Mechanisms of autophagy function and regulation in plant growth,development, and response to abiotic stress
Yongo Li,Xingmin Xu,Gung Qi,Dezhou Cui,Chen Hung,Xinxi Sui,Genying Li,*,Qingqi Fn,*
a Crop Research Institute, Shandong Academy of Agricultural Sciences, Jinan 250100, Shandong, China
b Yantai University, Yantai 264005, Shandong, China
Keywords:Autophagy Function Mechanism Development Abiotic stresses
ABSTRACT Autophagy is an evolutionarily conserved degradation pathway of lysosomes(in mammals)and vacuoles(in yeasts and plants)from lower yeasts to higher mammals.It wraps unwanted organelles and damaged proteins in a double-membrane structure to transport them to vacuoles for degradation and recycling.In plants, autophagy functions in adaptation to the environment and maintenance of growth and development.This review systematically describes the autophagy process, biological functions, and regulatory mechanisms occurring during plant growth and development and in response to abiotic stresses.It provides a basis for further theoretical research and guidance of agricultural production.
1.Introduction
Autophagy is a catabolic process used for removing harmful components from plants during normal development and under environmental stresses, such as drought, nutrient deficiency,oxidative stress, unfavorable temperature, salt, and hypoxia[1,2].Three types of autophagy have been described: macroautophagy, microautophagy, and mega-autophagy [3] (Fig.1).In the process of autophagy, damaged proteins and unnecessary organelles in cells are wrapped in a double-layer membrane structure to form autophagosomes, which fuse with vacuoles to degrade their contents [4,5].Macroautophagy includes nonselective and selective macroautophagy.The occurrence of selective macroautophagy requires the specific recognition of some intracellular damaged or redundant organelles, which are surrounded by autophagy membranes and transported to vacuoles for degradation.The completion of this recognition process usually requires the participation of specific cargo receptor proteins, which interact with one key member of autophagyassociated proteins (ATGs), ATG8, via their ATG8–interacting motif (AIM) to help in substrate recognition.An ATG8-containing autophagy membrane can be used as a docking platform allowing autophagy receptors to selectively identify and bind cargoes to be degraded, thus mediating the selective macroautophagy process.However, nonselective macroautophagy does not require autophagy receptors to selectively degrade cargoes [6].In microautophagy, the vacuole membrane is depressed and wraps the phagocyte, and then the phagocyte is degraded in the vacuole cavity [7].Mega-autophagy refers to an extreme autophagy process realized by the infiltration or rupture of the vacuole membrane, which in plants usually occurs in programmed cell death (PCD).During mega–autophagy, the vacuole membrane becomes more permeable and then breaks, releasing vacuole hydrolases into the cytoplasm.These degrade cytoplasmic substances and even the cell wall,thus inducing PCD [3].Among these processes, macroautophagy is the most investigated [4].
The autophagy process goes through several stages: induction,cargo recognition, phagophore formation, phagophore expansion and closure,and autophagosome fusion and breakdown[8].During the past few decades,over 40 conserved ATGs in the central autophagy machinery have been identified in yeast, animals, and plants[3].These ATGs are conventionally divided into four protein complexes:the ATG1/ATG13 kinase complex for the initiation of autophagy,the ATG9 complex for nucleation and phagophore expansion,the autophagy-specific class III phosphatidylinositol 3-kinase(PI3K) complex for phagophore decoration, and the ATG8/12 conjugation system for autophagosome maturation [9].
2.The role of autophagy in plant growth and development
2.1.Root development
Regulation of primary and secondary plant root development is necessary for root establishment [10].Recent studies [11,12,13]show that autophagy is involved in the regulation of plant root development.Under conditions of phosphate starvation, the S-domain receptor kinase Arabidopsis receptor kinase2 (ARK2)phosphorylates and activates E3 ligase containing a U Box/armadillo repeat sequence (PUB9), leading to ubiquitination of the auxin/indoleacetic acid (AUX/IAA) protein or other auxin inhibitors.These ubiquitinated auxin inhibitory factors are then selectively transported to autophagosomes to be degraded, releasing auxin response factor(ARF)or other signals to promote auxin accumulation and lateral root development.However, application of the autophagy inhibitor 3-MA inhibited the growth of lateral roots and blocked auxin accumulation in roots [11,12].These studies show that autophagy promotes root growth and development by regulating the accumulation of auxin.In untreated Arabidopsis,overexpression of ATG8f reduced the number of lateral roots, but the length of the primary roots did not change.In the absence of exogenous zeatin application,ATG8f-overexpressing plants showed primary root lengths similar to those of control plants and more adventitious roots.Thus, ATG8 affects zeatin-mediated rootstructure regulation [13].
Recently, autophagy was found [14,15] to be involved in glucose-mediated regulation of the root meristem growth.Glucose is a nutrient signal regulating the activity of the plant root meristem [16].At high sugar concentrations, the accumulation of reactive oxygen species (ROS) oxidizes IAA and impairs meristem activity and growth.But in atg mutants, transmission of the high-glucose signal to peroxisomes is interrupted, reducing the accumulation of IAA oxidized by ROS and accelerating root growth[15].Thus, autophagy further regulates root meristem activity by regulating the ROS and IAA produced by peroxisomes.
Organ-specific autophagy regulation increases the root:shoot ratio under sulfur limitation.Sulfate deficiency leads to the down-regulation of target of rapamycin (TOR) expression in shoots, in turn activating autophagy and increasing carbon distribution to roots [17,18].When roots are stimulated by sugar, the TOR signaling pathway is activated, inhibiting autophagy and maintaining the activity of the meristem at the top of the root to support root growth [19], thus increasing the utilization of new sulfate resources in soil [18].
Autophagy is involved in the regulation of the hydrophilic rate of Arabidopsis roots.Autophagosomes aggregated in hydrophilic root bending after Arabidopsis seedlings were transferred to a water potential gradient stress system, normal medium–water stress medium (NM-WSM) [20].By contrast, the roots of atg2,atg5, atg8b, atg8i, or atg9 mutants did not show hydrophilic bending in the NM-WSM system.During the hydrophilic reaction,H2O2accumulated in root bending at a rate similar to that of autophagy[20], indicating that H2O2is induced and participates in the regulation of autophagy in the water potential gradient stress system.In Populus tomentosa, ATG genes and the ATG8 protein were expressed at different stages of primary and secondary root development [21].Ultrastructural observation showed that autophagosomes accumulated during root xylem differentiation, indicating that autophagy is associated with root morphogenesis.
2.2.Grain development
Grain development determines yield and quality.Autophagy functions in grain development.During seed development in Arabidopsis, many ATG genes are upregulated [22].ATG8f is highly expressed in phloem companion cells and embryos of the Arabidopsis pericarp.Autophagy was observed in embryos labeled with green fluorescent protein (GFP)-ATG8 [22].In maize, ATG genes were highly expressed in the endosperm, and the lipidated form of ATG8(a sign of autophagy activity)continued to be highly expressed 18–30 days after anthesis [23].
Autophagy regulates endosperm starch synthesis and grain size.In rice, ATG7 knockout inhibited the degradation of endosperm starch,leading to chalky grain with lower starch content and smaller size [24].Overexpression of ATG8b caused starch grains in the endosperm to increase in number and become denser and increased the number of kernels per spike [25].In wheat, autophagy caused PCD of pericarp cells, with interference with ATG8 leading to premature and smaller grain [26].
Autophagy also promotes the formation of 12S globulin and the transport of trace elements from vegetative organs to seeds.In the Arabidopsis atg5 mutant, the amount of 12S globulin in seeds decreased, but the amount of 12S globulin precursors increased,indicating that autophagy is involved in transporting precursors to protein storage vacuoles, where they are processed into their mature forms [22].Autophagy deficiency hindered the transport of metal elements, such as Fe, Zn, and Mn, from vegetative organs to grain [27].
Thus, autophagy affects seed yield and quality by regulating starch, protein synthesis, seed size, and trace-element transport.
2.3.Male reproductive system development
The degradation of tapetum cells requires autophagy during pollen development.In rice, atg7 or atg9 mutants show a malesterility phenotype [28].Many autophagy structures can be observed in tapetum cells of the anther mononuclear stage under transmission electron microscopy, but not in the atg7 mutant.Tapetum cells provide nutrients for pollen development via PCD[29].The tapetum was degraded completely in wild-type plants but only partially in the atg7 mutant, and the liposomes, plastids,and mitochondria remained in the cytoplasm [28].
Pollen release is another key process in male sterility [30].The last step of pollen release is dehiscence,during which the stomata that separate from the anther leaflets are broken [31,32], and the epidermal cells around the fissure undergo PCD [33].In tomato,many autophagosomes appear around the cleft in the process of PCD, indicating that autophagy is involved in this process [34].
Autophagy is involved in pollen germination.In the initial stage of tobacco pollen germination, many autophagosomes gathered around the germination hole, and the pollen germination rate decreased in tobacco lines with interference with ATG2, ATG5, or ATG7 [35].There were residual mitochondrial convex layers in the cells around the germination hole of the tobacco ATG interference strains.The autophagy marker ATG8 and mitochondrial markers were partially co-located, and the mitochondria-specific phospholipid cardiolipin became aggregated.Thus, mitochondria can be used as autophagy degradation substrates in the process of tobacco pollen germination.
Autophagy negatively regulates the senescence of leaves and flowers and ATG genes participate in the senescence process of plant leaves.In Arabidopsis, ATG7, ATG8, and ATG18 are highly expressed at the beginning of leaf senescence [1,36,37].ATG11 was necessary for mitochondrial degradation when Arabidopsis leaves were senescing [38].In maize, the lipidated form of ATG8 was highly accumulated in the yellowing area of aging leaves[39].Arabidopsis atg4, atg5, atg7, atg10, or atg12 mutants showed marked yellowing of leaves [40,41].In wheat, interference with ATG6 or application of the autophagy inhibitor 3-methyladenine(3-MA)accelerated leaf senescence[42].In Petunia hybrida,silencing ATG6 and PI3K accelerated petal senescence and reduced flower number[43].Autophagy inhibited the senescence and PCD of Arabidopsis leaves by negatively regulating the salicylic acid signaling pathway [44].
Thus, autophagy supports the growth and development of the whole plant mainly by regulating the development of roots and seeds,the fertility of male reproductive organs,and the senescence of leaves and flowers (Fig.2).
3.The role of autophagy in abiotic stress response
As sessile organisms, plants must cope with abiotic stresses such as starvation, high temperature, drought, and salt [45–47].To better adapt to these environmental stresses,plants have developed a set of reaction mechanisms [47].Plants can resist external environmental stresses by using autophagy to degrade and recycle damaged intracellular proteins and organelles[48].Abiotic stresses can induce autophagy, and autophagy-deficient plants are extremely sensitive to external environmental stresses [48].Here we review the roles of autophagy in responses to abiotic stresses(Table 1).
3.1.Starvation
During a period of nutrient deficiency, storage substances and organelles in plant cells are massively degraded [49–51].Under carbon starvation, Arabidopsis atg5 and atg7 mutants presented delayed growth,decreased amino acid levels,increased respiration,and decreased net protein synthesis [52].Under nitrogen starvation, ammonium, amino acids, and proteins accumulated in the leaves of Arabidopsis atg mutants [53,54] and ATG8-mediated autophagy transferred the 26S proteasome to vacuoles for degradation [51].Under dark conditions, chloroplasts in Arabidopsis leaves underwent autophagic degradation[50].Tobacco ATG genes were expressed and autophagosomes were increased at night[55].Starch synthesized in chloroplasts during the day can be degraded by autophagy at night and used for respiration.However, when autophagy is inhibited by chemical inhibitors or genetic manipulation,small starch granular structures accumulate in the cytoplasm[55].Thus, autophagy promotes the degradation of storage substances and organelles under starvation.When plants change from heterotrophic to autotrophic growth, the composition of enzymes in peroxisomes changes in the process of photomorphogenesis after germination[56].In Arabidopsis,excessive peroxisomes accumulated in the hypocotyl cells of autophagy-deficient mutants,indicating that autophagy helps to maintain peroxisome homeostasis and cell remodeling [57].
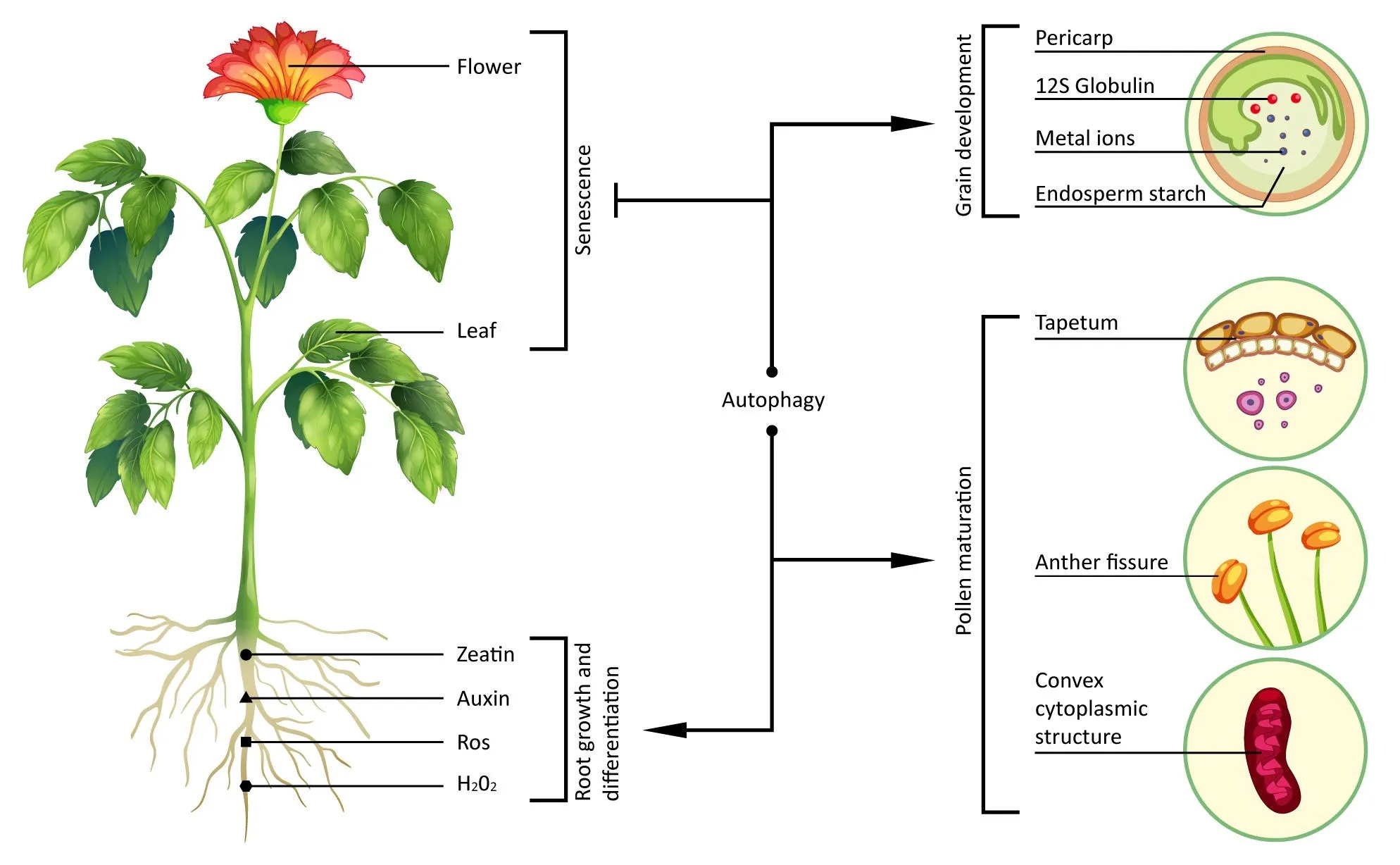
Fig.2.Schematic diagram of autophagy roles in plant growth and development.Autophagy negatively regulates PCD to delay leaf and flower senescence, promotes the production of auxin and zeatin, and reduces levels of ROS and H2O2 to promote the growth and differentiation of roots.Autophagy also promotes grain development by regulating endosperm starch synthesis, 12S globulin processing, pericarp degradation, and transfer of metal ions.Autophagy-mediated degradation of the tapetum, the convex cytoplasmic structure, and fissure epidermal cells promotes pollen maturation.
Autophagy is also involved in the recycling of the trace nutrients zinc and sulfur in plant cells[58–60].In Arabidopsis,zinc deficiency caused autophagosome accumulation[61].Arabidopsis atg5 and atg10 mutants underwent premature senescence compared with wild-type plants under zinc deficiency,indicating that autophagy was necessary for zinc cycling [58].Recent studies have shown that autophagy functions in sulfur metabolism.In the Arabidopsis atg5 mutant, sulfur transport from the rosette to the seed is blocked [59].The selective autophagy receptor, neighbor of the BRCA1 gene 1 (NBR1), functions in plants’ response to sulfur deficiency,and overexpression of NBR1 increased the tolerance of Arabidopsis to sulfur deficiency [60].
3.2.Heat stress
Heat stress impairs plant growth by causing abnormal protein folding and denaturation [62].These proteins are degraded by autophagy [63].In a recent study [64], the NBR1-mediated selective autophagy pathway was involved in the denaturation or degradation of damaged proteins under heat stress.Under heat stress, an Arabidopsis nbr1 mutant showed decreased heat resistance and accumulation of insoluble and highly ubiquitinated proteins.Further investigation showed that the autophagy receptor NBR1 interacted with ATG8 to remove ubiquitinated proteins,suggesting that the autophagy receptor NBR1 targets the degradation of ubiquitinated proteins.
Heat stress induced the expression of ATG genes and the accumulation of autophagosomes [65].ATG8 co-precipitated with several types of heat shock proteins (HSPs), and autophagy defects caused the accumulation of HSPs, indicating that autophagy promotes the degradation of HSPs [66].High-temperature stress promoted autophagy of anther cells wall and microspores[67].Pollen development and anther dehiscence were severely impaired after ATG2, ATG5, ATG7, or ATG10 knockout, indicating that autophagy functions in tapetum degeneration and pollen development during high-temperature stress.
ATG8 was translocated to the swollen Golgi cisternal membrane under short-term acute heat stress[68].ATG8 proteins recruit clathrin to stimulate the budding of ATG8-positive vesicles from dilated cisternae in a noncanonical autophagy pathway,supporting Golgi body reconstruction after recovery from heat stress [68,69].These results broaden the fundamental scope of autophagy in plant response to heat stress.
3.3.Cold stress
In contrast to heat stress,cold stress has been the subject of few studies of the regulation of plant autophagy.Under low temperature, the expression of ATG6a/c was down-regulated in rice [70],whereas the expression of ATG6 was up-regulated in barley [71],showing that ATG6 functions in plant response to low temperature stress.NBR1-mediated selective autophagy seems also to beinvolved in the response of plants to cold stress.In Solanum lycopersicum,brassinolide and positive regulator BRASSINAZOLE–RESISTANT 1 (BZR1) promoted autophagy and the accumulation of the autophagy receptor NBR1 to degrade ubiquitinated proteins under low-temperature stress [72].
3.4.Drought stress
The global economic loss caused by drought in the past decade is estimated at $30 billion [73].Drought stress promoted the upregulation of ATG18a expression and increased activation of autophagy in Arabidopsis [74].In the crop species tomato and wheat,drought stress increased the expression of ATG genes, the formation of autophagosomes and the lipidated form of ATG8, and then activated autophagy [42,65,75].Under drought or osmotic stress,other plants such as apple [76,77], banana [78], barley [71], millet[79],peach[80], pepper[81],rice[82],and sweet orange[83]also showed up-regulated ATG gene expression.
Autophagy is required for plant drought tolerance.In Arabidopsis, knockout of ATG5 or ATG7 and knockdown of ATG18a inhibited autophagy, leading to a decrease in drought tolerance [64,74].Interfering with tomato ATG8d or ATG18h and wheat ATG6 also reduced drought tolerance [42,75].Autophagy can increase drought resistance in plants via a variety of signal molecules.Brassinosteroids (BRs) function in response to stresses.BRI1-EMS SUPPRESSOR 1 (BES1)is the core component of BR signaling pathways [84].BES1 is recruited into autophagosomes to be degraded under drought stress; and the degradation of BES1was abolished in an atg7 mutant [84], suggesting that autophagic degradation of BES1 represses BR signaling pathways under drought stress.Autophagy also increased plant drought resistance by degrading plasma membrane intrinsic protein 2;7 (PIP2;7) [85,86].PIP2;7 is a member of the aquaporin family that mediates water transport.A decrease of PIP2;7 reduced water loss, and was induced by drought stress [87,88].Applying an autophagy inhibitor or knocking out ATG5 inhibited the degradation of PIP2;7 in Arabidopsis[85].Drought stress increased heme production, and excess heme is toxic to cells [89,90].The selective autophagy receptor tryptophan-rich sensory protein (TSPO) combined with heme to form TSPO-heme, which was transported to vacuoles for degradation under drought stress[91].Thus, autophagy can remove heme to increase plant drought resistance.Drought stress promotes autophagy by regulating the abscisic acid(ABA)signaling pathway.Drought stress induced ABA accumulation,leading to stomatal closure and expression of stress-responsive genes [92].Pyrabactin resistance/pyrabactin resistance-like/regulatory components of ABA receptor (PYR/PYL/RCAR) function in ABA signaling [93].PYR1, PYL4, and PYL8 can be ubiquitinated and degraded relying on vacuolar protein sorting 23A(VPS23A)and fyve domain protein required for endosomal sorting1 (FREE1), thus suppressing ABA signaling [94].However, autophagy is responsible for VPS23A and FREE1 degradation, thereby removing the degradation of PYR1, PYL4, and PYL8 [95].
3.5.Salt stress
Soil salinity is the main abiotic factor limiting crop yield[96,97].Globally,about 900 million hectares of land is saline–alkaline land and more than 30% of irrigated crops are affected by saline–alkaline stress [96,98].
Salt stress induces plant autophagy,which promotes the degradation and recycling of proteins, providing the macromolecules and energy needed for plant survival.Interference with ATG2 or ATG7 inhibited wheat autophagy and reduced tolerance to salt stress [99].Overexpression of sweet orange ATG18a in Arabidopsis increased its salt tolerance relative to the wild type [83].Overexpression of ATG18a increased salt tolerance in Populus tremula;and reduced oxidative damage to the cell membrane [100].The number of autophagosomes in the rice atg10b mutant is smaller than that in the wild-type, and the mutant is sensitive to salt stress.Overexpression of ATG10 in apple increased autophagy activity in roots and salt tolerance in transgenic plants [101].Reducing the expression of ATG8f reduced Na+retention in Brassica napus roots under salt stress, reducing the plant’s salt tolerance[102].
Autophagy has different functions under differing degrees of salt stress.At low NaCl concentrations, the germination of Arabidopsis atg9 and atg2 mutants is faster than that of the wildtype,whereas the germination rates of atg5 and atg7 mutants show the opposite trend.At high NaCl concentrations, the expression levels of wheat ATG4a, 4b, 8a, 8 g and 8 h are up-regulated [103],and the germination rate of all atg mutant strains decreases[104].Overexpression of a GFP-ATG8F-HA fusion protein led to electrolyte leakage,cell membrane system damage,and other phenomena, inhibiting plant growth under moderate NaCl concentrations [13].
4.Regulation mechanisms of autophagy
4.1.Transcriptional regulation
To date, about 40 ATG genes have been identified in yeast and their homologs have been found in plants [105].Abiotic stresses regulate the expression of ATG genes in plants at the transcriptionlevel, as in Arabidopsis, wheat, rice, and tomato [82,103,106,107].Transcriptional regulation of ATG genes permits plants to respond to environmental stresses.Some transcription regulatory factors of ATG genes are described in Table 2.

Table 2 Transcriptional regulators of autophagy in plants.
HSPs are produced during the stress response and function by stabilizing or refolding proteins.Heat shock transcription factor(Hsf) regulates the expression of stress response genes (including genes encoding HSPs).According to their protein structures, plant Hsfs are divided into three conserved evolutionary categories:HsfA, B, and C [108].HsfA1a is a positive transcription factor for autophagy[65].Under drought stress,tomato HsfA1a is trimerized and activated.The activated form of HsfA1a binds to the heat shock elements in the ATG10 and ATG18f promoters to activate autophagy.After overexpression of HsfA1a, the number of autophagosomes and the transcript levels of ATG10 and ATG18f increases.Thus, HsfA1a is a positive transcription factor for autophagy [65].
WRKYs (named for its N-terminal conserved WRKYGQK sequence)are a large family of transcription factors(TFs)that regulate many plant physiological processes and support plant growth and adaption to environmental stresses[109].Under abiotic stresses,WRKYs induce the expression of ATG genes[110,111].WRKY33 is also involved in the regulation of heat–induced autophagy.In tomato and Arabidopsis, heat stress promoted the expression of ATG genes and autophagosome accumulation, and inhibition of autophagy lead to decreased heat tolerance [110].Silencing of tomato WRKY33a or WRKY33b reduced the expression levels of ATG5 and ATG7 and the formation of autophagosomes, thereby reducing tomato heat resistance.Thus, WRKY33 promotes autophagy by regulating ATG genes at the transcription level.
BRs function in stress response and in plant growth and development[112,113].BZR1 is a transcription factor in the BR signaling pathway that functions in the regulation of autophagy [72,114].BZR1 increases the tolerance of tomato to low temperature and nitrogen deficiency by activating autophagy.Bzr1 mutants show decreased tolerance to cold and nitrogen deficiency, while BZR1-overexpressing plants show the opposite.In a yeast one-hybrid experiment and chromatin immunoprecipitation (ChIP), BZR1 bound to the promoters of ATG2,ATG6,and NBR1A/B[72,114].Thus,BZR1-mediated BR signaling positively regulates autophagy.
Elongated hypocotyl 5 (HY5) belongs to the basic domain/leucine zipper motif (bZIP) transcription factor family, and is associated with the development and regulation of the light and nutrition signal pathway [115].When plants are transferred from a light to a dark environment, the expression of ATG genes is upregulated and autophagy is initiated[116].HY5 gene deletion leads to the increase of ATG genes’ expression and to autophagy activation and increases resistance to darkness and nitrogen starvation.In Arabidopsis, HY5 bound to the promoters of ATG5 and ATG8e to inhibit autophagy[116].Thus,HY5 negatively regulates autophagy at the transcription level.
In plants,bZIP TFs function in resisting various biotic or abiotic stresses, participating in light signaling pathways and flowers and seed development [117].TGA motif-binding protein 9 (TGA9) is a member of the bZIP family that up-regulates the expression levels of ATG8b,ATG8e,and other ATG genes by binding to the TGA motif in their promoters,thus promoting autophagy in Arabidopsis[118].Under sucrose starvation and osmotic stress, TGA9 overexpression activated autophagy, indicating that TGA9 is a positive regulatory factor of autophagy and increases seedling tolerance to starvation[118].TGA9-mediated autophagy was inhibited in atg5 mutant protoplasts or in cells treated with the autophagy inhibitor 3-MA,indicating that it employs the typical autophagy mechanism[118].
The ethylene pathway also participates in the regulation of autophagy [119,120].In soybean, sugar and nitrogen starvation induce up-regulated expression of ATG genes and ethyleneresponsive genes.1-Aminocyclopropane-1-carboxylic acid (ACC),the precursor of ethylene, promoted the expression of ATG8i[119,120].Application of an ethylene inhibitor reduced ATG8s expression in Petunia petals[120,121].Drought stress or ACC treatment increased ethylene response factor 5(ERF5)expression.Overexpression of ERF5 in tomato increased drought resistance [121].Under drought stress, ERF5 bound to the promoters of ATG8d and ATG18h and activated gene expression to promote autophagy[75].Thus,ERF5 promotes ATG gene expression at the transcription level, thus activating autophagy and increasing plant drought resistance.
Histone deacetylase 9 (HDA9) is a member of the HDA family that inhibits gene transcription by deacetylation, and functions in autophagy-mediated leaf senescence [122–124].In Arabidopsis,HDA9 and POWERDRESS (PWR) interact and are transported from the cytoplasm to the nucleus, where they combine with the binding elements W–boxes of the WRKY53, PWR, and ATG9 promoters to inhibit the expression of ATG9 [124].This finding suggests that HDA9 negatively regulates autophagy by inhibiting ATG9 expression.
Endoplasmic reticulum (ER) stress induces autophagy at the transcription level [125].Inositol-required enzyme 1b (IRE1b) is a protein that regulates the cell’s response to ER stress[126].IRE1b functions as an RNA splicing factor of bZIP60 mRNA, leading to bZIP60 splicing and activation, followed by transfer of the active bZIP60 to the nucleus[127].In the nucleus,it functions in the transcription of ER stress-responsive genes [128].When autophagy is induced by heat or ER stress, the number of autophagosomes in the ire1b mutant decreased relative to those in wild-type plants[125,129].Thus, IRE1b mediates autophagy induced by heat or ER stress.Given that IRE1b regulates bZIP60, it may be speculated that bZIP60 participates in ER stress-induced autophagy by regulating the transcription of ATG genes.
4.2.Post-translational regulation
4.2.1.Protein–protein interactions
Protein interaction is a form of post-translational regulation that operates in plant growth, development, and adaption to vari-ous environmental stresses by promoting or inhibiting autophagy.Several proteins interacting with ATGs are described in Table 3.NBR1 is an autophagic receptor that interacts with ATG8 via the AIM domain in the process of degrading certain substrates in the cytoplasm [64,72].Arabidopsis and tomato nbr1mutants are sensitive to various stress conditions (drought, salt, and heat stress)[64].Silencing NBR1 triggered the accumulation of ubiquitinated insoluble proteins and reduced autophagy under cold stress,revealing the role of NBR1 in resisting cold stress [72].NBR1 is involved in the autophagy regulation process caused by sulfur deficiency,and overexpression of NBR1 modified the plant response to sulfur deficit[60].These findings suggest that NBR1 increases plant stress resistance by interacting with ATG8 to promote autophagy.
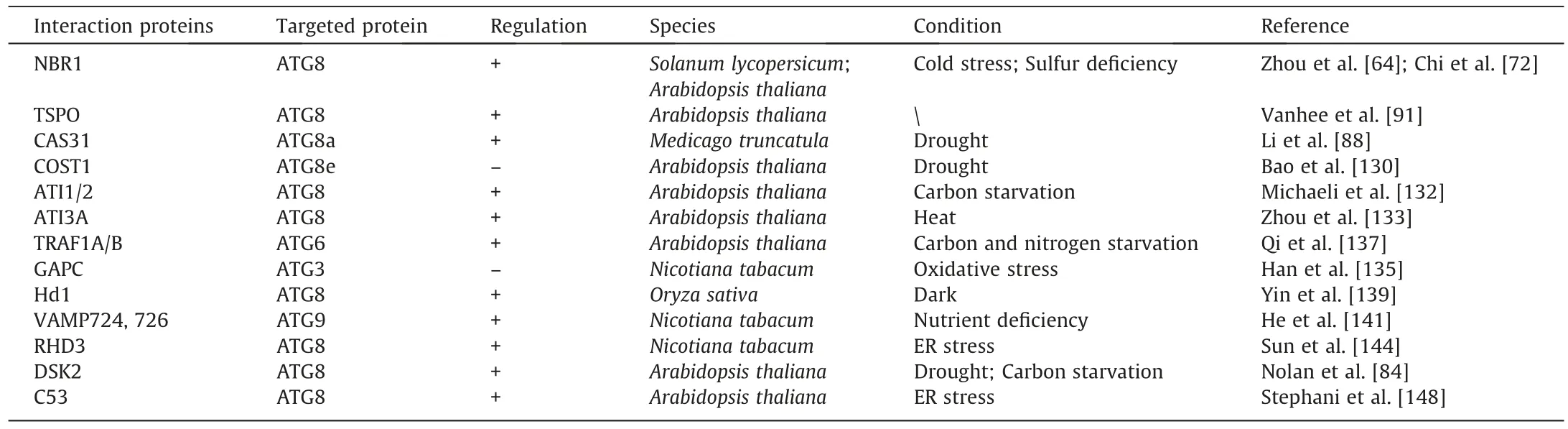
Table 3 Summary of protein–protein interactions in autophagy regulation.
TSPO, as a selective autophagy receptor, is degraded via its interaction with ATG8 [91].In an Arabidopsis wild-type strain,TSPO induced a decrease in the level of PIP2;7, but there was no change in TSPO-deficient plants [85].In the atg5 mutant strain,the abundance of PIP2;7 was also not affected.Thus, TSPOinduced degradation of PIP 2;7 depends on selective autophagy.
Cold acclimation-specific 31(CAS31)is another PIP2;7 degradation receptor.In Medicago sativa,CAS31 interacted specifically with ATG8a to promote autophagy[88].Drought stress increased CAS31 expression[88].Overexpression of CAS31 increased plant tolerance to drought stress,and the tolerance of cas31 mutants to drought is reduced.Dehydration treatment caused PIP2;7 degradation and mutation of cas31 or application of an autophagy inhibitor repressed such degradation, indicating that autophagy promotes the degradation of PIP2;7 and that CAS31 can be used as a selective autophagy receptor with PIP2;7, transferring it into vacuoles for degradation.Thus CAS31 interacts specifically with ATG8a to promote the autophagic degradation of PIP2;7.
Constitutive stress protein 1 (COST1) is a plant-specific protein first identified in Arabidopsis that functions in regulating stress response.In the cost1 mutant, genes responding to drought, salt,and cold stress were upregulated, presenting a drought-resistant phenotype [130].Further investigation revealed that interaction between COST1 and ATG8e negatively regulated autophagy.Overexpression of COST1 reduced the abundance of ATG8e,whereas the transcript level was not affected, suggesting that the decrease in ATG8e levels mediated by COST1 functions via post-translational regulation [130].Thus, interaction between COST1 and ATG8e reduces ATG8e levels and autophagy, thus reducing plant drought resistance.
ATG8 interacting protein 1/2 (ATI1/2) is a plant-specific autophagy receptor [131].During carbon starvation, ATI1/2 interacts with plastid-associated proteins and ATG8 to mediate the transport and degradation of plastid components to vacuoles [132].
ATG8 interacting protein 3 (ATI3) is present only in dicotyledonous plants.In Arabidopsis, there are three types of ATI3: ATI 3A/B/C.Of these,ATI3A promotes selective autophagy via the interaction between the AIM motif WxxL and ATG8 [133].Overexpression of ATI3A increased heat resistance in Arabidopsis,and the heat resistance of the ati3a mutant was decreased.These findings suggest that ATI3A interacts with ATG8 to promote autophagy, thus increasing heat tolerance in Arabidopsis.
In recent years,the enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPC) has been recognized [134] for its function in producing energy and providing intermediates for cell metabolism.GAPC participated in the regulation of autophagy by interacting with ATG3 in tobacco and potato [135].Methyl viologen (MV) is a well-known plant oxidative stress inducer.MV treatment inhibited the interaction between GAPC and ATG3 in tobacco [136].Silencing of GAPC activated autophagy, whereas overexpression of GAPC inhibited the formation of autophagosomes.Three GAPC genes (GAPC1, GAPC2, and GAPC3), have been identified in potato.Silencing of the GAPC genes led to PCD in the apical meristem of the tuber, presenting as a loss of apical dominance.GAPC1, 2,and 3 interacted with ATG3 to inhibit autophagic cell death and maintain apical dominance.Thus, GAPC is a negative regulator of autophagy at the post-translational level.
Tumor necrosis factor receptor-related factor(TRAF)family proteins regulate autophagy by interacting with ATG6 [137].In Arabidopsis, the double mutant traf1a/b presented an early senescence phenotype under carbon and nitrogen starvation,while the complementary line TRAF1A/B completely rescued the sensitivity of the double mutant to starvation stress.TRAF1A and TRAF1B were induced and located in autophagosomes under carbon starvation.During nitrogen and sucrose starvation, fewer autophagosomes accumulatde in the double mutant traf1a/b line than in the wild–type line.Thus, TRAF1A/B promoted autophagy by interacting with ATG6,thereby increasing plant tolerance to carbon and nitrogen starvation.
Heading date 1(HD1)is the key factor in the photoperiodic control of plant flowering time [138].Under dark conditions,autophagy-mediated HD1 degradation regulates rice flowering.A loss of autophagy leads to the accumulation of HD1 and delays flowering.In the dark, nuclear-localized HD1 is regarded as a substrate of autophagy, and vacuole degradation is carried out by the ATG8 protein.The interaction between HD1 and ATG8 is necessary for the autophagic degradation of HD1 in the dark.The above research shows that autophagy regulates HD1 protein homeostasis to control rice flowering [139].
Soluble N-ethylmaleimide sensitive factor attachment protein receptor (SNARE) functions in regulating autophagy [140].Two SNAREs, vesicle–associated membrane protein 724 (VAMP724)and VAMP 726 have been identified in Arabidopsis [141].Phenotypic analysis shows that mutants of Arabidopsis vamp724 or vamp726 are sensitive to nutrient deficiency conditions.Living cell imaging of vamp724 or vamp726 mutants expressing yellow fluorescent protein (YFP)-ATG8e shows abnormal autophagy structures and impaired autophagy flux outside vacuoles.Further transient co-expression, immunoprecipitation, and double immunogold transmission electron microscopy analysis showed that ATG9 interacts and co-locates with VAMP724 or VAMP726 during autophagosome formation [141].In short, VAMP724 or VAMP726 interact with ATG9 in Arabidopsis to respond to nutritional deficiency by regulating autophagy.
Root hair defect 3(RHD3)is a member of the dynamin-like GTP enzyme family, which comprise evolutionarily conserved proteins that can mediate ER homotype fusion[142].RHD3 plays an important role in ER stress signaling [143].The Arabidopsis rhd3 mutant is not sensitive to ER and salt stress.RHD3 promotes selective autophagy through the interaction between the AIM motif and ATG8, thus improving plant tolerance to ER stress [144].
Cyclin-dependent kinase 5 regulatory subunit associated protein 3 (CDK5RAP3, also known as C53) is an ER-specific receptor protein that functions biologically as a tumor suppressor[145,146].C53 is a highly conserved protein,being found in vertebrates,invertebrates,and plants[147].In plants,C53 interacts with ATG8 through a novel ATG8 binding motif SAIM (Shuffled AIM,IDWG/D), and participates in the response to ER stress [148,149].Subcellular localization showed that C53 co-located with ATG8 in cells and both proteins moved to punctate autophagosomes under ER stress[145].Thus,the interaction between C53 and ATG8 initiates autophagy to cope with ER stress.
BES1 is the core TF in the BR signaling pathway,which regulates mainly the growth and development of plants by influencing the expression of BR-responsive genes[150].DOMINANT SUPPRESSOR OF KAR 2 (DSK2) is a ubiquitin-binding receptor that operates in the protein degradation pathway [151].The interaction between DSK2 and ATG8 promotes the autophagic degradation of BES1 under drought or starvation conditions [84].
4.2.2.Phosphorylation
Protein phosphorylation is a post-translational modification in eukaryotes.It activates or deactivates ATGs via conformational changes in their structure, thus regulating autophagy activity[152,153] (Fig.3).

Fig.3.Schematic diagram of phosphorylation regulation in plant autophagy.Under starvation conditions,Raptor is phosphorylated,which inhibits TOR activity,resulting in the dephosphorylation of ATG13, thus promoting the binding of ATG1 and ATG13 and activating autophagy.On the one hand, starvation can also cause phosphorylation of Raptor via KIN10 of SnRK1, thus inhibiting TOR activity, leading to dephosphorylation of ATG13, promoting the combination of ATG1 and ATG13, and then activating autophagy; on the other hand, starvation can induce phosphorylation of ATG1 via KIN10 of SnRK1, and then promotes the binding of ATG1 and ATG13, thus activating autophagy.Under starvation conditions, TOPP causes dephosphorylation of ATG13, promotes the formation of the ATG1-ATG13 complex, and then promotes autophagy.Under nutrition sufficiency,Raptor cannot be phosphorylated,and then TOR kinase is activated,resulting in phosphorylation of ATG13 inhibiting autophagy.BIN2 negatively regulates the BR signaling pathway and directly phosphorylates Raptor, leading to the inhibition of TOR and activation of autophagy.BIN2 phosphorylates DSK2,strengthening the binding between DSK2 and ATG8, thus promoting autophagy.
TOR is an atypical serine/threonine protein kinase that is highy conserved in evolution, supporting the normal growth and development of plants and their adaptation to various external influences[154,155].Plant TOR is a negative regulator of autophagy.In Arabidopsis, the autophagy genes vacuolar protein sorting 15(VPS15), VPS34, ATG7, ATG8, ATG9, and ATG13 were up-regulated after treatment with a TOR inhibitor(AZD8805)[156].After interference with the TOR gene,many ATG genes were up-regulated and the number of autophagosomes increased[157].By contrast,overexpression of TOR in Arabidopsis reduced autophagy activity during salt and osmotic stress [158].
ATG13 is the target protein of TOR [158].In Arabidopsis, S (serine)248,S397,S404,S406,S407,and S558 of ATG13 are phosphorylated by TOR [159].Under starvation, inactivation of TOR will cause dephosphorylation of ATG13, promoting the formation of autophagosomes[160].Thus,TOR regulates autophagy by mediating ATG13 phosphorylation [161].
Sucrose non-fermentation related protein kinase 1 (SnRK1) is a heterotrimeric complex that functions in life regulation under energy and nutrition deficit conditions [162,163].Plant SnRK1 is homologous to yeast sucrose non-fermenting1 (SNF1) and mammalian adenosine monophosphate-activated protein kinase(AMPK),and is reported[164]to activate autophagy under various stress conditions.In Arabidopsis, the catalytic subunit of SnRK1 comprises three subtypes: SNF1 kinase homolog 10 (KIN10),KIN11, and KIN12.KIN10 is responsible for the main activities of the SnRK1 complex.Overexpression of KIN10 in Arabidopsis increases the number of autophagosomes and activates autophagy[165].However, in the kin10 mutant, the activation of autophagy by most abiotic stresses is blocked.During carbon starvation,phosphorylation of ATG1a in the KIN10-overexpressing strain increased relative to the wild type.These findings indicate [165] that SnRK1 promotes autophagy by increasing Arabidopsis ATG1 phosphorylation.SnRK1 regulates autophagy via the TOR signaling pathway[166].In Arabidopsis, knockout or overexpression of KIN10 inhibits or activates autophagy[167].However,autophagy is still activated in the kin10 mutant after TOR signaling is suppressed.When TOR signaling is activated, overexpression of KIN10 cannot activate autophagy.Thus, SnRK1 is expressed upstream of TOR in regulating autophagy.A further finding that KIN10 interacts with a regulatory protein of TOR(RAPTOR)in vivo and phosphorylates RAPTOR in vitro to inhibit TOR activity shows that SnRK1 inhibits TOR activity by phosphorylating RAPTOR,leading to activation of autophagy[48,168].
In Arabidopsis,BR activated TOR kinase to promote plant growth[169].Autophagy is activated by blocking the BR signaling pathway.Brassinosteroid insensitive 2 (BIN2) kinase, a glycogen synthase kinase 3 (GSK3)-like kinase, which negatively regulates BR signaling and phosphorylates RAPTOR1b at conserved serine residues in the typical BIN2 phosphorylation motif [170].Mutation of serine to alanine at amino acid 916 of RAPTOR1b blocks the phosphorylation of RAPTOR1b by BIN2, inhibiting autophagy and increasing the phosphorylation of the TOR substrate, ATG13a.Thus, when BRs are deficient, BIN2 phosphorylates and inhibits RAPTOR1b activity,activating autophagy.Active BR signaling inhibits BIN2, inhibiting BIN2–mediated phosphorylation of RAPTOR1b; in turn, TOR activity and ATG13a phosphorylation are increased, resulting in decreased autophagy [170].BIN2 phosphorylates DSK2, strengthening the binding between DSK2 and ATG8, thus promoting the autophagic degradation of BES1 [84].
In Arabidopsis, autophagy activity in mutants of type I protein phosphatase 7 (topp7) and topp8 was blocked, showing insensitivity to carbon starvation [171].TOPP caused dephosphorylation of ATG1a, promoting the formation of the ATG1a-ATG13a complex which promoted autophagy.
4.2.3.S-nitrosylation
S-nitrosylation is a post-translational modification of proteins.It is the process that a nitric oxide (NO) group is covalently linked to the free sulfhydryl group of specific cysteine residues of the target protein, thus forming S–nitrosothiol (SNOI).S-nitrosylation modification regulates various biological processes or signal pathways in many organisms by changing the biochemical activity,stability, subcellular localization, and protein–protein interactions of target proteins [172,173].
Plants may respond to hypoxia stress with NO-mediated Snitrosylation, thereby maintaining intracellular homeostasis[174].A regulator of plant S-nitrosoglutathione (GSNO) levels, Snitrosoglutathione reductase 1 (GSNOR1), is also regulated by NO-mediated S–nitrosylation [175].Hypoxia stress promotes the S-nitrosylation of GSNOR1, inhibiting the enzyme activity of GSNOR1 [176].The Arabidopsis gsnor1 mutant is not sensitive to hypoxia stress [177].In further studies [174,177] in Arabidopsis,GSNOR1 was S–nitrosylated at the conserved Cys-10 residue,exposing the AIM domain,which interacted with ATG8 to promote the selective autophagic degradation of GSNOR1 under hypoxia stress.Thus, the S–nitrosylation–induced selective autophagy of GSNOR1 functions in plant response to hypoxia stress.
4.2.4.Ubiquitination
Ubiquitin functions in regulating the stability and function of ATGs during autophagy [178].The RING-type E3 ligases, SEVEN IN ABSENTIA OF ARABIDOPSIS 1(SINAT1),SINAT2,and SINAT6,regulate autophagy by regulating the ubiquitination of ATG6 and ATG13 under various nutritional conditions [137,179].Under nutrient-rich conditions,SINAT1 and SINAT2 interact with TRAF1a and TRAF1b, resulting in the ubiquitination and degradation of ATG6 and ATG13, thus inhibiting autophagy.Under conditions of long-term nutritional deficiency, SINAT1 and SINAT2 recruit SINAT6, which competitively binds SINAT1 and SINAT2 with ATG6 and ATG13 via a truncated RING finger domain, thus maintaining the stability of ATG6 and ATG13 and activating autophagy[137,179].Thus SINAT family proteins regulate the ubiquitination and stability of ATG6 and ATG13 and thereby autophagy.
4.2.5.S-sulfhydration
Sulfur compounds such as hydrogen sulfide (H2S) function in plant development and stress response [180,181].In Arabidopsis,L-CYS DESULFHYDRASE 1 (DES1) is the only l-cysteine sulfhydryl enzyme identified in the cytosol.It promotes the degradation of cysteine and the production of intracellular H2S, which acts as a signaling molecule in post–translational modification of proteins by sulfurhydration to increase the antioxidant capacity [182].Mutation of DES1 inhibited the production of H2S in Arabidopsis cytosol[182].The lack of DES1 promotes the accumulation and lipidation of ATG8, in turn promoting autophagy.However, application of sulfide inhibited the accumulation and lipidation of ATG8 in the des1 mutant, inhibiting autophagy [183].Thus, DES1-mediated desulfurization in the cytosol negatively regulates autophagy.
4.2.6.Acetylation
Histone acetyltransferases (HATs) and HDAs precisely regulate histone acetylation [184,185].HATs activate transcription by acetylation, and HDAs inhibit transcription by deacetylation[185].In Arabidopsis, histone deacetylase 9 (had9), a reduced potassium dependency 3 (RPD3)-like histone deacetylase, functions in leaf senescence [124].The expression levels of ATG9,ATG2, ATG8e, and ATG13 in the hda9 mutant were up-regulated,indicating that HDA9 negatively regulates the expression of ATG genes in Arabidopsis during senescence.Under sufficient light or nitrogen, HDA9 interacted with HY5 to inhibit the expression of ATG5 and ATG8e by deacetylation of Lys9 and Lys27 of histone 3[116].This finding suggests that HDA9 inhibits autophagy by histone deacetylation.
4.2.7.Lipidation
Lipidation is a post-translational modification that covalently modifies proteins with specific lipids and functions in the regulation of autophagy [58,186].In the process of autophagy, ATG8 is lipidated and combined with phosphatidylethanolamine (PE) to form ATG8-PE, which is located on the autophagosomes[187,188].The degree of lipid formation of ATG8 can thus be used as a marker for autophagy.Stress promotes the formation of ATG8-PE [189].The ATG12-ATG5 and ATG8 ubiquitin-like conjugation systems determine the lipidation of ATG8 for autophagosome formation[190].ATG5 and ATG7 are the key components of these two conjugation systems [191].Knockout of ATG5 or ATG7 blocked the formation of ATG8–PE and inhibits autophagy, whereas overexpressing ATG5 or ATG7 promoted the formation of ATG8-PE and activated autophagy [40,191,192].Thus, ATG5 or ATG7 promotes the lipidation of ATG8 by regulating the ATG12-ATG5 and ATG8 conjugation systems, thus activating autophagy.
Under the action of cysteine protease ATG4, glycine at the carbon terminal of ATG8 is exposed and then covalently linked with PE to form ATG8-PE.ATG4 cleaves ATG8–PE, located on the autophagosome intracellular membrane, into ATG8 and PE for recycling [193].In Arabidopsis atg4 mutants, ATG8 did not bind to PE, thus inhibiting autophagy [194].
4.3.Other regulation of autophagy
4.3.1.DNA methylation
DNA methylation is an epigenetic modification mediated by DNA methyltransferases (methyltransferases, chromomethylases,and domain-rearrangement methylases) that occurs in the sequences CG,CHG and CHH(H stands for A,T,or C)in eukaryotes[58].Expression profiling[58]showed that DNA methylation regulates ATG genes in plants.Almost all tomato ATG loci are enriched in various cytosine sequence regions.
Genome-wide characterization of DNA methylation in Arabidopsis revealed [195] that almost every ATG gene had methylation modification.The methylation profile of a mutant of the DNA methyltransferase gene, domain-rearranged methyltransferase 2 (drm2), showed that the level of methylated CG in ATG6 and ATG7 was lower than that in wild–type Arabidopsis[196].A full DNA methylation analysis showed that the promoter region of ATG8f was under-methylated, and in Arabidopsis, the expression of ATG8f was induced under TOR inhibition[197].In tobacco,interference with the DNA methyltransferase, chromomethylase3(CMT3) gene, resulted in increased expression of Beclin 1 (an ATG6 homolog)[198].Thus,DNA methylation operates in the regulation of autophagy in plants.
4.3.2.Second messengers
Second messengers are signal regulating molecules in the process of plant growth and development and in responses to abiotic stresses [199].Autophagy is also regulated by second messengers including ethylene, ROS, and polyamines [200,201].In plants with ethylene-dependent petal senescence, exogenous ethylene treatment accelerated ethylene production and petal senescence,while inhibiting ethylene biosynthesis by chemical or genetic engineering methods delayed petal senescence[202,203].In Petunia flower senescence, there is marked autophagy, and pollination induces the expression of ATG8 in Petunia, associated with an increase in ethylene.Ethylene inhibitor treatment delayed the induction of ATG8 in pollinated flowers, and ethylene treatment rapidly increased the expression of ATG8 in the Petunia petals [120].Knockout of ATG6 gene accelerated petal senescence, and genes associated with ethylene synthesis were highly expressed [204].Thus, ethylene is a regulator of autophagy in petal senescence of Petunia.
Spermidine(Spd)is a ubiquitous plant polyamine that acts as a plant growth regulator,functioning in plant response to salt stress[205,206].Salt stress increased the activity of enzymes involved in Spd biosynthesis and the content of Spd[207].In cucumber,exogenous application of Spd increased salt tolerance and the expression of ATG genes and the formation of autophagosomes,whereas interference with ATG4 or ATG7 reduced Spd–mediated salt tolerance[208].Thus, Spd increases cucumber salt tolerance by activating autophagy, and is a positive regulator of autophagy.
Under abiotic stresses,ROS such as superoxide anion,hydrogen peroxide, singlet oxygen, and hydroxyl radical, play a dual role in plant biology.They can be used as second messengers to induce a variety of signal cascade reactions at low concentrations, and at high concentrations can cause severe oxidative damage to DNA,RNA, and proteins in cells [209,210].ROS can regulate autophagy by targeting upstream factors or key autophagy genes.Redox signaling regulates the kinase activity of the autophagy upstream regulator SnRK1.In vitro,the activity of KIN10(the catalytic subunit of SnRK1) in Arabidopsis depended strongly on the redox state [211].ATG4 protease activity was inhibited by ROS to ensure the lipidation of ATG8 and the process of autophagy in Arabidopsis and Chlamydomonas reinhardtii under stresses [212,213].Peroxisome aggregation and high ROS accumulation were observed in Arabidopsis atg mutants, leading to disordered ROS homeostasis and stomatal defects in guard cells [214].Interference with mitochondrial alternative oxidase(AOX1a)reduced the level of autophagy in tomato,resulting in an increase in H2O2concentration[75].Autophagy reduced ROS levels by degrading the damaged organelles(mitochondria, chloroplasts, and peroxisomes) that produce excessive ROS under environmental stresses [215–217].Thus, ROS not only promotes autophagy as a second messenger but also is eliminated by autophagy [166].
In summary, ROS can promote autophagy by regulating upstream signal molecules at low concentrations, and autophagy can remove ROS to maintain cell homeostasis at higher concentrations.When ROS are excessive, beyond the clearance range of autophagy, they will cause irreversible damage to cells.Usually,autophagy and ROS are in balance, allowing plants to resist environmental stresses.
5.Concluding remarks and future perspectives
Autophagy participates in many ways in the regulation of plant growth and development, and abiotic stresses.Many functional ATG genes and regulatory networks have been identified and described, providing theoretical support for improving crop resistance and laying a foundation for further study of the mechanism of autophagy.We have seen that autophagy functions in tapetum degradation, pollen release, and pollen germination and in these processes, provides necessary nutrients for male reproductive development by degrading intracellular components and cell structures.Developing male-sterile lines via autophagy to increase the efficiency of crop breeding is a future challenge.
However, mining ATG genes and studying their mechanisms have been concentrated in Arabidopsis.In crops, especially wheat,research progress in autophagy is relatively slow.At present, the study of autophagy in plant cells is at only the basic theoretical research stage.Although many ATG genes affecting plant growth,development,and stress resistance have been identified,exploiting them to improve field crops is challenging.
CRediT authorship contribution statement
Yongbo Li:Writing – original draft, Project administration.Xiangmin Xu:Data curation.Guang Qi:Formal analysis.DezhouCui:Investigation.Chen Huang:Validation.Xinxia Sui:Validation.Genying Li:Writing – review & editing.Qingqi Fan:Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Shandong Natural Science Foundation (ZR2020QC114), the National Natural Science Foundation of China (32001542, 32001545), the Agricultural Variety Improvement Project of Shandong Province (2021LZGC013), and the Shandong Academy of Agricultural Sciences Innovation Project(CXGC2023A01, CXGC2023C02).
- The Crop Journal的其它文章
- OsSPL10 controls trichome development by interacting with OsWOX3B at both transcription and protein levels in rice (Oryza sativa L.)
- Ectopic expression of OsNF-YA8, an endosperm-specific nuclear factor Y transcription-factor gene, causes vegetative and reproductive development defects in rice
- ZmDRR206 functions in maintaining cell wall integrity during maize seedling growth and defense response to external stresses
- The plasmodesmata-associated β-1,3-glucanase gene GhPdBG regulates fiber development in cotton
- The MabHLH11 transcription factor interacting with MaMYB4 acts additively in increasing plant scopolin biosynthesis
- Evolutionary genetics of wheat mitochondrial genomes

