Goldilocks principle of minimally invasive surgery for gastric subepithelial tumors
Wei-Jung Chang, Lien-Cheng Tsao, Hsu-Heng Yen, Chia-Wei Yang, Hung-Chi Chang, Chew-Teng Kor, Szu-Chia Wu, Kuo-Hua Lin
Abstract
Key Words: Gastric subepithelial tumors; Endoscopic resection; Laparoscopic resection; Tumor size
INTRODUCTION
Gastric subepithelial tumors (SETs) include a broad spectrum of benign and malignant lesions, of which gastrointestinal stromal tumors (GISTs) are the most common. Current guidelines recommend the complete resection of gastric SETs if the size is > 2 cm, malignant features are present, or the patient is symptomatic and would prefer surgical management[1-5]. As small GISTs pose a risk for malignancy, endoscopic resection (ER) could be a good alternative method for obtaining a histological diagnosis and therapeutic resection compared with periodic surveillance[6]. With recent advancements in endoscopic and laparoscopic management, different approaches to minimally invasive surgery have been adopted and tailored to individual cases.
Among the minimally invasive approaches, laparoscopic surgery has proven to be feasible with faster recovery, shorter hospital stays, and equivalent oncological safety compared to open surgery. Initially, open surgery was considered the main treatment for extensive tumors, and laparoscopic surgery was reserved for small tumors (< 5 cm). However, with advanced laparoscopic techniques, tumor size is no longer a restricting factor; even large tumors can be successfully removedvialaparoscopic surgery[7-9]. For certain tumors in unfavorable locations[10], Hikiet al[11] introduced laparoscopic and endoscopic cooperative surgery (LECS), with the combined advantages of endoscopy and laparoscopy[12],which helps to achieve precise localization, minimal resection, and functional preservation. Endoscopic submucosal dissection (ESD) techniques have advanced in the resection of tumors located deeper than the submucosal layer[13]. The ER of small gastric SETs (< 5 cm) involves a shorter surgery and less intraoperative blood loss in selected cases of intraluminal tumors[14,15]. By considering complications, such as perforation or bleeding, we modified the LECS procedure as a backup laparoscopic surgery to provide timely management, which required more operative time but reduced postoperative morbidity[16].
Although different minimally invasive approaches can be applied to gastric SETs, the effectiveness and safety of ER,laparoscopic resection (LR), or hybrid methods have not been well established. Thus, in this study, we aimed to use the Goldilocks principle to determine the best type of treatment for gastric SETs by comparing the clinical outcomes of ER,LR, and our hybrid method, as this information can be crucial in improving options for minimally invasive surgery considering the risks and potential benefits in this setting.
MATERIALS AND METHODS
Patients and study design
We conducted a retrospective study of patients with gastric SETs who underwent ER or LR at the operating theater in our institution between January 2013 and December 2021. Medical records were retrospectively reviewed to define the patient/tumor characteristics and operative outcomes. Based on pathologic diagnosis, the tumor was further divided into two groups: benign disease and malignant or malignant potential disease. All patients underwent endoscopic ultrasonography (EUS) or abdominal computed tomography (CT) to evaluate the tumor size, invasion depth, and characteristics before resection.
Ethical permission
The study was approved by the Institutional Review Board of Changhua Christian Hospital (approval No. 220117) and registered at ClinicalTrials.gov (NCT05452265). This work has been reported in line with the “Strengthening the Reporting of Observational studies in Epidemiology (STROBE)” criteria[17]. All relevant data are included in the paper and its Supporting Information files.
Patient management strategy
Complete resection of gastric SETs is recommended if the tumor size is > 2 cm, malignant features are present, or if the patient is symptomatic, declined periodical surveillance, and preferred to undergo diagnostic and therapeutic resection.Patients with gastric SETs in the superficial layer underwent ER in the endoscopic room and those with a high probability of surgical intervention were evaluated both by endoscopists and general surgeons preoperatively[16,18]. ER with backup surgery was indicated for patients with endoscopic intent, and a small tumor size tolerated the endoscopic retrieval. On the other hand, LR was indicated for patients with surgical intent and those with the following conditions, which were not suitable for ER: (1) Large tumor size with difficult endoscopic retrieval; (2) Symptoms of gastrointestinal tract bleeding with difficulty in endoscopic visualization; (3) Suspicion of tumor rupture that required intra-abdominal exploration; and (4) Histologic diagnosis of GIST with initial treatment of target therapy. Open surgery was performed in patients who were not amenable to laparoscopy due to pulmonary compliance and cardiovascular disease, had large tumors that eventually needed a large incision wound for specimen extraction, and had tumors with suspected multivisceral involvement.
Inclusion and exclusion criteria
In total, we included 194 patients who underwent tumor resection under general anesthesia at the operating theater, with 100 and 94 patients in the ER and LR groups, respectively. We excluded three patients in the ER group due to anatomic changes in the stomach following previous surgery and four patients in the LR group due to converted open surgery.Among the four patients who underwent converted open surgery, one had splenic metastasis with difficult dissection plain intraoperatively (tumor size 12.5 cm, posterior aspect of the upper third stomach), two had difficulty in tumor localization of the upper third stomach with one receiving preoperative endoscopic tattoo (size 4 cm, posterior side and size 1 cm, lesser curvature side with tattooing), and one was concerned with post-gastrectomy stenosis with further gastro-gastrostomy (size 7 cm, posterior aspect of the lower third stomach).
ER only, ER with backup surgery, and LR were performed according to the protocols in our previous studies[16,18]. In the ER group, most patients (92, 92%) underwent endoscopic submucosal dissection (ESD) and endoscopic mucosal resection if necessary for an R0 attempt, except for eight cases of submucosal tunneling ER (STER). Some cases with iatrogenic perforation could be successfully repaired by endoscope (Video 1). In the LR group, most patients (92, 97.9%)underwent wedge gastrectomy, except for two cases who underwent distal gastrectomy with Roux-en-Y reconstruction(2, 2.1%), given the risk for postoperative stenosis. In addition, 12 patients (12.8%) underwent intraoperative endoscopeassisted LR to localize the tumor more precisely.
Statistical analysis
Categorical and continuous variables are expressed as number (proportion) and median and interquartile range (IQR),respectively. Chi-squared test was used to compare categorical variables, and Kruskal-WallisHtest was used to compare continuous variables. A logistic regression model was used to assess the association between patient characteristics and likelihood of a treatment strategy. Odds ratios were calculated using a crude multivariate analysis and a 1:1 propensitymatched dataset. Forest plots provide a data visualization method to present multivariate adjustment factors for the likelihood of undergoing treatment. Linear regression models were used to assess the impact of the three treatment strategies on clinical outcomes (procedure time, length of hospital stay, and Clavien grade ≥ III complications).Furthermore, we used the area under the curve (AUC) to assess the discriminative ability of tumor size and Youden’s index to determine the optimal cut-off tumor size. Kaplan-Meier curves and log-rank tests were used to compare the disease-free survival rates and overall survival between the ER and LR groups during long-term surveillance. Statistical analyses were performed using SAS (version 9.4; SAS Institute Inc., Cary, NC, United States), and a visualization plot was constructed using the R software (version 4.1.0; Comprehensive R Archive Network: http://cran.r-project.org). All twosidedPvalues less than 0.05 were considered statistically significant.
RESULTS
A total of 194 patients were included: 100 in the ER group and 94 in the LR group. In the ER group, 73 patients underwent ER only, and 27 underwent further backup laparoscopic surgery due to uncontrolled bleeding or incidental perforation.There were no significant differences in sex, the layer of tumor origin, length of hospitalization, or major postoperative complications between the ER and LR groups (Table 1). In the ER group, patients who were slightly younger (56vs62 years) had a significantly higher percentage of small tumor sizes of ≤ 2 cm (72%vs16%) and shorter procedure times (75vs130 min). In the LR group, significant differences were observed in the percentage of tumors > 3 cm in size (84%vs28%), tumors in the middle third of the stomach (14.9%vs6%), exophytic tumor growth (56.4%vs11%), and pathology of malignancy or malignant potential (85.1%vs45%). Multivariable analysis results showed that patients tended to undergo laparoscopic surgery rather than ER when the following factors were present: tumor size of > 2 cm (3-5 cm adjusted odds ratio (aOR) 6.643, > 5 cm aOR 30.158), tumor in the middle or lower third of the stomach (aOR 2.625), exophytic growth(aOR 6.0782), or pathology of malignancy (aOR 3.552). However, it is possible that these factors resulted from preoperative selection bias (Figure 1A).
A total of 27 patients who underwent backup surgery after incomplete ER were compared with those in the LR group(Table 2). No significant differences were observed in age, sex, tumor location, the layer of tumor origin, exophytic tumor growth, or major postoperative complications; however, a higher percentage of tumors of size > 2 cm (84%vs40.7%),pathology of malignancy or malignant potential (85.1%vs59.3%), prolonged procedure duration (130vs185 min), and a shorter length of hospital stay (6vs7 d) were observed in the LR group. Multivariable analysis showed that patients tended to undergo laparoscopic surgery rather than an initial ER attempt if the tumor size was > 2 cm (aOR 5.81), if the tumor was in the middle or lower third of the stomach (aOR 5.22), and if there was pathology of malignancy (aOR 4.37);these results were statistically significantly different (Figure 1B).
To compare the operative outcomes between ER with backup surgery and LR, the predictor of a prolonged procedure was tumor location in the middle or lower third of the stomach, and the predictor of a prolonged stay was advanced age.However, these factors were not significant after propensity-score matching. More importantly, patients who underwent ER with backup surgery had longer procedures (56.4 min) and prolonged hospital stays (2 d) on average (Table 3).Furthermore, the optimal cut-off point for the tumor size for laparoscopic surgery was 2.15 cm, with an AUC of 0.841,sensitivity of 84%, and specificity of 74% (Figure 2).
Among patients with major complications (Clavien grade ≥ III), there were five patients (5.3%) in the ER group (three graded IIIa and two graded IIIb), none after backup surgery, and three patients (3.2%) in the LR group (two graded IIIa and one graded IVa). In the ER group, three patients were graded IIIa, two patients had gastric ulcer bleeding and received endoscopic hemostasis, and one patient had massive pneumoperitoneum without peritonitis and received sonography-guided air tapping. Two patients with grade-IIIb perforation had delayed perforation and underwent laparoscopic surgery[15]. In the LR group, two patients with grade IIIa had delayed gastric emptying after laparoscopic wedge gastrectomy and received endoscopic duodenal tube insertion for enteral feeding on postoperative days (POD) 18 and 19,respectively. One patient with grade IVa developed pneumonia with acute respiratory failure on POD 3 and received intensive critical care thereafter. All patients recovered from the complications and were discharged.
The pathology of the gastric SETs is shown in Table 4. Before laparoscopic surgery, the rate of endoscopic ultrasoundguided fine-needle aspiration (EUS-FNA) was 17% (16/94), and the diagnostic accuracy was 10.6% (10/94), with one complication and one perforation. Nine patients had cytopathological diagnoses of GIST, and two patients received neoadjuvant target therapy with imatinib at a dose of 400 mg/day for one year preoperatively. Overall, the percentage of malignant pathology (85.1%vs45%,P< 0.001), particularly the composition of GIST with intermediate and high risk(39.7% vs. 11.6%), and the recurrence rate (6.4%vs0,P= 0.012) were significantly higher in the LR group, except for disease-related mortality (Table 4). In the ER group, the majority of the patients diagnosed with GIST were categorized into the very low-risk group (60.5%), followed by the low-risk (27.9%), and the intermediate-risk (11.6%) groups,according to the NIH classification[18]. They underwent long-term surveillance for a mean duration of 27.5 mo, and no recurrence was detected. One patient, diagnosed with neuroendocrine tumor grade 1, received further gastrectomy and adjuvant chemotherapy without recurrence during a 64-month follow-up period. In the LR group, 78 patients were diagnosed with GISTs, with five cases of complicated bleeding and two ruptures, and were categorized into the very lowrisk (11, 14.1%), low-risk (36, 46.2%), intermediate-risk (20, 25.6%), and high-risk (11, 14.1%) groups. A total of 12 patients(15.4%) received post-operative adjuvant imatinib therapy. They had long-term surveillance for a mean duration of 37.3 mo; four cases were reported to have recurrences (two local recurrences, one liver metastasis, and one mesenteric metastasis) with a mean disease-free survival of 12.3 mo. Four patients who were still alive during follow-up received target therapy, and one patient with mesenteric metastasis underwent further surgery for tissue proof and occult obstruction. One patient diagnosed with lipoleiomyosarcoma had liver metastasis, with a disease-free survival time of 23.5 mo. The liver metastasis progressed, and the patient died of inferior vena cava syndrome, with an overall survival of 47 mo. Another patient with a neuroendocrine tumor also had liver metastasis, with a disease-free survival time of 66.5 mo. She received octreotide treatment but died due to multiple distant metastases, with an overall survival of 80 mo. The remaining five patients died from other disorders, with an overall mortality rate of 7.4% and a disease-related mortality rate of 2.1%. Overall, although the disease-free survival and survival rates were slightly decreased in the LR group during long-term surveillance, the difference was not significant (log-rankP= 0.600, 0.200) (Figure 3).
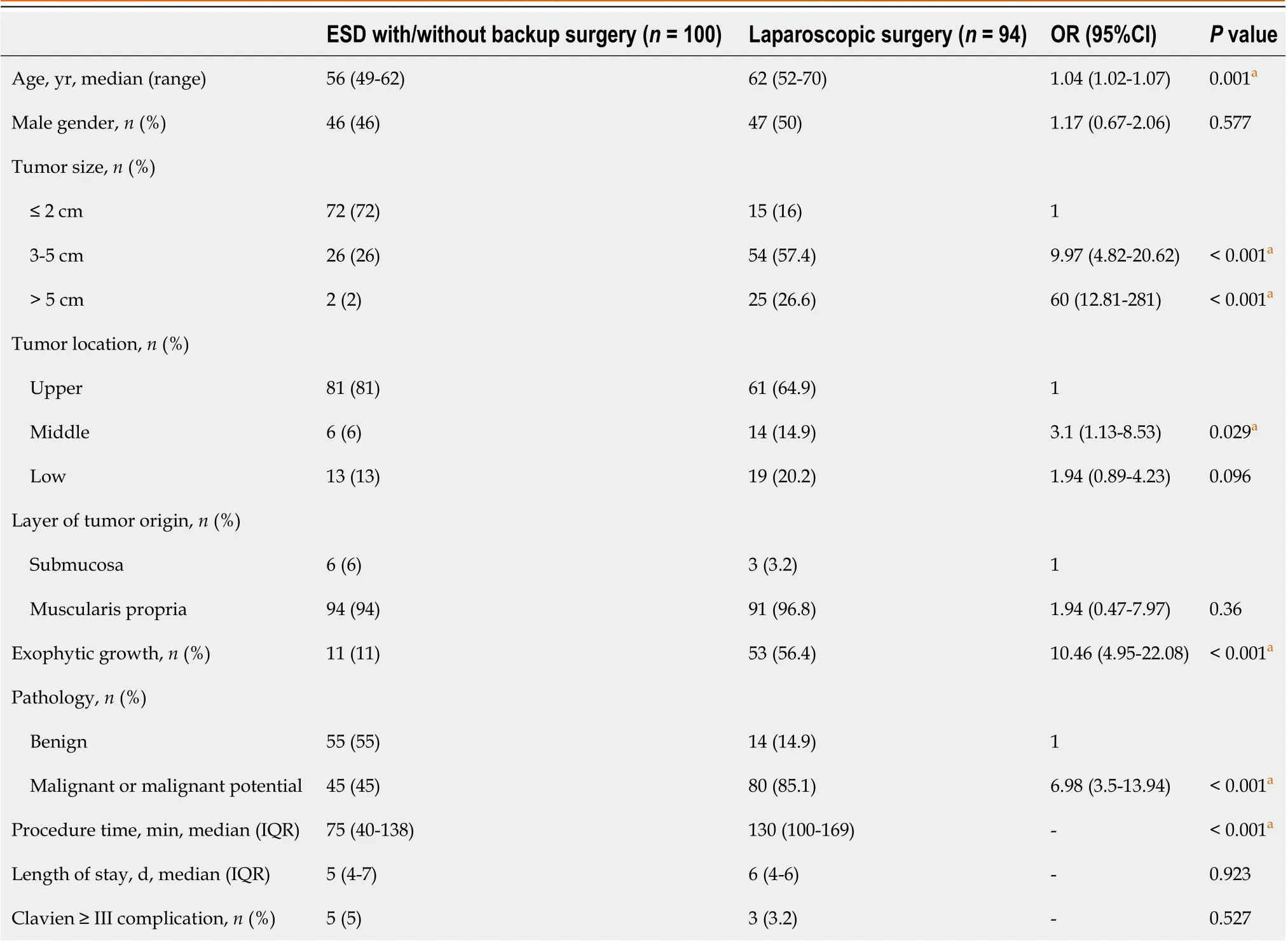
Table 1 Characteristics of patients undergoing endoscopic submucosal dissection and laparoscopic surgery
DISCUSSION
For gastric SETs, complete tumor removal with a free margin, minimal resection of the normal stomach, and avoiding pseudocapsule rupture are the goals of the present treatment. With recent advances in endoscopic techniques, ER has become an alternative option because the endoscopic approach maintains the integrity and anatomical function of the stomach without damaging the abdominal wall. More studies have compared clinical outcomes between ER and LR for gastric SETs of size ≤ 5 cm, and ER has the advantages of lower invasiveness, a shorter procedure duration[14,15,19-23],faster recovery[20,21,23,24], and a shorter hospital stay[20,21,24,25] in selected cases with smaller tumor sizes[14,15,19,20,24,25] and intraluminal growth[14,15,20]. However, ER-related complications are still a greater concern for prolonged hospital stays than those for LR[22]. From our experience, we also found that ER had the advantage of a shorter procedure duration than LR, while similar outcomes for hospital stays and major postoperative complications were observed. Where backup surgery was required to address incomplete ER, which could effectively reduce post-ER morbidity[16], prolonged procedures and hospital stays were observed compared with LR. Thus, ER is considered an effective and less invasive approach in selected cases if the tumor can be successfully resected.
Although ER tends to be used to manage smaller tumors, debates regarding the usual size for minimally invasive surgery for gastric SETs continue. For ER, a systematic review has recorded the average diameter of gastric GISTs, which ranges from 1.1 to 3.8 cm[14]. Recently, the European Society of Gastrointestinal Endoscopy (ESGE) guidelines have suggested that ER for gastric GISTs of size < 3.5 cm with intraluminal growth is an alternative to laparoscopic surgery[6].With advances in endoscopic techniques for the closure of large gastric wall defects, a Chinese study found that it is feasible to treat giant gastric SETs of size ≥ 6 cm by ER with favorable long-term outcomes, although the minimum diameter of the tumor was associated withen blocresection[26]. In our early period of ER, there were two cases of tumors> 5 cm in size, and we found it difficult to perform endoscopic retrieval of the entire tumor. Although piecemeal ER was feasible for complete resection, we tended to useen blocresection for smaller tumors, with a rate of 90.4% based on our data.
On the other hand, laparoscopic surgery was initially suggested for tumors < 5 cm in size with a risk for tumor rupture and concern for oncological safety[27]. However, with the development of techniques and energy devices, laparoscopic surgery is no longer limited by the tumor size. Laparoscopic surgery provides a clear and broad field of vision thatfacilitates more sophisticated dissection and timely treatment of intraoperative bleeding, thereby realizing equivalent oncological safety and even better 5-year disease-free survival for large tumors (> 5 cm) compared to open surgery[7-9].In our study, 26.6% of the tumors in the LR group were large tumors, and we also used this for two cases with preoperative target therapy for one year. We continued adjuvant therapy with imatinib at a dose of 400 mg/d postoperatively, and the patients were still disease-free at 25 and 39.5 mo during follow-up. Nevertheless, laparoscopy was limited by its decreased tactile feedback and difficulty in tumor localization; therefore, endoscopic assistance is suggested for small tumors (≤ 1.8 cm) and intraluminal growth types[28]. In our study, intraoperative endoscopy was required to precisely localize the tumors in 12 patients (12.8%) in the LR group. Overall, from our practical experience, we found that the optimal cut-off point for the tumor size for laparoscopic surgery is 2.15 cm.
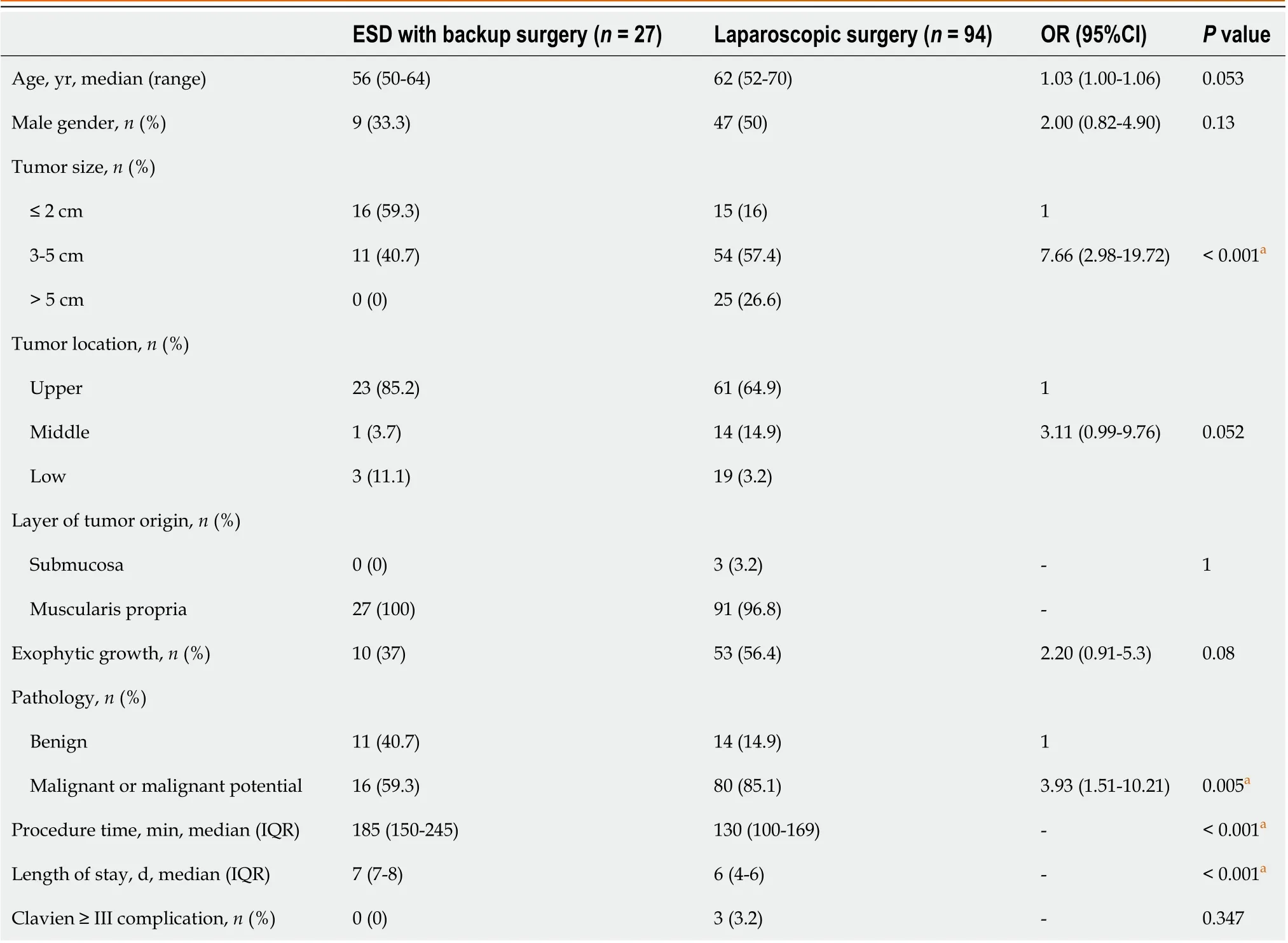
Table 2 Characteristics of endoscopic submucosal dissection with backup surgery and laparoscopic surgery
In our study, patients with tumors in the middle or lower third of the stomach tended to undergo laparoscopic surgery rather than ER or backup surgery, which might have resulted from the initial selection bias of endoscopists and surgeons considering the tumor size. However, the location in the upper third of the stomach has been reported as a risk factor for perforation due to the relatively thin gastric wall and difficultly in endoscopic angulation[18,29]. With the technical expertise of experienced endoscopists, gastric SETs in the upper third of the stomach could be successfully removed with ER. In addition, we considered that ER for gastric SETs in the anterior wall of the stomach body had a high probability of surgical intervention because intragastric gas leakage into the peritoneal cavity without a soft tissue boundary made endoscopic repair more difficult, owing to a poor visual field and a gradually distended abdominal wall[16]. A Japanese study reported that surgeons found it difficult to endoscopically repair large defects on the anterior gastric wall and resorted to laparoscopic surgery[15]. These reasons may explain why tumors in the middle or lower third of the stomach tend to be treatedvialaparoscopic surgery.
Tumors with exophytic growth have a high risk for perforation[16], and their size may be larger than those without exophytic growth. In the present study, exophytic growth was significantly different between the ER and LR groups;however, no significant difference was observed between the backup surgery and LR groups. Patients with exophytic tumor growth eventually underwent laparoscopic surgery because of the high risk for incidental perforation after ER and easy localization during laparoscopic exploration. Based on the above findings, we assumed that exophytic tumor growth was undoubtedly indicative of LR.
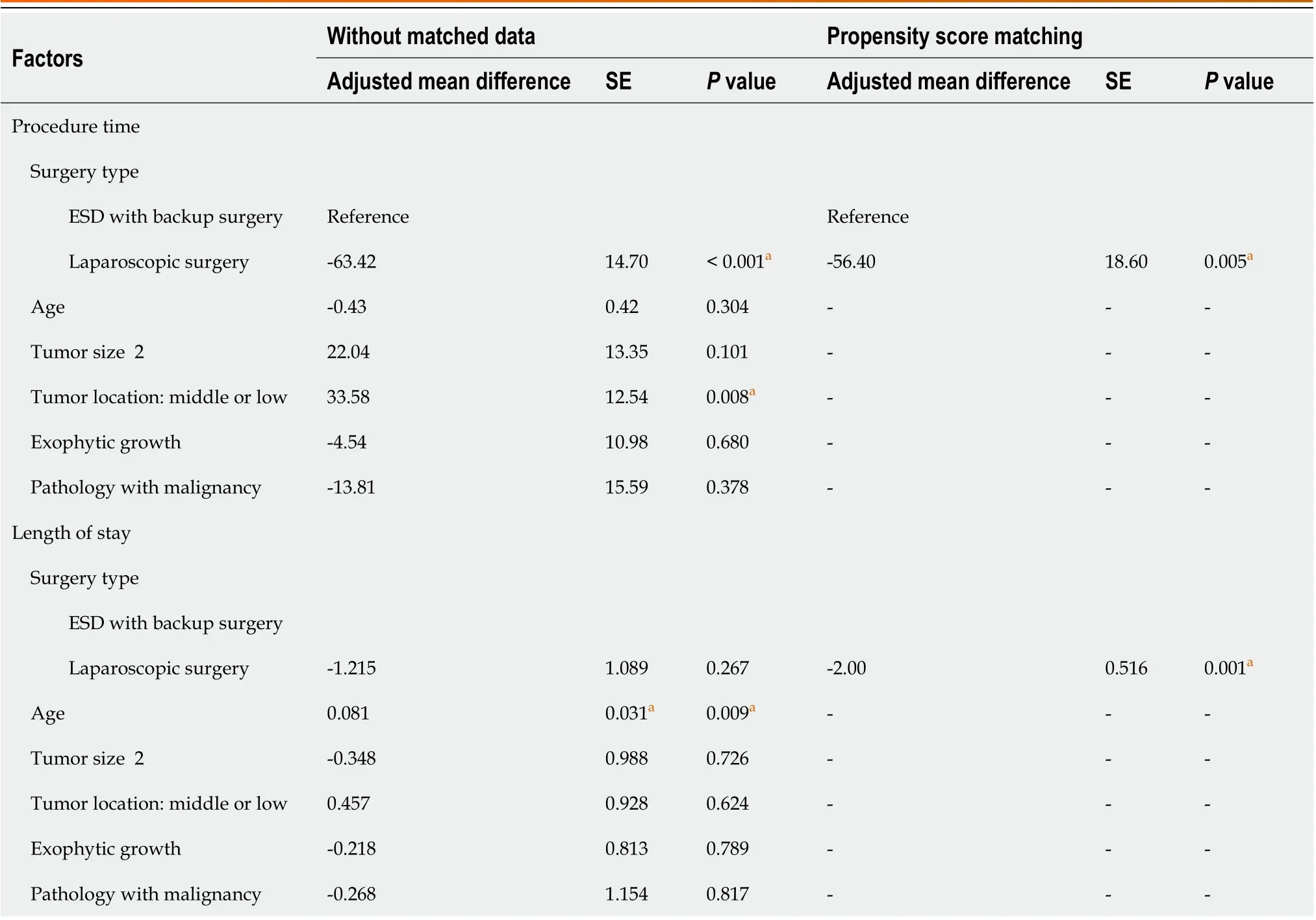
Table 3 Factors affecting procedure time and length of stay in endoscopic submucosal dissection with backup surgery and laparoscopic surgery
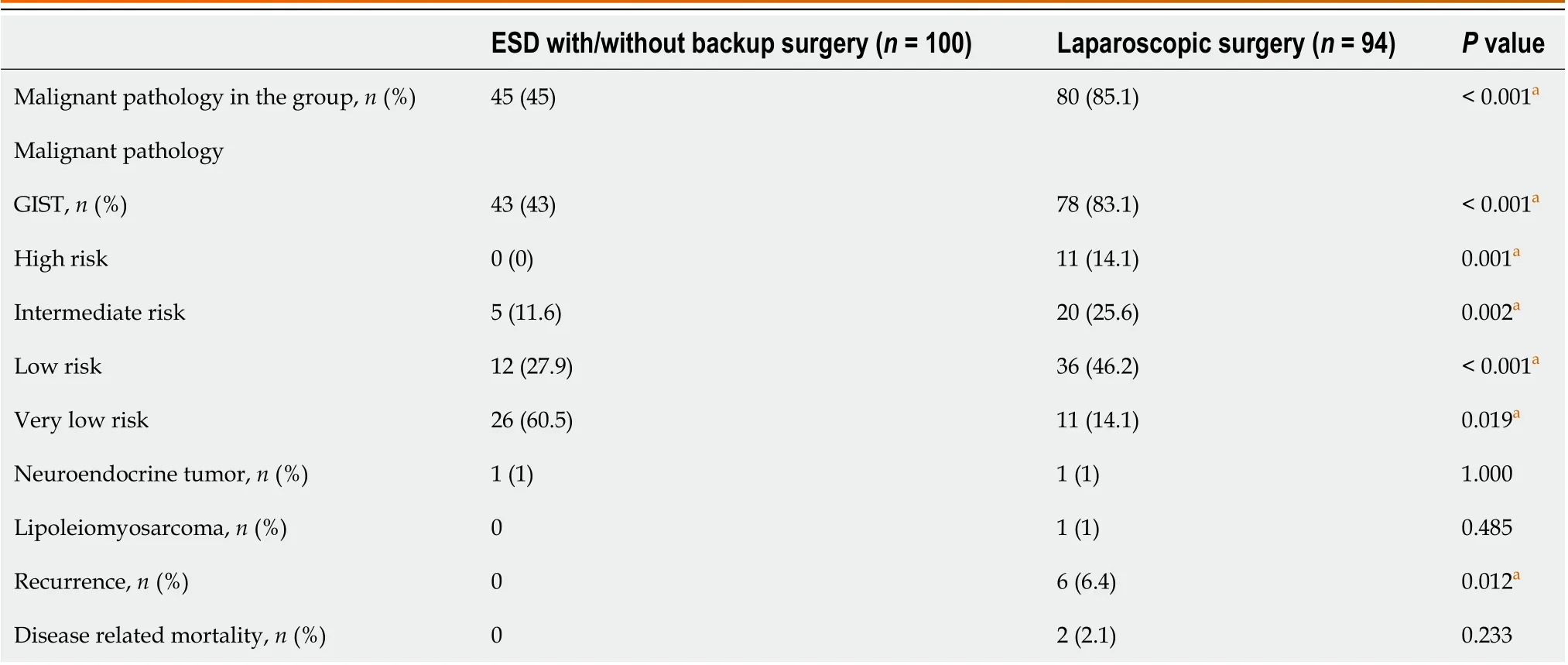
Table 4 Malignant pathology and clinical outcomes after endoscopic submucosal dissection and laparoscopic surgery
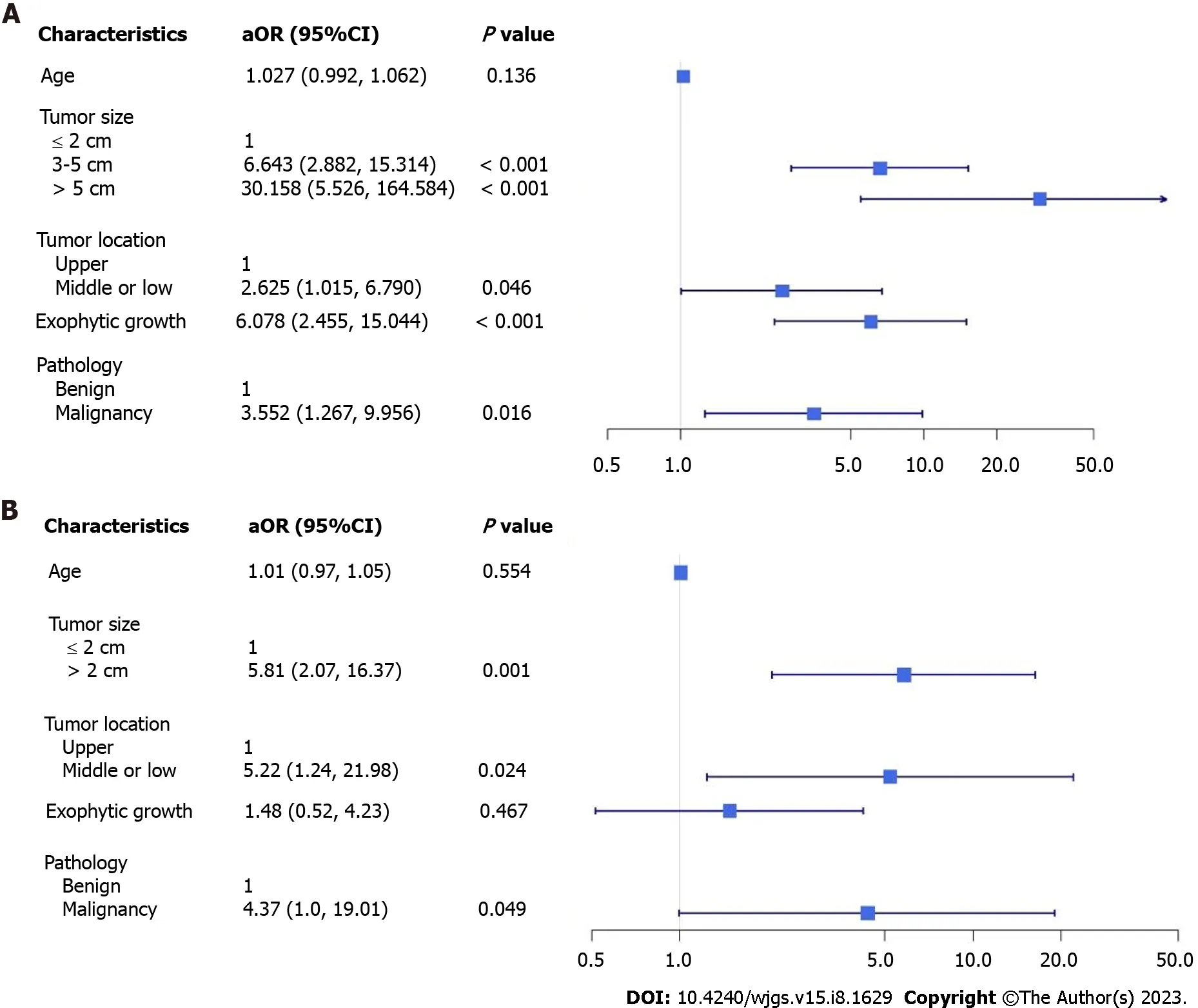
Figure 1 Multivariate adjustment factor for the likelihood of patients undergoing laparoscopic surgery compared to endoscopic submucosal dissection patients and endoscopic submucosal dissection with backup surgery. A: Endoscopic submucosal dissection patients; B:Endoscopic submucosal dissection with backup surgery. aOR: adjusted odds ratio; CI: Confidence interval.
Furthermore, patients with malignant tumors or malignant potential tended to undergo LR (85.1%vs45%; OR, 6.98;P< 0.001). For malignant pathology, oncological safety is still a concern for ER because the complete resection rate has been found to be lower in ER than that in surgery[23,25]. Nevertheless, ER for small tumor sizes has still achieved R0 resection rates of up to 97%, according to a systematic review and meta-analysis[14,30,31], and no significant differences in longterm oncological outcomes for GISTs have been observed between ER and LR [23]. For gastric GISTs, tumor size is a key factor for recurrence risk rather than resection status, if no macroscopic residual tumor exists[24,29,32] and should be cautiously considered for different strategies of minimally invasive surgery. A previous study suggested that gastric GISTs that were completely resected endoscopically carry a lower stratified risk for aggressive clinical outcomes[24,32,33], and we found similar results in our study. In the ER group, the majority (60.5%) of the GISTs had a very low risk due to their small sizes and lack of disease recurrence during follow-up. In the LR group, 39.7% of the GISTs were categorized as intermediate- or high-risk. Overall, a higher recurrence rate (6.4%) and disease-related mortality rate (2.1%) were observed in the LR group; however, no significant difference was observed during long-term surveillance. Although all the patients underwent complete resection, theen blocresection rate after ER was only 90.4%, which is not of high concern for LR. For tumors with suspected aggressive behavior of malignant pathology preoperatively, we preferred laparoscopic surgery to achieve similar oncological outcomes.
This study has some limitations. First, it was conducted at a single center with a relatively small sample size. Second,selection bias existed between the ER and LR groups because endoscopists and surgeons evaluated the patients preoperatively to make collaborative decisions regarding minimally invasive surgical methods. A few patients with gastric SETs in the superficial submucosal layer who underwent ER in the endoscopic room were not included in this database. Third,although GISTs are the most common type of malignant pathology, we focused on gastric SETs and other malignant pathologies. Thus, we focused on perioperative clinical outcomes after ER and LR, whereas the long-term outcomes of malignant pathologies require further analysis. Fourth, different ER and LR methods were chosen by endoscopists and surgeons according to tumor characteristics and location. However, we were unable to perform a detailed comparison of the different methods because of the small sample size.
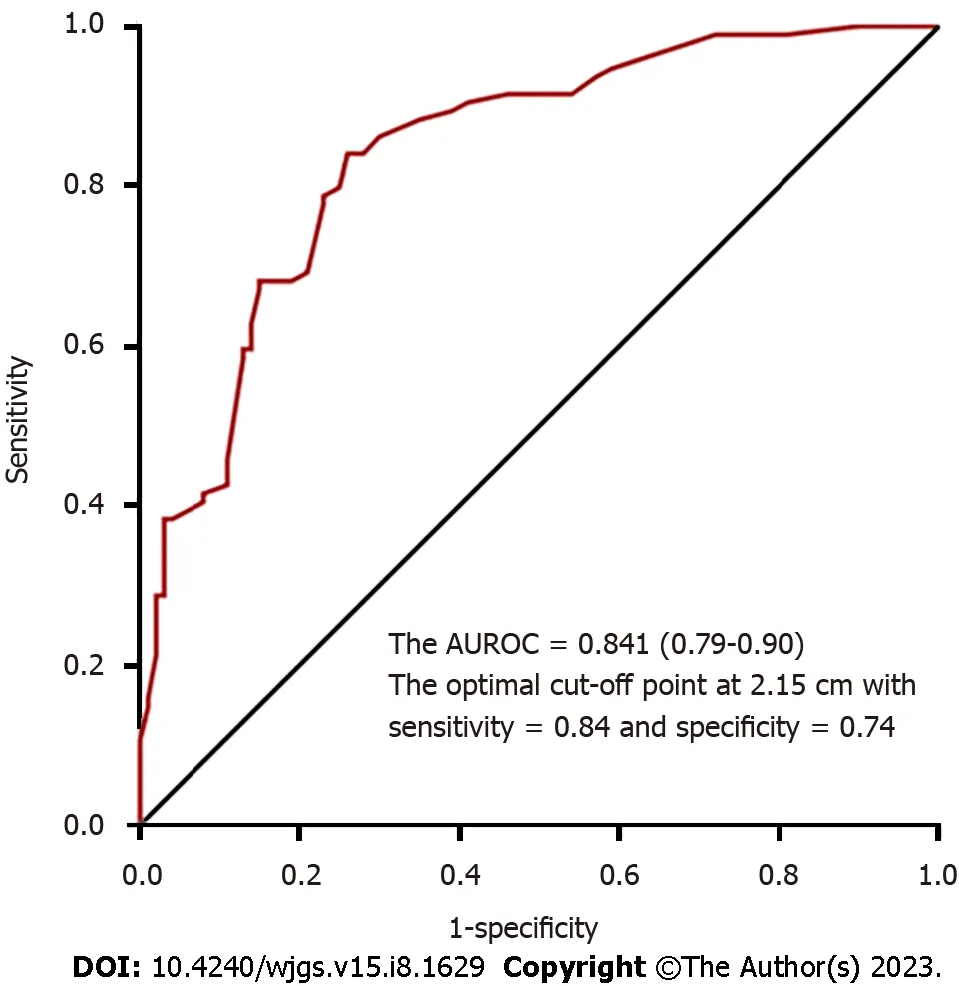
Figure 2 Optimal cut-off point for tumor size for laparoscopic surgery. AUROC: Area under the receiver operating characteristic curve.
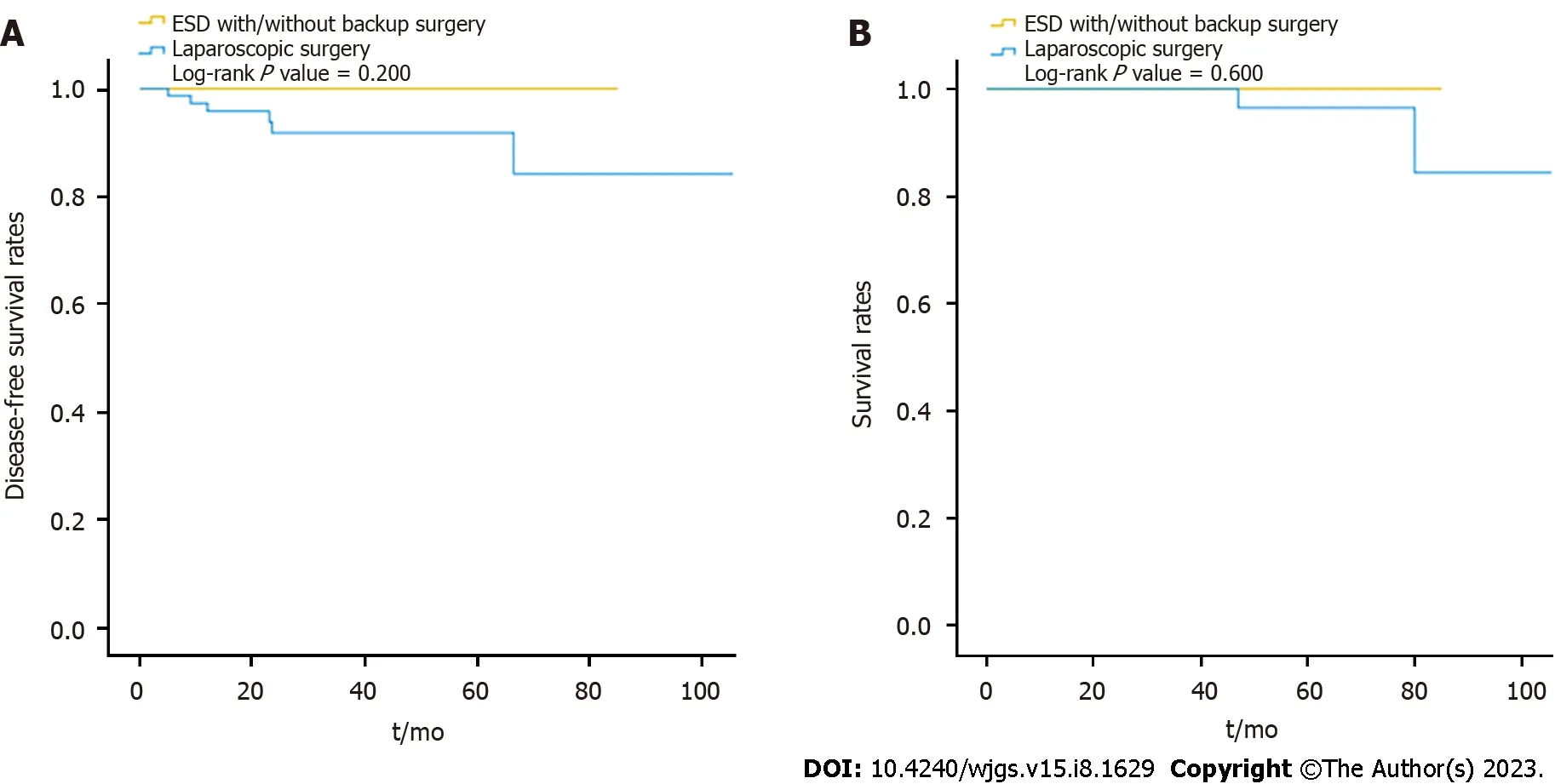
Figure 3 Comparison of disease-free survival rates and survival rates between the endoscopic and laparoscopic resection groups during long-term surveillance. A: Disease-free survival rates; B: Survival rates. ESD: Endoscopic submucosal dissection.
CONCLUSION
There are different approaches to minimally invasive surgery for gastric SETs with the objective of achieving better perioperative clinical outcomes. ER was indicated for smaller tumor sizes and intraluminal growth, whereas LR was indicated for larger tumor sizes, with an optimal tumor size cut-off point of 2.15 cm, tumors located in the middle or lower third of the stomach, exophytic tumor growth, and more aggressive malignant behavior. Multidisciplinary teamwork is an effective strategy for selecting suitable treatments, leading to better clinical outcomes.
ARTICLE HIGHLIGHTS
Research background
With recent advancements in endoscopic and laparoscopic management of gastric subepithelial tumors (SETs), different approaches to minimally invasive surgery have been adopted to improve the clinical outcomes.
Research motivation
To treat gastric SETs, the effectiveness and safety of endoscopic resection (ER), laparoscopic resection (LR), or our hybrid method were compared in terms of procedure duration, duration of hospital stay, and major complications.
Research objectives
This retrospective study compared the differences between ER and LR, and between ER with backup surgery and LR, in terms of demographic data, tumor characteristics, and perioperative outcomes. Thus, Goldilocks principle was used to determine the best type of minimally invasive surgery for gastric SETs.
Research methods
This retrospective review of records was performed on all patients of gastric SETs with high probability of surgical intervention undergoing tumor resection in the operating theater between January 2013 and December 2021. All patients were divided into two groups, either group of ER or group of LR.
Research results
Totally, 194 patients were divided into the ER group (n= 100) and LR group (n= 94). In the ER group, 27 patients required backup laparoscopic surgery after an incomplete ER. The patients in the ER group had small tumor sizes and shorter procedure durations while the patient in the LR group had large tumor sizes, exophytic growth, malignancy, and tumors that were more often located in the middle or lower third of the stomach. Both groups had similar durations of hospital stays and a similar rate of major postoperative complications. For the patients in the ER group who underwent backup surgery required longer procedures (56.4 min) and prolonged stays (2 d) compared to the patients in the LR group without the increased rate of major postoperative complications. The optimal cut-off point for the tumor size for laparoscopic surgery was 2.15 cm.
Research conclusions
ER was indicated for a smaller tumor and intraluminal growth, whereas LR was indicated for a larger tumor (optimal cutoff point: 2.15 cm), tumors located in the middle or lower third of the stomach, exophytic growth, and more aggressive malignancy behavior. Backup surgery is preserved for incomplete ER to effectively reduce associated morbidities.
Research perspectives
Multidisciplinary teamwork adopts different strategies to yield the efficient clinical outcome according to the tumor characteristics.
FOOTNOTES
Author contributions:Yen HH, Chang HC, and Lin KH designed research; Tsao LC, Yen HH, Yang CW, Chang HC, and Lin KH performed research; Tsao LC, Yen HH, and Kor CT contributed new reagents/analytic tools; Kor CT and Wu SC analyzed the data;Chang WJ and Lin KH wrote the paper.
Institutional review board statement:The study was approved by the Institutional Review Board of Changhua Christian Hospital(approval No. 220117).
Informed consent statement:This retrospective study had a waiver of informed consent due to retrospective nature.
Conflict-of-interest statement:The authors declare that they have no conflict of interest.
Data sharing statement:No additional data are available.
STROBE statement:The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Taiwan
ORCID number:Wei-Jung Chang 0000-0002-0647-2864; Lien-Cheng Tsao 0000-0002-2153-4517; Hung-Chi Chang 0000-0002-0038-3895; Chew-Teng Kor 0000-0002-5665-9635; Szu-Chia Wu 0000-0003-3815-2176; Kuo-Hua Lin 0000-0003-3360-9747.
S-Editor:Yan JP
L-Editor:A
P-Editor:Wu RR
 World Journal of Gastrointestinal Surgery2023年8期
World Journal of Gastrointestinal Surgery2023年8期
- World Journal of Gastrointestinal Surgery的其它文章
- Initial suction drainage decreases severe postoperative complications after pancreatic trauma: A cohort study
- Vascular complications of chronic pancreatitis and its management
- Historical changes in surgical strategy and complication management for hepatic cystic echinococcosis
- Post-transplant biliary complications using liver grafts from deceased donors older than 70 years:Retrospective case-control study
- Prognosis after splenectomy plus pericardial devascularization vs transjugular intrahepatic portosystemic shunt for esophagogastric variceal bleeding
- Prognostic scores in primary biliary cholangitis patients with advanced disease
