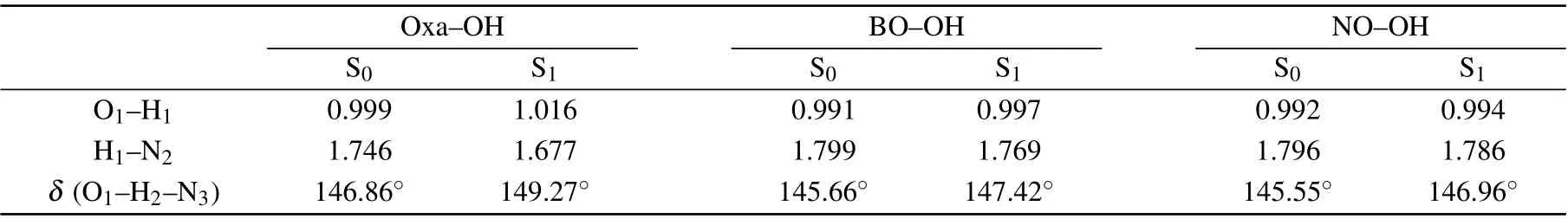
Table 1.The partial parameters of Oxa-OH,BO-OH and NO-OH in S0 and S1 states based on the DFT/TDDFT methods.
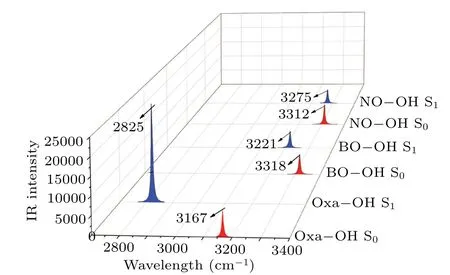
Fig.2.Infrared vibrational spectra of Oxa-OH,BO-OH and NO-OH configuration in the S0 and S1 states.
Figure 2 represents the infrared spectra of O-H bands in the S0and S1states.It can be seen that the O-H stretching vibration frequencies for Oxa-OH, BO-OH and NO-OH are 3167 cm-1, 3318 cm-1and 3312 cm-1in the S0state, respectively, and upon photoexcitation, it varies to 2825 cm-1,3221 cm-1and 3275 cm-1in the S1state.Overtly, the bathochromic-shift produced by hydrogen bond in the S1state could be found,which indicates that the intramolecular hydrogen bond is strengthened in the S1state.[30-32]Interestingly,the bathochromic-shift of 342 cm-1, 97 cm-1, and 37 cm-1for the O-H stretching band was induced by the intramolecular hydrogen bonds of these structures, revealing that the difference value of hydrogen bond energy between S0and S1states decrease withπ-conjugation.
3.2.Absorption and emission spectra
Figure 3 displays the computational electronic spectra of Oxa-OH,BO-OH and NO-OH configuration.It can be noted that their absorption peaks are at 334 nm,382 nm and 387 nm,which agree with the experimental results of 347 nm,369 nm,and 372 nm,respectively.Besides,the calculated peak of normal fluorescence of these molecules were 377 nm, 445 nm,and 460 nm.A bathochromic-shift of 43 nm, 63 nm, 88 nm is relative to the absorption peak, which should be attributed to the Stokes shift.Distinctly,the bathochromic-shift is gradually increasing.In addition, it can be seen from Fig.3 that another fluorescence peak is located at 467 nm, 486 nm, and 504 nm, which is ascribed to the keto-form of triple configurations.Figure 4 shows the frontier MOs of the Oxa-OH,BO-OH and NO-OH,which could qualitatively discuss molecular configuration distribution and charge transfer in the S1state.[33,34]It is clear that the uniform distribution of charge on both sides of the molecular skeleton indicates that the charge transfer pattern of these configurations is typicalπ-π∗transition.The transfer of molecules from HOMO to LUMO shows obvious charge transfer characteristics.The charge distribution around N atom decreased significantly, while the charge distribution around O atom increased significantly, demonstrating the intramolecular hydrogen bond gradually strengthened,and thus promoting the occurrence of proton transfer process.These results show that the ESIPT process is due to intramolecular charge transfer.In Table 2,the HOMO and LUMO predominantly occupy the transition component with 94.6%, 97.8%,and 96.5% from the S0to S1state for Oxa-OH, BO-OH,and NO-OH, respectively.The relevant oscillator strength is 0.6919, 1.2700, and 1.3965, and the excitation energy is 334 nm,367 nm,and 387 nm.
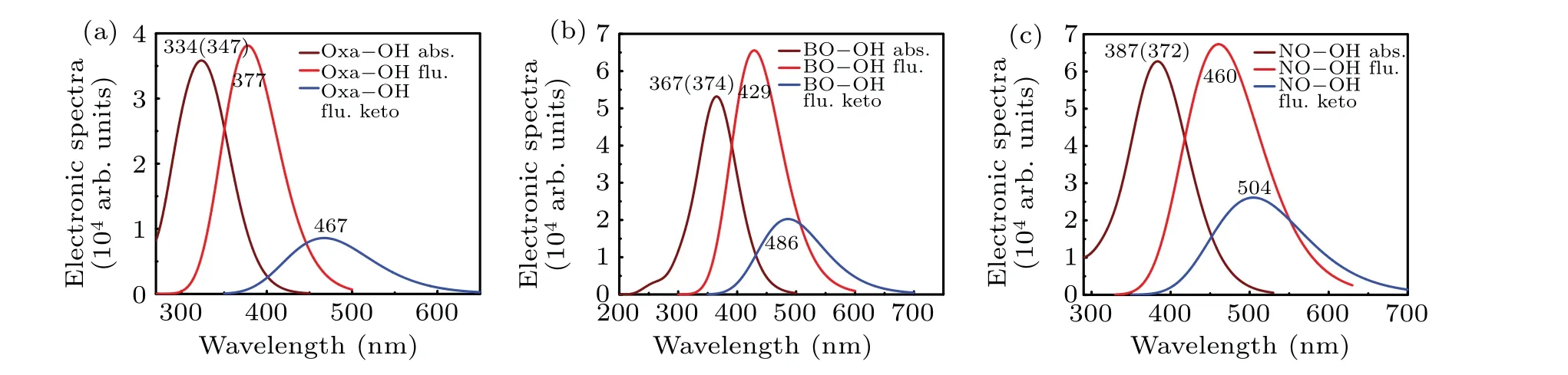
Fig.3.Calculated absorption and fluorescence spectra of(a)Oxa-OH,(b)BO-OH and(c)NO-OH in chloroform solvent.

Fig.4.The calculated frontier MOs of Oxa-OH,BO-OH and NO-OH.

Table 2.Transition types with different excitation energies λ (nm)of Oxa-OH,BO-OH,and NO-OH respectively and their oscillator strengths(f)and transition compositions.
3.3.Non-covalent interactions analysis
Figure 5 exhibited the obtained RDG isosurfaces, which can discern the influence of different substituents on the hydrogen bond strength in real space.[35-38]The strength of hydrogen bond interactions can be clearly described by analyzingρ(r)and RDG of the electron density.The RDG function can be indicated as
Additionally,the electron density matrix equation is obtained on the basis of Bader theory
Previous work shows that multiple weak interactions can be clearly found,namely,negative values of sign(λ2)ρrefer to hydrogen bonding effects,positive sign(λ2)ρstands for steric effect and values near zero exhibit the van der Waals(VDW)interactions.We find that the spike peaks of these configurations are located at-0.048,-0.042 and-0.040 in the S0state.The interaction type is classified as hydrogen bond interaction,and the peak value is O1-H2···N3.Upon photoexcitation to the S1state,O1-H2···N3shift to-0.056,-0.044 and-0.042, which promulgates that the hydrogen bond was strengthened in the S1state.

Fig.5.RDG versus sign(λ2)ρ and low-gradient isosurfaces for Oxa-OH, BO-OH and NO-OH configurations.Weak interactions can be detected according to RDG.
3.4.Potential energy curves
To further discuss the effect of different substituents on the ESIPT process of Oxa-OH, BO-OH, and NO-OH, the PESs in the S0and S1states were provided by the DFT and TDDFT methods, which are displayed in Fig.6.Besides,the O-H bond length ranges from 0.99 °A to 1.99 °A with a step size of 0.1 °A.It can be found that their barrier height in the S state is 9.5 kcal/mol, 10.1 kcal/mol, and 9.8 kcal/mol.It asseverates that it is demanding for these configurations to spontaneous PT in ground state.When the excited molecule transmits to the S1state, we found that the barrier height of the Oxa-OH, BO-OH, and NO-OH in chloroform solvent dropped to 1.4 kcal/mol, 6.1 kcal/mol, and 7.4 kcal/mol, respectively.Evidently,the ESIPT reaction is more likely to take place in the S1state.Even more noteworthy,the barrier height of BO-OH and NO-OH increase when addingπ-conjugationsubstitution, while the calculated peak of enol and keto fluorescence of NO-OH is distinctly bathochromic-shift relative to the Oxa-OH configuration.In addition,the inverse reaction of ESIPT with three different substitutional configurations has to overcome a much higher energy barrier than the forward reaction.Therefore,we believe that the reverse ESIPT reaction cannot be spontaneous.

Fig.6.Potential-energy curves of S1 and S0 states for(a)Oxa-OH,(b)BO-OH and(c)NO-OH as functions of the O-H bond length.
4.Conclusions
In this paper, the influence of differentπ-conjugationsubstitutions on ESIPT and photophysical properties have been investigated in chloroform solvent by the DFT and TDDFT methods.Through the analysis of bond length,bond angle and infrared vibration spectrum, it indicates that the hydrogen bonding interaction in excited state is strengthened, which is beneficial to the ESIPT processes.Interestingly,the barrier height of NO-OH is higher when addingπconjugation-substitution,while the calculated peak of enol and keto fluorescence of NO-OH is distinctly bathochromic-shift relative to the Oxa-OH configuration,which means that theπconjugation-substitution can diversify the fluorescence color.
Acknowledgements
Project supported by the National Natural Science Foundation of China (Grant No.11974152), the Shenyang High level Innovative Talents Program (Grant No.RC200565), the Science program of Liaoning Provincial Department of Education(Grant No.LJKZ0097),the Intercollegiate cooperation project of colleges and universities of Liaoning Provincial Department of Education.
- Chinese Physics B的其它文章
- Matrix integrable fifth-order mKdV equations and their soliton solutions
- Comparison of differential evolution,particle swarm optimization,quantum-behaved particle swarm optimization,and quantum evolutionary algorithm for preparation of quantum states
- Explicit K-symplectic methods for nonseparable non-canonical Hamiltonian systems
- Molecular dynamics study of interactions between edge dislocation and irradiation-induced defects in Fe-10Ni-20Cr alloy
- Engineering topological state transfer in four-period Su-Schrieffer-Heeger chain
- Spontaneous emission of a moving atom in a waveguide of rectangular cross section

