产气荚膜梭菌的主要毒素及其在肉鸡坏死性肠炎致病机理中的作用研究进展
雷蕾 帅柯 杨汉博 肖昌

摘 要:肉鸡坏死性肠炎(necrotic enteritis,NE)是产气荚膜梭菌(Clostridium perfringens)引发的一种严重的肠道疾病,给肉鸡业造成了巨大的经济损失。为深入了解肉鸡坏死性肠炎的致病因子和致病机理,本文对产气荚膜梭菌的主要毒素及其在肉鸡坏死性肠炎致病机理中的作用进行了概述,为该病的临床诊断、监测和防控提供参考。
关键词:肉鸡;产气荚膜梭菌;毒素;坏死性肠炎;致病机理
中图分类号:S831.4 文献标志码:A 文章编号:1001-0769(2023)01-0018-08
肉鸡坏死性肠炎(necrotic enteritis,NE)是一种常见的由产气荚膜梭菌(Clostridium perfringens)引发的一种严重的肠道疾病[1]。产气荚膜梭菌是肉鸡肠道菌群中的一员,是一种条件性致病菌,也可导致人和动物发病,是一种食源性致病菌。随着全球肉鸡饲养量大幅增长,产气荚膜梭菌诱发的坏死性肠炎已成为持续性挑战,造成了巨大的经济损失。与此同时多国禁止使用抗生素生长促进剂,该病的发病率再次升高,导致肉鸡的体重或增重降低,影响肉鸡的生产力和肉品质。因此,有必要深入了解产气荚膜梭菌及其所产毒素对肉鸡坏死性肠炎的致病机理,从而为该病的防控打下基础。
1 产气荚膜梭菌的生物学特性
产气荚膜梭菌是一种革兰阳性菌,呈棒状,无运动性,可形成孢子。其大小随生长环境而变化,例如,在淀粉培养基中,菌体较大,而在葡萄糖培养基中则较小。产气荚膜梭菌对寒冷有较强抵抗力,其芽孢耐热[2]。产气荚膜梭菌可水解明胶,能将硝酸盐还原为亚硝酸盐;在含硫培養基中,由于硫元素被还原,会产生黑色菌落。其特征性鉴定试验为乳糖发酵试验,即剧烈乳糖发酵,因为其在牛乳中可产生大量气体。该菌能产生大量超氧化物歧化酶,因此可在微氧条件下生长[3-4]。产气荚膜梭菌能形成芽孢,可以在各种环境中生存,常见于废水、尘埃、空气以及健康的人和动物的肠道[5]。该菌是肉鸡肠道正常菌群的一员,与其他肠道微生物呈竞争性生长关系,属于条件性致病菌[6]。产气荚膜梭菌也是常见的食源性致病菌之一,可导致人发生胃肠炎等疾病[7]。
2 产气荚膜梭菌的毒素类型及其所致疾病
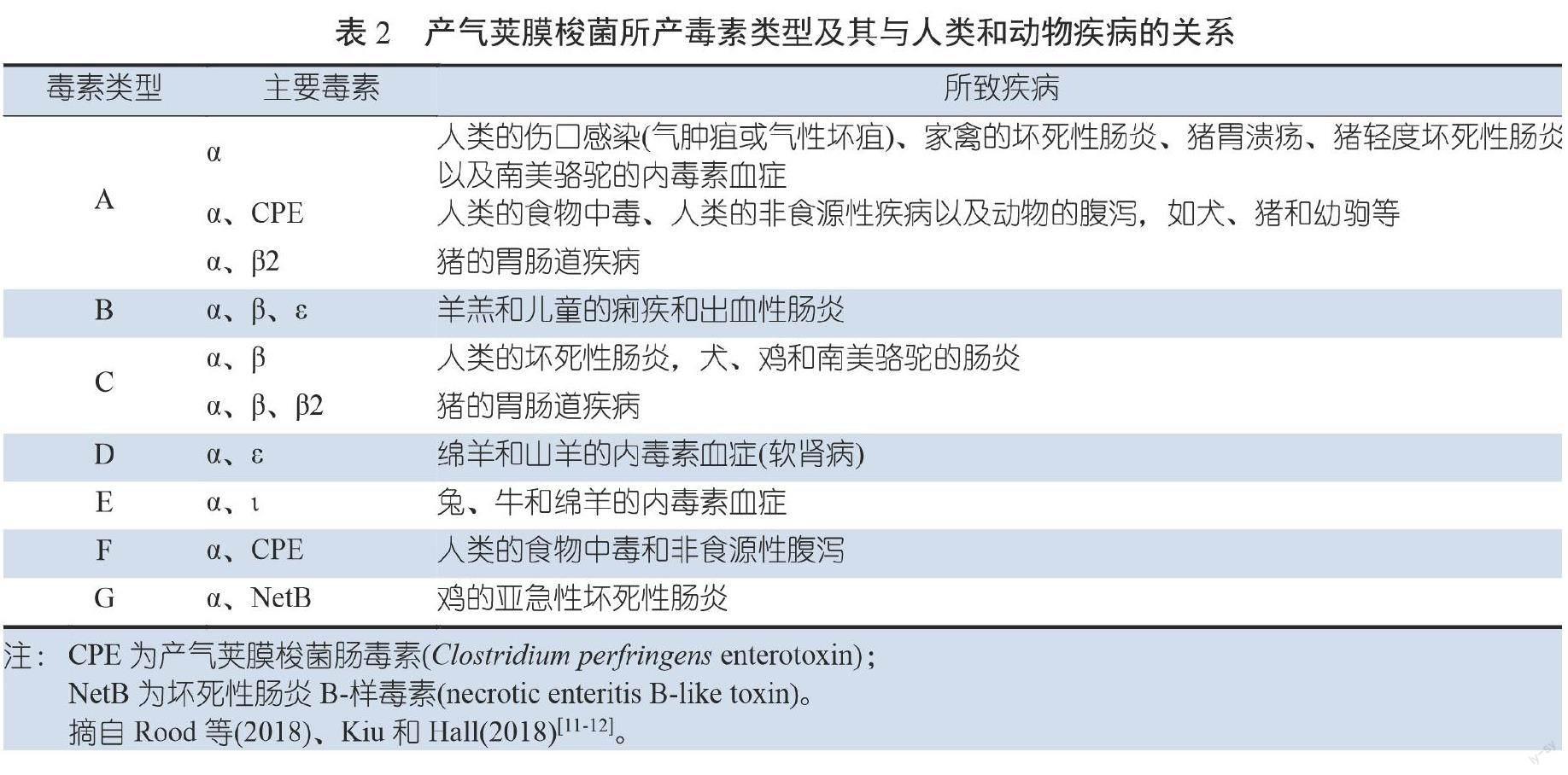
产气荚膜梭菌释放的毒素在肉鸡坏死性肠炎致病机理中起着重要作用。迄今为止,已经鉴定出20多种不同类型的产气荚膜梭菌毒素[8]。根据是否存在产气荚膜梭菌alpha(α)、beta(β)、epsilon(ε)、iota(ι)毒素以及最近增加的产气荚膜梭菌肠毒素(Clostridium perfringens enterotoxin,CPE)、β2-毒素和坏死性肠炎B-样毒素(necrotic enteritis B-like toxin,NetB)编码基因,禽类产气荚膜梭菌可分为7种类型(表1)[9-10],与肉鸡密切相关的产气荚膜梭菌类型为A、C和G型。每种毒素类型与人和动物疾病的关系见表2。
2.1 α-毒素
在所有毒素类型中,α-毒素是唯一所有类型产气荚膜梭菌都能产生的毒素。产气荚膜梭菌毒素基因见于染色体和质粒。早期研究发现,α-毒素具有酶活性,可以催化在其作用位点发生的反应[12]。
α-毒素是一种磷脂酶C和神经鞘磷脂酶,可水解细胞膜的磷脂,从而导致细胞死亡。因此,α-毒素具有细胞毒性、溶血活性、皮肤坏死性和致死性等特性[13]。α-毒素是导致气性坏疽的主要原因,但其对坏死性肠炎的作用尚有争议[14]。
α-毒素在A型产气荚膜梭菌的致病机理中起重要作用。研究发现,患病鸡体内的α-毒素水平高于健康鸡的[15]。接种A型产气荚膜梭菌的肉汤培养上清液或纯化的α-毒素接种无特定病原鸡,成功诱导了鸡死亡和肠道病变,而用α-毒素抗血清和α-毒素后并未导致鸡死亡[16]。
尽管A型产气荚膜梭菌通常见于环境和健康肠道,但侵袭性菌株可产生多得多的α-毒素。Rehman等[17]在体外研究中发现,α-毒素可影响肠黏膜的屏障功能。但这些研究使用的是粗制的或部分纯化的毒素,因此,毒素中可能存在其他的蛋白共同发挥作用[18]。Cooper和Songer[19]研究发现,尽管使用重组α-毒素进行免疫可部分保护产气荚膜梭菌诱发的坏死性肠炎,但坏死性肠炎病变的产生与体外α-毒素的产量并无关联。
但是,使用从发病鸡和健康鸡上分离的产气荚膜梭菌进行的另一项研究发现,α-毒素产量并无差异。2006年以前,研究人员认为坏死性肠炎主要的毒力因子是α-毒素,但其后的研究表明,不携带α-毒素基因的突变株也能导致肉鸡发生坏死性肠炎[18]。因此,尚不能确定α-毒素是否是坏死性肠炎的主要毒力因子。
α-毒素可与细胞膜上的一种神经节苷脂糖-β-氨丙基(ganglioside sugar-β-aminopropy,GM1a)结合,诱导二酰基甘油在细胞中蓄积,导致酪氨酸激酶A被激活,从而诱导释放白介素-8[20]。α-毒素可促进胆固醇生成,其可与另一种毒素——产气荚膜梭菌溶素O(perfringolysin O,PFO,也称为θ-毒素)结合[21]。PFO属于胆固醇依赖性细胞溶素家族。该毒素家族的成员具有40%~80%的结构同源性和相似的生物特性[22]。产气荚膜梭菌产生的这些亚基可在具有胆固醇来源的细胞表面上聚合,然后插入跨膜区,从而产生一个穿孔[23],允许细胞膜内外的粒子和大分子穿越[24]。
在利用气肿疽的实验动物模型进行的研究发现,A型产气荚膜梭菌分泌的PFO可损伤宿主组织以及该部位的炎性细胞。该毒素通过传播可扩散进入全身血液循环,使多形核白细胞上的黏附分子发生改变。研究人员认为这会导致白细胞的细胞周期停滞和局部组织缺氧[25]。
2.2 坏死性肠炎B-样毒素
坏死性肠炎B-样毒素(necrotic enteritis B-like toxin,NetB)是β-穿孔毒素中的α-溶血素家族成员,分离自发生坏死性肠炎的鸡体内的A型产气荚膜梭菌菌株。之所以取名为NetB,是因为其与产气荚膜梭菌β-毒素具有相似性。研究发现,某些A和G型产气荚膜梭菌分离株产生的穿孔毒素NetB(而非α-毒素)是坏死性肠炎致病机理中一种非诱发性毒力因子[26]。
研究发现,野生型菌株与分离自发生坏死性肠炎的肉鸡的α-毒素阴性突变株均可诱发相似的坏死性肠炎[27]。使用鸡肝癌细胞(leghorn male hepatocellular cells,LMH)开展的体外试验也显示,NetB毒素可导致细胞变圆和裂解。这些结果对以往的一种观点提出了挑战,即α-毒素是坏死性肠炎致病机理中的唯一毒力因子。但是,在发生坏死性肠炎的鸡体内也检出了NetB阴性细菌[28],同时,在另一个实验性坏死性肠炎模型中,NetB阴性产气荚膜梭菌未能诱发坏死性肠炎[29]。
感染不携带α-毒素基因突变株的肉鸡在攻毒后发生坏死性肠炎,这表明,α-毒素并不是导致肉鸡发病的唯一致病因素[18]。NetB毒素的发现开启了肉鸡坏死性肠炎研究的新征程。研究人员已在几个田间病例中鉴定出该毒素[30-32]。NetB毒素与其他穿孔毒素的序列相似性不高,如产气荚膜梭菌的β-毒素和δ-毒素(序列相似性分别为38%和40%),金黄色葡萄球菌的α和γ-溶血素[33]。NetB毒素的7个亚基在细胞膜上聚合,形成一个穿孔,这与α-溶血素的装配和作用相似[34]。存在胆固醇时,NetB毒素的毒力增强,不过,该毒素与细胞结合的受体尚不明确[22]。
在不同的国家,健康和患病家禽体内含NetB毒素的分离株比例不同。研究发现,在澳大利亚,分离自患病鸡的70%的产气荚膜梭菌有NetB基因,所有这些分离株在体外均能产生NetB毒素[35]。在美国,分离自患病鸡的58%产气荚膜梭菌和分离自健康鸡的9%菌株含有NetB基因[31]。在丹麦开展的一项研究显示,分离自健康鸡的61%的产气荚膜梭菌含有NetB基因;而患病鸡中只有52%的分离株含有该基因[30]。
存在NetB基因并不能说明该细菌会产生NetB毒素,因为不是所有的NetB基因阳性分离株都会在体外产生该毒素。但是,来自患病鸡的分离株产生该毒素的可能性高于来自健康鸡的分离株[36]。
产气荚膜梭菌中许多独立基因的激活以及毒力因子的释放均受控于一个双组份信号转导系统。该系统包含一个传感器分子VirS以及一个效应器分子VirR。VirS是一种跨膜蛋白,其胞外域可感受细胞的外部环境,并促进胞内结构域的自体磷酸化,导致细胞质中的VirR發生磷酸化反应。NetB毒素受VirSR(VirS/VirR)系统调控,在接种细菌对数生长后期(培养4 h后)产生[27]。
VirSR的编码基因最初是在PFO、α-毒素和唾液酸酶释放的调控中被发现,其还可以调控几种参与大分子降解(从而为细菌提供营养物质)的基因。通过该系统进行调控的其他基因似乎也参与了营养物质的摄入和代谢[37]。目前尚不清楚是什么因子激活了VirS来促进毒力因子的释放。如果可以抑制毒素释放,就可以预防发生坏死性病变。
研究发现,产气荚膜梭菌含有3个抗原基因座,这些基因座可能在坏死性肠炎致病机制中起作用。基因座NELoc1位于质粒上,长度42 kb。另外两个基因座NELoc2和NELoc3较短,分别为11.2 kb和5.6 kb,NELoc3也位于质粒上,NELoc2位于染色体上[38]。
2.3 产气荚膜梭菌产生的其他毒素
研究发现,β-毒素与动物的出血性黏膜溃疡形成有关[13],具有细胞裂解性、皮肤坏死性等特性。但是,尚不清楚其作用模式。
β2-毒素基因位于质粒上[13],首次发现C型产气荚膜梭菌,该菌分离于患有坏死性小肠结肠炎的仔猪体内[39]。目前已从健康和患病的家禽体内分离到了β2-毒素[40]。但是,β2-毒素在动物肠道疾病中的作用尚有争议。2007年,在荷兰开展的一项研究表明,携带非典型cpb2基因的产气荚膜梭菌与蛋鸡的亚急性坏死性肠炎有关[41]。
某些产气荚膜梭菌分离株中的肠毒素与人的胃肠道疾病相关。肠毒素基因etx位于质粒上,其编码的ε-毒素以无活性形式分泌,然后通过蛋白酶水解作用转化为毒性形式[13]。肠毒素是一种穿孔毒素,可与细胞紧密连接的组分闭合蛋白(claudin)结合,在细胞表面形成一个前孔,然后插入细胞[42]。这些穿孔的形成可使钙离子进入细胞。在低浓度时,该过程可诱导细胞凋亡;在高浓度时,该过程可导致肿瘤病变,细胞体积变大,并能诱发炎性细胞死亡[43]。这种毒素会导致皮肤坏死,导致犬、马和人出现腹泻相关疾病[44]。
肠毒素在产气荚膜梭菌的孢子形成过程中产生,并在蛋白酶消化后被活化,该过程会去除24个N末端氨基酸[44],其特性包括细胞毒性、红斑性和致死性[13]。ε-毒素分离自绵羊、山羊和小鼠的D型产气荚膜梭菌分离株[45],但也见于B型产气荚膜梭菌分离株,是产气荚膜梭菌所产生的重要毒力因子之一[46]。
ι-毒素属二元毒素家族成员,由两个独立的多肽(Ia和Ib)构成,这两个多肽协同作用可破坏肌动蛋白细胞骨架,导致细胞死亡[44]。这些蛋白质以前体分子的形式分泌,需要对N末端区域进行蛋白水解来激活。Ib可与宿主细胞脂蛋白相互作用,一旦结合,Ia可与Ib发生相互作用,促进ι-毒素的内吞,从而破坏宿主细胞的细胞骨架[47]。
产气荚膜梭菌产生的其他毒素可能有助于坏死性肠炎的发病过程,如Perfrin、TpeL和μ-毒素[26]。Perfrin是最近发现的一种细菌素,可能是坏死性肠炎的一种毒力因子。细菌素具有抗菌作用[48],Perfrin对其他产气荚膜梭菌分离株具有抗菌活性,抑制其他分离株的生长,从而促进具有该毒素的侵袭性分离株的生长[49]。
TpeL毒素(最初发现于C型产气荚膜梭菌中)见于A型产气荚膜梭菌,研究发现其可以增加坏死性肠炎的严重程度。该毒素至少有A、B、C和D四个活性结构域,其中B结构域可与细胞结合。该毒素被细胞内吞后,D结构域可插入内吞小体膜中。细胞质组分可以激活C结构域,导致毒素被裂解,并释放出A结构域。A结构域可激活细胞质中的GTP酶。TpeL可修饰Rac1和Ras,以介导其细胞毒性作用[29]。这些小的GTP酶分子可在肌动蛋白细胞骨架重排和细胞增殖中起作用[50]。
A型产气荚膜梭菌也可能含有μ-毒素。μ-毒素是一種透明质酸酶,可降解胞外基质中的透明质酸。研究人员认为其可提高产气荚膜梭菌的毒力,其作用机制是通过提高细胞通透性增强其他毒素的生物学效应[51]。
3 结论与展望
产气荚膜梭菌可产生多种毒素,每种毒素类型均与特定的人或动物疾病相关,这提示产气荚膜梭菌毒力与毒素的产生相关[52]。深入了解每种毒素的结构和功能,对研究肉鸡坏死性肠炎的致病机理至关重要,不仅可以深刻认识肉鸡坏死性肠炎病理变化的发生、发展和转归,还可以为坏死性肠炎的临床诊断或监测工具开发提供理论依据。当前,已经基于这些研究结果建立了基因检测方法[53-55]和ELISA检测方法[56-58],此外,找到引发坏死性肠炎的关键致病毒素,将有利于开发有效的亚单位疫苗[59-60]或抗体[61-62],这对无抗生素时代肉鸡坏死性肠炎的防控意义重大。
参考文献
[1] PARISH W E.Necrotic enteritis in the fowl(Gallus gallus domesticus).I.Histopathology of the disease and isolation of a strain of Clostridium welchii[J].Journal of Comparative Pathology,1961,71:377-393.
[2] SARKER M R,SHIVERS R P,SPARKS S G,et al.Comparative experiments to examine the effects of heating on vegetative cells and spores of Clostridium perfringens isolates carrying plasmid enterotoxin genes versus chromosomal enterotoxin genes[J].Applied and Environmental Microbiology,2000,66(12):5549.
[3] GEISSMANN T A,TEUBER M,MEILE L.Transcriptional analysis of the rubrerythrin and superoxide dismutase genes of Clostridium perfringens[J].Journal of Bacteriology,1999,181(22):7136-7139.
[4] JEAN D,BRIOLAT V,REYSSET G.Oxidative stress response in Clostridium perfringens[J].Microbiology,2004,150(6):1649-1659.
[5] KHELFA D E D G,ABD EL-GHANY W,SALEM H.Recent status of Clostridial enteritis affecting early weaned rabbits in Egypt[J].Life Science Journal,2012,9(4):2272-2279.
[6] VAN IMMERSEEL F,DE BUCK J,PASMANS F,et al.Clostridium perfringens in poultry:An emerging threat for animal and public health[J].Avian Pathology,2004,33(6):537-549.
[7] MAY F J,POLKINGHORNE B G,FEARNLEY E J.Epidemiology of bacterial toxin-mediated foodborne gastroenteritis outbreaks in Australia,2001 to 2013[J].Communicable Diseases Intelligence Quarterly Report,2016,40(4):E460-E469.
[8] LI J H,ADAMS V,BANNAM T L,et al.Toxin plasmids of Clostridium perfringens[J].Microbiology and Molecular Biology Reviews:MMBR,2013,77(2):208-233.
[9] SONGER J G.Clostridial enteric diseases of domestic animals[J].Clinical Microbiology Reviews,1996,9(2):216-234.
[10] ROOD J I,ADAMS V,LACEY J,et al.Expansion of the Clostridium perfringens toxin-based typing scheme[J].Anaerobe,2018,53:5-10.
[11] KIU R,HALL L J.An update on the human and animal enteric pathogen Clostridium perfringens[J].Emerging Microbes & Infections,2018,7(1):1-15.
[12] MACFARLANE M G,KNIGHT B C.The biochemistry of bacterial toxins:The lecithinase activity of Cl.welchii toxins[J].The Biochemical Journal,1941,35(8/9):884-902.
[13] PETIT L,GIBERT M,POPOFF M R.Clostridium perfringens:Toxinotype and genotype[J].Trends in Microbiology,1999,7(3):104-110.
[14] AWAD M M,BRYANT A E,STEVENS D L,et al.Virulence studies on chromosomal alpha-toxin and theta-toxin mutants constructed by allelic exchange provide genetic evidence for the essential role of alpha-toxin in Clostridium perfringens-mediated gas gangrene[J].Molecular Microbiology,1995,15(2):191-202.
[15] HOFSHAGEN M,STENWIG H.Toxin production by Clostridium perfringens isolated from broiler chickens and capercaillies (Tetrao urogallus) with and without necrotizing enteritis[J].Avian Diseases,1992,36(4):837-843.
[16] FUKATA T,HADATE Y,BABA E,et al.Influence of Clostridium perfringens and its toxin in germ-free chickens[J].Research in Veterinary Science,1988,44(1):68-70.
[17] REHMAN H,IJAZ A,SPECHT A,et al.In vitro effects of alpha toxin from Clostridium perfringens on the electrophysiological parameters of jejunal tissues from laying hens preincubated with inulin and N-acetyl-L-cysteine[J].Poultry Science,2009,88(1):199-204.
[18] KEYBURN A L,SHEEDY S A,FORD M E,et al.Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens[J].Infection and Immunity,2006,74(11):6496-6500.
[19] COOPER K K,SONGER J G.Necrotic enteritis in chickens:A paradigm of enteric infection by Clostridium perfringens type A[J].Anaerobe,2009,15(1/2):55-60.
[20] ODA M,KABURA M,TAKAGISHI T,et al.Clostridium perfringens alpha-toxin recognizes the GM1a-TrkA complex[J].The Journal of Biological Chemistry,2012,287(39):33070-33079.
[21] MOE P C,HEUCK A P.Phospholipid hydrolysis caused by Clostridium perfringens α-toxin facilitates the targeting of perfringolysin O to membrane bilayers[J].Biochemistry,2010,49(44):9498-9507.
[22] POPOFF MR.Clostridial pore-forming toxins:Powerful virulence factors[J].Anaerobe,2014,30:220-238.
[23] SHEPARD L A,SHATURSKY O,JOHNSON A E,et al.The mechanism of pore assembly for a cholesterol-dependent cytolysin:Formation of a large prepore complex precedes the insertion of the transmembrane beta-hairpins[J].Biochemistry,2000,39(33):10284-10293.
[24] BILLINGTON S J,JOST B H,SONGER J G.Thiol-activated cytolysins:Structure,function and role in pathogenesis[J].FEMS Microbiology Letters,2000,182(2):197-205.
[25] BRYANT A E,BERGSTROM R,ZIMMERMAN G A,et al.Clostridium perfringens invasiveness is enhanced by effects of theta toxin upon PMNL structure and function:The role of leukocytotoxicity and expression of CD11/CD18 adherence glycoprotein[J].FEMS Immunology and Medical Microbiology,1993,7(4):321-336.
[26] KEYBURN A L,BOYCE J D,VAZ P,et al.NetB,a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens[J].PLoS Pathogens,2008,4(2):e26.
[27] CHEUNG J K,KEYBURN A L,CARTER G P,et al.The VirSR two-component signal transduction system regulates NetB toxin production in Clostridium perfringens[J].Infection and Immunity,2010,78(7):3064-3072.
[28] CHALMERS G,BRUCE H L,HUNTER D B,et al.Multilocus sequence typing analysis of Clostridium perfringens isolates from necrotic enteritis outbreaks in broiler chicken populations[J].Journal of Clinical Microbiology,2008,46(12):3957-3964.
[29] TIMBERMONT L,HAESEBROUCK F,DUCATELLE R,et al.Necrotic enteritis in broilers:An updated review on the pathogenesis[J].Avian Pathology:Journal of the W.V.P.A,2011,40(4):341-347.
[30] MOHAMED E ABD EL-HACK,MOHAMED T EL-SAADONY,AHMED R ELBESTAWY,et al.Necrotic enteritis in broiler chickens:Disease characteristics and prevention using organic antibiotic alternatives - a comprehensive review[J].Poultry Science,2022,101(2):101590.
[31] MARTIN T G,SMYTH J A.Prevalence of netB among some clinical isolates of Clostridium perfringens from animals in the United States[J].Veterinary Microbiology,2009,136(1/2):202-205.
[32] JOHANSSON A,ASP?N A,KALDHUSDAL M,et al.Genetic diversity and prevalence of netB in Clostridium perfringens isolated from a broiler flock affected by mild necrotic enteritis[J].Veterinary Microbiology,2010,144(1/2):87-92.
[33] NAGAHAMA M,OCHI S,ODA M,et al.Recent insights into Clostridium perfringens beta-toxin[J].Toxins,2015,7(2):396-406.
[34] SAVVA C G,FERNANDES D A COSTA S P,BOKORI-BROWN M,et al.Molecular architecture and functional analysis of NetB,a pore-forming toxin from Clostridium perfringens[J].The Journal of Biological Chemistry,2013,288(5):3512-3522.
[35] KEYBURN A L,YAN X X,BANNAM T L,et al.Association between avian necrotic enteritis and Clostridium perfringens strains expressing NetB toxin[J].Veterinary Research,2010,41(2):21.
[36] ABILDGAARD L,SONDERGAARDd TE,ENGBERG RM, et al.In vitro production of necrotic enteritis toxin B,NetB,by netB-positive and netB-negative Clostridium perfringens originating from healthy and diseased broiler chickens[J].Veterinary Microbiology,2010,144(1/2):231-235.
[37] SHIMIZU T,BA-THEIN W,TAMAKI M,et al.The virR gene,a member of a class of two-component response regulators,regulates the production of perfringolysin O,collagenase,and hemagglutinin in Clostridium perfringens[J].Journal of Bacteriology,1994,176(6):1616-1623.
[38] LEPP D,ROXAS B,PARREIRA V R,et al.Identification of novel pathogenicity loci in Clostridium perfringens strains that cause avian necrotic enteritis[J].PLoS One,2010,5(5):e10795.
[39] BACCIARINI L N,BOERLIN P,STRAUB R,et al.Immunohistochemical localization of Clostridium perfringens beta2-toxin in the gastrointestinal tract of horses[J].Veterinary Pathology,2003,40(4):376-381.
[40] LEBRUN M,FIL?E P,MOUSSET B,et al.The expression of Clostridium perfringens consensus beta2 toxin is associated with bovine enterotoxaemia syndrome[J].Veterinary Microbiology,2007,120(1/2):151-157.
[41] ALLAART J G,DE BRUIJN N D,VAN ASTEN A J A M,et al.NetB-producing and beta2-producing Clostridium perfringens associated with subclinical necrotic enteritis in laying hens in the Netherlands[J].Avian Pathology,2012,41(6):541-546.
[42] GAO Z J,MCCLANE B A.Use of Clostridium perfringens enterotoxin and the enterotoxin receptor-binding domain (C-CPE) for cancer treatment:Opportunities and challenges[J].Journal of Toxicology,2012,2012:981626.
[43] MCCLANE B A,CHAKRABARTI G.New insights into the cytotoxic mechanisms of Clostridium perfringens enterotoxin[J].Anaerobe,2004,10(2):107-114.
[44] SONGER J G.Bacterial phospholipases and their role in virulence[J].Trends in Microbiology,1997,5(4):156-161.
[45] GARCIA J P,ADAMS V,BEINGESSER J,et al.Epsilon toxin is essential for the virulence of Clostridium perfringens type D infection in sheep,goats,and mice[J].Infection and Immunity,2013,81(7):2405-2414.
[46] ALVES G G,MACHADO D E ?VILA R A,CH?VEZ-OL?RTEGUI C D,et al.Clostridium perfringens epsilon toxin:The third most potent bacterial toxin known[J].Anaerobe,2014,30:102-107.
[47] ADAMS V,LI J H,WISNIEWSKI J A,et al.Virulence plasmids of spore-forming bacteria[J].Microbiology Spectrum,2014,2(6):2.6.04.
[48] NISHIE M,NAGAO J I,SONOMOTO K.Antibacterial peptides bacteriocins:An overview of their diverse characteristics and applications[J].Biocontrol Science,2012,17(1):1-16.
[49] TIMBERMONT L,DE SMET L,VAN NIEUWERBURGH F, et al.Perfrin,a novel bacteriocin associated with netB positive Clostridium perfringens strains from broilers with necrotic enteritis[J].Veterinary Research,2014,45(1):40.
[50] NAGAHAMA M,OHKUBO A,ODA M,et al.Clostridium perfringens TpeL glycosylates the rac and ras subfamily proteins[J].Infection and Immunity,2011,79(2):905-910.
[51] CANARD B,GARNIER T,SAINT-JOANIS B,et al.Molecular genetic analysis of the nagH gene encoding a hyaluronidase of Clostridium perfringens[J].Molecular and General Genetics MGG,1994,243(2):215-224.
[52] SMEDLEY J G III,FISHER D J,SAYEED S,et al.The enteric toxins of Clostridium perfringens[M]//Reviews of Physiology,Biochemistry and Pharmacology.Berlin,Heidelberg:Springer Berlin Heidelberg,2004:183-204.
[53] WISE M G,SIRAGUSA G R.Quantitative detection of Clostridium perfringens in the broiler fowl gastrointestinal tract by real-time PCR[J].Applied and Environmental Microbiology,2005,71(7):3911-3916.
[54] MOORE R J.Necrotic enteritis predisposing factors in broiler chickens[J].Avian Pathology,2016,45(3):275-281.
[55] BAILEY M A,MACKLIN K S,KREHLING J T.Use of a multiplex PCR for the detection of toxin-encoding genes netB and tpeL in strains of Clostridium perfringens[J].ISRN Veterinary Science,2013,2013:865702.
[56] COOPER K K,SONGER J G,UZAL F A.Diagnosing clostridial enteric disease in poultry[J].Journal of Veterinary Diagnostic Investigation,2013,25(3):314-327.
[57] LEE K W,LILLEHOJ H S,JEONG W,et al.Avian necrotic enteritis:Experimental models,host immunity,pathogenesis,risk factors,and vaccine development[J].Poultry Science,2011,90(7):1381-1390.
[58] LEE Y,KIM W H,LEE S J,et al.Detection of chicken interleukin-10 production in intestinal epithelial cells and necrotic enteritis induced by Clostridium perfringens using capture ELISA[J].Veterinary Immunology and Immunopathology,2018,204:52-58.
[59] YUAN B H,SUN Z F,LU M M,et al.Immunization with pooled antigens for Clostridium perfringens conferred partial protection against experimental necrotic enteritis in broiler chickens[J].Vaccines,2022,10(6):979.
[60] LEE K W,LILLEHOJ H S,PARK M S,et al.Clostridium perfringens alpha-toxin and NetB toxin antibodies and their possible role in protection against necrotic enteritis and gangrenous dermatitis in broiler chickens[J].Avian Diseases,2012,56(1):230-233.
[61] ALI SHAMSHIRGARAN M,GOLCHIN M,MOHAMMADI E.Lactobacillus casei displaying Clostridium perfringens NetB antigen protects chickens against necrotic enteritis[J].Applied Microbiology and Biotechnology,2022,106(19):6441-6453.
[62] ABADEEN Z U,JAVED M T,JAMIL T,et al.Ameliorative effects of anti-clostridial egg yolk antibodies (IgYs) in experimentally-induced avian necrotic enteritis[J].Animals,2022,12(10):1307.

