Inhibitory effect of water soluble propolis on oxidative damage in rats with ulcerative colitis
ZHOU Hua, ZHANG Min, JIANG Wen-tao
Department of Physiology, Anhui Medical College, Hefei 230601, China
Keywords:Water-soluble propolis Ulcerative colitis Oxidative stress Intestinal mucosal barrier Natural products
ABSTRACT Objective: To investigate the effects of water-soluble propolis (WSP) on the levels of colonic mucosal inflammation and oxidative stress in rats with ulcerative colitis (UC).Methods:The UC rat model was made by sodium dextran sulfate (DSS).Forty-eight male rats were randomly divided into six groups: the normal group (N group), the control group (C group),the positive control group (P group), the low-dose propolis group (L group), he mediumdose propolis (M group) and the high-dose propolis group (H group).The protective effect of WSP on DSS induced UC rats was evaluated by preadministration and re-construction model.Body mass, disease activity index (DAI), blood stool, colon length, intestinal mucosal damage index (CMDI) of rats were observed.The indexes of medulla peroxidase (MPO), superoxidase(SOD), malondialdehyde (MDA) and glutathione peroxidase (GSH-Px) in colon tissue were determined by ELISA.Results: the oxidative stress level of colon tissue in the model group was increased and neutrophils were activated.After medium and high concentration propolis intervention, the oxidative stress level was reduced and the colonic inflammatory injury was relieved.Conclusion: WSP can improve the activity of SOD, GSH-Px and other endogenous antioxidant enzymes, reduce oxidative stress products, inhibit the activity of neutrophil and other mechanisms to relieve intestinal inflammation in experimental UC rat model.
1.Introduction
Ulcerative colitis (UC) is a debilitating, idiopathic chronic inflammatory colon disease characterized by chronic repeated inflammation of the intestinal mucosa, which can lead to symptoms such as bloody diarrhea and severe abdominal pain.The ups and downs of tissue damage and repair eventually lead to a loss of mucosal function and the development of colitis-related cancers.It has been proved that the occurrence and development of UC are related to multiple factors, in which reactive oxygen species (ROS)and pro-inflammatory cytokines are triggers, which trigger and aggravate the damage of intestinal mucosal barrier integrity[1], thus leading to the influx of intestinal pathogens and the subsequent abnormal activation of intestinal mucosal immune response[2].This can lead to tissue damage and eventually lead to various clinical symptoms of UC.
At present, glucocorticosteroids, aminosalicylic acid and adrenal glucocorticokinin are often used to treat UC, but most of them have side effects.New alternative therapies are a research hotspot in UC therapy, and natural products are sources in this field.According to reports, dietary polyphenols can reduce the damage of ROS to intestinal mucosa, enhance intracellular “self-defense”, and fight against oxidative stress and inflammation[3].
Propolis is rich in flavonoids (a widely available class of biologically active polyphenol compounds).Recent studies have reported that bee glue has an anti-ulcer effect and can promote mucosal healing and granuloma formation[4].Propolis significantly inhibits the expression and release of macrophage cytositis cytokines in rats, alleviates intracellular ROS accumulation, and plays an anti-inflammatory role in inflammatory diseases[5].These antiinflammatory properties make propolis a promising natural product for improving UC.Previous studies have shown that propolis can relieve colon inflammation in UC rats, however, the mechanism by which propolis alleviates UC remains to be elucidated.
In this study, UC rat models were prepared with dextran sulfate sodium (DSS) to investigate the antioxidant and anti-inflammatory effects of water-soluble propolis on inflamed colonic mucosa.It provides a new therapeutic option for the clinical treatment of inflammatory bowel diseases such as UC.
2.Materials and Methods
2.1 Animals
2.2 Drugs and Reagents
DSS (MW: 36,000-50,000; MP Biomedicals, CANADA);superoxide dis-mutase (SOD) ELISA test kit, glutathione peroxidase(GSH-Px) ELISA test kit, All above were purchased from CUSABIO Company.ROS ELISA kit, malondialdehyde (MDA) and Myeloperoxidase (MPO) test kit were purchased from Jiancheng Institute of Biomedical Engineering, Nanjing.Sulfasalazine (Sulfa)was purchased from Hefei Zhihongtek Biotechnology Co., LTD.Paraformaldehyde and hematoxylin eosin are purchased from Biosharp.Water soluble propolis purchased from Guangzhou Jiehe Bee Industry Co., LTD.(Lot number: 20210325-B).
2.3 Animal grouping
48 male SD rats were properly fed for 1 week before experiment and caged with wood chips in an animal room (23±2) ℃ ambient temperature and 12 h light - dark cycling).They were divided into normal group (group N), control group (group C), positive control group (group P) and low dose group (group C).group L), medium dose group (group M) and high dose group (group H), with 8 animals in each group.
2.4 Administration Method
The experiment adopts the method of pre-administration to reconstruct the model.During the whole experiment, groups N and C were given orally distilled water, group P was given orally salazepidine 100 mg/kg , and groups L, M and H were given orally propolis at the dosage of 50, 100 and 200 mg/kg, respectively.After 7 d of administration, group N was free to drink distilled water for 7 d.Groups C, P, L, M and H were given 5% DSS solution for 7 d.Finally, free drinking of DSS solution was stopped, and all groups were fed with distilled water for drinking for 2 d (Figure 1).The rats were killed by posterior cervical vertebrae dislocation.
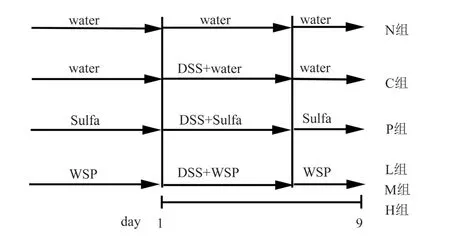
Fig 1 Ulcerative colits model design
2.5 Weight change and stool blood monitoring
Starting from the first day after drinking DSS, the body weight,stool blood status and stool status of rats in each group were observed at the same time every day, and the data were recorded in detail for 9 d, and the animals were killed on the 10th day.The day 1 weight was labeled as the pre-experiment starting weight.Weight change (%) = [(Day x weight Day 1 weight) / Day 1 weight]100%
2.6 Disease activity index score
From day 2 after drinking DSS, disease activity index (DAI) was evaluated according to the following criteria based on weight change,fecal traits and stool blood status.DAI= score of body weight loss +score of fecal traits + score of blood in stool[6].The scoring criteria are shown in Table 1.
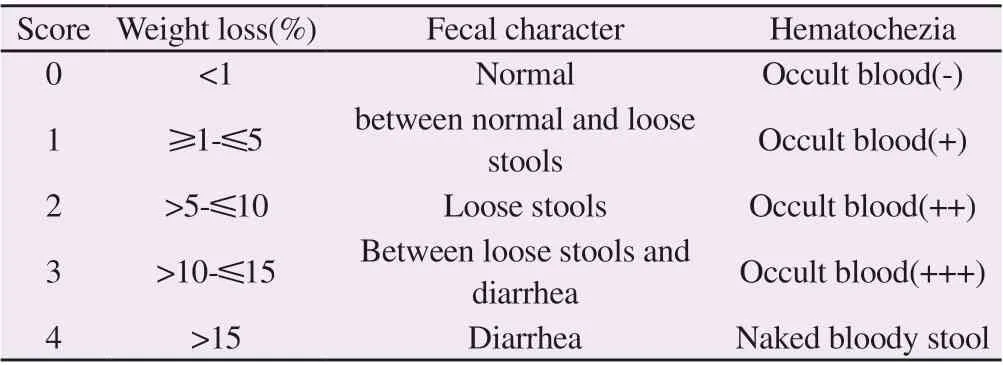
Tab1 Disease activity index score
2.7 Sample collection and processing
After the rats in each group were killed, the intestinal segment of the nodal intestine above 2 cm from the anus of the rats was completely removed from the abdominal cavity.The length of nodular segment was measured.The colon was dissected along the longitudinal axis, rinsed with pre-cooled physiological saline,cleaned the colon contents, drained on filter paper, and measured colonic mass /length (weight/length, W/L) ratio = colonic mass (g)/colonic length (cm)[7], and colonic mucosal damage index (CMDI)[8] were evaluated, and the scoring criteria were shown in Table 2.
Here the raven left him and he was handed over once more to the care of the first band of slaves, while a large bat flickered71 down and settled upon his head of its own accord, and so he was taken back to the marble bath, and had to go through the whole process again, only this time he began in deep water which receded72 daily inch by inch
Colon tissue was cut and part of colon tissue was fixed in EP tube with 4% paraformaldehyde.The remaining intestine was cut into EP tubes, homogenized with 5 times the volume of normal saline, left for 2 h, separated from the center at 10 000 r/min at 4 ℃ for 10 min,and the supernatant was taken and stored at 80 ℃.

Tab2 Colonic mucosal injury index (CMDI) score
2.8 Histopathologic observation of colon
The fixed colon tissue was rinsed overnight with water, dehydrated with gradient alcohol, transparent with xylene, embedded in paraffin, prepared into 5 μm thick slices, stained by conventional hematoxylin-eosin (HE), observed under an optical microscope for pathological slices, and performed histological score (HS) score[9].The score criteria of colon histological injury are shown in Table 3.

Tab3 Colon histological score
2.9 Determination of oxidative stress index levels in rat colonic tissue
Each group of intestinal tissue was removed from the ice box at 80 ℃, weighed 100 mg and put into the ice box.The tissue was prepared into 10% tissue homogenate with ice saline, and then 1/4 tissue homogenate with 1% volume of dilute release was extracted.The activities of ROS, MDA, MPO, SOD and GSH-Px were determined by centrifuge at 14 000 r/min for 15min at 4 ℃, supernuents were taken, and the activities of ROS, MDA, MPO,GSH-PX were determined according to the method described in the test box instructions.
2.10 Statistical analysis
3.Results
3.1 WSP improves colon inflammation induced by DSS in UC rats
In this study, the rats were treated with WSP and Sulfa for 16 d, and the UC model was established by drinking 5% DSS solution on the 8th day.The DSS solution was continuously treated for 7 days, and then the DSS solution was stopped for 2 d.During the simulated UC period, the active disease and remission stage alternately appeared.Table 4Resultsshow that compared with group N, rats in group C showed significant weight loss on the second day after drinking DSS(F=4.323, P<0.01), and intervention with low, medium and high concentrations of propolis was performed.Compared with group C, rats in group C showed significant weight loss on the second day after drinking DSS solution.Group H could significantly inhibit the body weight loss caused by DSS (F=4.323, P<0.05).On the 4th and 6th days of DSS solution drinking, groups M (F=106.134, P<0.05)and L (F=28.156, P<0.01).Weight loss due to DSS was significantly inhibited.
Table 5Resultsshowed that DAI (0.88±0.35) of rats in group C was significantly higher than that in group N (0.25±0.46) (F=1.909,P<0.05) on the second day of DSS administration, and compared with that in group C, DAI was significantly higher than that in group C.DAI scores of rats in groups M (0.38±0.52) and H (0.38±0.52)were decreased (F=1.909, P<0.05).On the 8th day after drinking DSS solution, DAI (6.75±0.46) in group L was significantly lower than DAI (7.38±0.52) in group C (F=249.503, P<0.01).
The length of the nodule was negatively correlated with the severity of the severity of the experimental nodule inflammation, and the shortening of the nodule was an indicator of the severity of the nodule inflammation.As shown in FIG.2AB, compared with group N (21.03±0.79), The colonic length of rats in group C (17.71±0.44)was significantly shortened (F=73.312, P<0.01).Compared with group C, the colonic length of rats in group M (18.49±0.29) and group H (20.89±0.52) was significantly extended (F=75.350,P<0.01), but there was no statistical significance between group L and group C (P>0.05) (FIG.2AB).Compared with group P, there were significant differences in groups L, M and H (P<0.01).Colonic mucosal congestion, edema, local erythema and erosion of rats in group C could be seen by naked eyes.Compared with groupN (0.25±0.46), the CMDI score of rats in group C was increased(3.63±0.52) (F=58.154, P<0.01) (Figure 2C).Compared with group C, CMDI scores in groups M (2.75±0.46) and H (1.63±0.52)were lower (F=58.154, P<0.01) (Figure 2C).Compared with group P, there was significant statistical difference between group L and group M (P<0.01), but no statistical significance in group H(P>0.05).Compared with group N (0.07±0.00), the W/L ratio of the nodal intestine in group C (0.08±0.01) was significantly higher (F=5.716,P<0.05) (Figure 2D).Compared with group C, the colon W/L ratio in groups L, M and H showed an increasing trend, but there was no statistical difference (P>0.05).There was no significant difference in colon W/L ratio between groups L, M and H compared with group P(P>0.05).
Tab4 Effects of WSP on body weight change in each group of rats
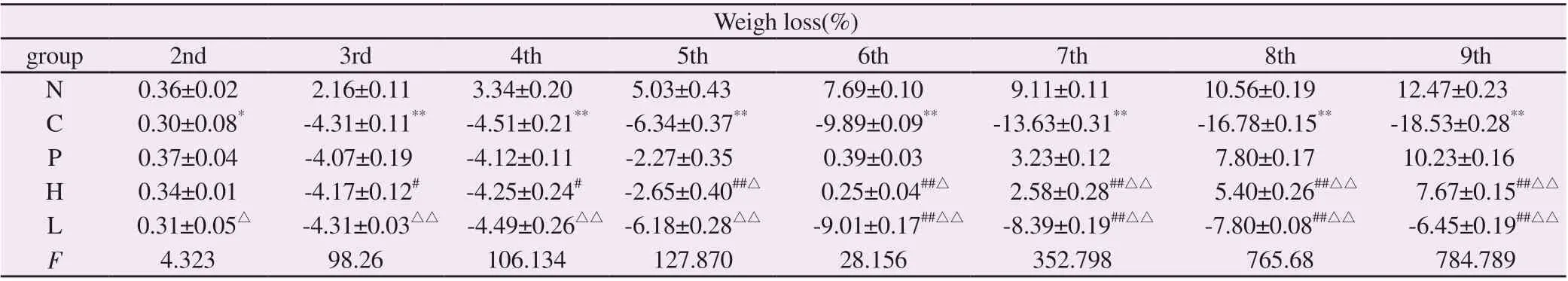
Tab4 Effects of WSP on body weight change in each group of rats
Note: Compared with group N, *P<0.05, **P<0.01; Compared with group C, #P<0.05, ##P<0.01; Compared with group P, △P<0.05, △△P<0.01.
Weigh loss(%)group 2nd 3rd 4th 5th 6th 7th 8th 9th N 0.36±0.02 2.16±0.11 3.34±0.20 5.03±0.43 7.69±0.10 9.11±0.11 10.56±0.19 12.47±0.23 C 0.30±0.08* -4.31±0.11** -4.51±0.21** -6.34±0.37** -9.89±0.09** -13.63±0.31** -16.78±0.15** -18.53±0.28**P 0.37±0.04 -4.07±0.19 -4.12±0.11 -2.27±0.35 0.39±0.03 3.23±0.12 7.80±0.17 10.23±0.16 H 0.34±0.01 -4.17±0.12# -4.25±0.24# -2.65±0.40##△ 0.25±0.04##△ 2.58±0.28##△△ 5.40±0.26##△△ 7.67±0.15##△△L 0.31±0.05△ -4.31±0.03△△ -4.49±0.26△△ -6.18±0.28△△ -9.01±0.17##△△ -8.39±0.19##△△ -7.80±0.08##△△ -6.45±0.19##△△F 4.323 98.26 106.134 127.870 28.156 352.798 765.68 784.789
Tab5 Effects of WSP on DAI in each group of rats

Tab5 Effects of WSP on DAI in each group of rats
Note: Compared with group N, *P<0.05, **P<0.01; Compared with group C, #P<0.05, ##P<0.01; Compared with group P, △P<0.05, △△P<0.01.
DAI group 2nd 3rd 4th 5th 6th 7th 8th 9th N 0.25±0.46 0.63±0.74 0.75±0.46 0.50±0.53 0.38±0.52 0.25±0.46 0.25±0.06 0.13±0.35 C 0.88±0.35* 3.63±0.52** 5.63±0.52** 6.13±0.35** 6.50±0.53** 7.25±0.46** 7.38±0.52** 7.00±0.53**P 0.25±0.46 1.75±0.71 2.25±0.46 2.88±0.35 3.38±0.52 3.63±0.52 3.25±0.46 2.50±0.53 H 0.38±0.52# 2.25±0.71## 2.88±0.35## 3.25±0.71## 4.00±0.53##△ 4.25±0.46##△△ 3.88±0.35##△△ 3.38±0.52##△△L 0.50±0.53△ 3.38±0.74△△ 5.25±0.46△△ 5.88±0.35△△ 6.13±0.35△△ 6.88±0.35△△ 6.75±0.46##△△ 6.25±0.46##△△F 1.909 21.610 116.363 142.736 168.433 279.332 249.503 184.621

*P<0.05, **P<0.0 vs.N group;# P<0.05, ##P<0.01 vs.C group;△△P<0.01 vs.P group
3.2 WSP alleviates the degree of colonic tissue lesion in UC rats
The pathological slices stained by HE were observed.As shown in Figure 3A, the structure of colon mucosa, submucosa and crypt in group N was normal, and no inflammatory cell infiltration was observed.In group C, the structure of colonic mucosa and submucosa was destroyed, and the crypt structure was lost,accompanied by large inflammatory cell impregnation.Compared with group N (0.25±0.46), the HS score of group C was significantly higher (F=84.082, P<0.01) (FIG.3B).In group H, the structure of colon mucosa, submucosa and crypt was basically intact, no inflammatory cell infiltration was observed, and the degree of injury was obviously delayed.Inflammatory cell infiltration was still observed in group M, while structural destruction of mucosa and submucosa and inflammatory cell infiltration were still observed in group L (FIG.3A).As can be seen in FIG.3B, compared with group C, HS score of group H decreased (2.75±0.46) (F=84.082, P<0.01),and HS score of group M decreased (4.00±0.53) (F=84.082, P<0.01)(FIG.3B).There was no significant statistical difference in HS scores in group L (P>0.05).Compared with group P, the HS scores of group L (P<0.01), group M (P<0.01) and group H (P<0.05) had statistical differences.

Fig 3 Effect of WSP on pathological changes of colon in rats(HE staining, ×40)
3.3 WSP alleviates oxidative stress injury of colon tissue in UC rats
As shown in Figure 4A, SOD content in colon tissue homogenate of group C (22.77±3.18) significantly decreased compared with that of group N (44.82±2.80) (F=121.178, P<0.01).Compared with group C (22.77±3.18), the SOD content in group H (33.41±1.34) (P<0.01)and group M (25.28±1.65) (P<0.05) was significantly increased,while the change in group L was not statistically significant(P>0.05).Compared with group P (38.66±0.80), SOD content in colon tissue homogenate in groups H, M and L was significantly decreased (P<0.01).
As shown in Fig 4B, the ratio of GSH-Px content in group C(20.88±1.98) decreased significantly compared with group N(44.78±2.39) in the fabric homogenate of rats’ nodule (F=262.132,P<0.01).Compared with group C, the GSH-Px content in group H (36.42±2.33) (P<0.01) and group M (23.32±1.25) (P<0.05)increased significantly compared with group C (20.88±1.98).There was no statistical significance in L group (P>0.05).There were significant differences in GSH-Px content between groups H, M and L compared with group P (10.66±1.56) (P<0.01).
As shown in Figure 4D, ROS content in the jejunal tissue homogenate of rats in group N (138.07±2.59) was significantly higher in group C (259.57±5.81) than in group N (F=192.140,P<0.01).The ROS content in colon tissue homogenate of group H (235.93±2.77) (P<0.01) and M (254.86±2.91) (P<0.05) was significantly lower than that of group C (259.57±5.81).There was no statistical significance in L group (P>0.05).ROS content in colonic tissue homogenate of group H, group M and group L were significantly different from that of group P (146.85±3.58) (P<0.01).As shown in Figure 4E, compared with group N (2.53±0.09),MDA content in group C (6.96±0.10) was significantly increased(F=176.712, P<0.01).Compared with group C (6.96±0.10), MDA content in colon tissue homogenate in group H (4.46±0.20) (P<0.01)was significantly lower than that in group H (6.88±0.14) and group L (6.91±0.10) (P>0.05).Compared with group P (146.85±3.58), the MDA content in colon tissue homogenates in groups H, M and L had significant statistical difference (P<0.01).The results showed that DSS induced nodular enteritis significantly increased the level of oxidative stress in nodular tissue.Medium and high concentrations of propolis may play a concentration-dependent protective role on colonic mucosa through antioxidant function.
MPO, an enzyme almost exclusively present in neutrophils, is an oxygen-promoting and pro-inflammatory enzyme, and is a marker of acute inflammatory reactions.As shown in Figure 4C,compared with the MPO level (169.37±12.49) in the middle colon tissue homogenate of rats in group N, the MPO level in group C (240.61±5.28) was significantly higher (F=591.267, P<0.01).This indicates that a large number of neutrophils migrate to the inflammatory colon mucosa during UC stage, and the infiltration and activation of neutrophils lead to abnormal activation of immune response and damage the structure and function of intestinal mucosa.MPO water in colonic tissue homogenate of rats in group C (240.61±5.28) was significantly lower in group H (191.99±1.89)(P<0.01) and group M (230.64±4.48) than in group C (240.61±5.28).No statistical significance was found in L group (P>0.05).Compared with group P, the changes in groups L, M and H had significant statistical significance (P<0.01).

Fig 4 Effect of WSP on the levels of SOD, GHS-Px, MDA, ROS and MPO in colon tissue of rats in each group
4.Discussion
UC is a recurrent and long-term inflammatory disease commonly found in the digestive system, which is harmful to the physical and mental health of affected persons and may increase the risk of colorectal cancer.The drugs commonly used in bed can not induce the retarding and preventing recurrence of inflammation, and have side effects.The primary treatment of UC is to reduce symptoms,reduce morbidity and improve the quality of life.Therefore, the treatment of UC is still a field with major medical needs.As a result,there is growing interest in identifying alternative therapies and more tolerable treatments for the disease.
Natural products and their derivatives are recognized as the source of new drug research and development due to their extensive therapeutic effects and reduced side effects.There is increasing evidence[10] that phenols, especially flavonoid compounds, exhibit potent anti-inflammatory and antioxidant activities in vitro and in vivo, and are promising drugs for the treatment of UC.Studies have shown that foods rich in polyphenols in the diet[11] play an important role in inducing remission of IBD.Propolis is a natural antibiotic in nature.It is a resin rich in many kinds of bioactive substances (such as flavonoids and terpenes) collected by bees from many kinds of plants.It has good antioxidant properties.It can be used to clear the free group in the body, protect the cell membrane, strengthen the cell vitality, and regulate the function of the node organization[12].Over the past decade, propolis has been investigated for its beneficial potential in intestinal health, demonstrating its therapeutic value in the treatment of inflammatory bowel diseases.
4.1 Protective effect of WSP on inflammatory colon injury
The DSS induced colitis model is a useful model for screening antiinflammatory and antioxidant applications due to its high similarity to human UC in terms of proinflammatory factors and ROS production[13].In this study, 5% DSS induced rat UC model was used, and Sulfa was selected as the positive control in order to better evaluate the efficacy of WSP on UC.
In the empirical UC animal model, DAI is often used as an independent scale to measure the progression of colitis.It is scored according to the signs and symptoms of the disease, and is considered to be the main indicator to judge the degree of disease and prognosis.The results showed that DAI increased significantly in UC model group, while DAI decreased in rats given WSP and Sulfa, and the differences were statistically significant.To elucidate the protective effect of WSP in the course of enteritis.Colon length is another determinant of colon inflammation.In UC model group,colon length was significantly shortened.In rats with medium and high concentration WSP and Sulfa, the length of isolated nodular intestines increased significantly, which again demonstrated the ability of WSP to limit the damage of nodular intestines.
Histological analysis confirmed that severe colonic inflammation characterized by changes in colonic tissue structure was observed in rats after exposure to DSS, and the observed results were consistent with other research findings[14].In contrast, the colonic mucosa,submucosa and crypt structure were protected, inflammatory cell infiltration was reduced, and the damage to intestinal mucosa by DSS was limited, indicating that a certain concentration of WSP could protect colonic mucosa and alleviate colitis symptoms.
The main function of the colon is to absorb water and electrolytes,promote the formation of feces, and facilitate the use of creep to promote defecation, and the obstruction of the absorption and adduction of the colon will cause diarrhea[15].The W/L ratio of colon in each group was also measured in this experiment.We found that compared with rats in the UC model group, although there was no statistical significance in the colon W/L ratio of the propolis groups,it showed an increasing trend, indicating that during the regeneration of colon tissue, new blood vessels occurred and fibroblasts grew,resulting in thicker colon, and the W/L ratio of colon reflected the degree of tissue repair[16].The results suggest that WSP can promote the repair of ulcerative surface of colon mucosa and restore the absorption function of colon.
4.2 Mechanism of protective effect of WSP on colon inflammation
The intestinal mucosal barrier, generally considered to be the primary host defense system, forms a strong barrier against various infectious and non-infectious stimuli to maintain intestinal mucosal homeostasis.The key event in the occurrence of colitis is the destruction of intestinal mucosal barrier[17].The lesion of intestinal mucosal barrier function is related to the alteration of the permeability of the epithelium of the colon and its susceptibility to bacterial invasion and translocation.These events can trigger the release of inflammation and oxidizing mediators, thereby amplifying the colon injury.The severity of colitis can be modified by agents that affect neutrophil infiltration, ROS production, and pro-inflammatory cytoplasmic production.The purpose of this study was to investigate the role and mechanism of WSP in DSS induced colitis.
Cytoplasmic factors and oxygenation stimuli in the gastroenteric tract mediate the onset, progression, exacerbation, and recurrence of inflammatory processes in various enteric diseases.The activity of MPO in colon tissue is often used to measure the size of the immune response in UC rats.MPO is an intermediate marker for the number of median granules in inflammatory gastroenteric tissue.It has been reported in the literature that the severity of UC is related to MPO activity, and the activity of MPO is proportional to the number of medium granules recruited into the inflammatory colon, which can reflect the degree of neutrophil infiltration[18].Neutrophil infiltration promotes the progression of UC pathogenesis and induces the production of pro-inflammatory cytokines and ROS.MPO is an enzyme that exists almost exclusively in neutrophils.After neutrophils are activated, MPO is released into the extracellular or phagocytic body, leading to intestinal mucosal dysfunction and colon tissue injury, and accelerating the development of intestinal tract inflammation.In this experiment, by monitoring the MPO activity in the jejunal tissue homogenate of rats in all groups, it was found that the colon tissue MPO activity of rats in the UC model group was significantly increased, while the MPO content was significantly decreased after the administration of medium and high concentration WSP and Sulfa.These results indicate that WSP can inhibit the accumulation of neutrophils, inhibit the abnormal immune response of intestinal mucous membrane, protect the barrier of intestinal mucous membrane, and slow down intestinal inflammation.The migration of neutrophils to the inflamed colonic mucosa leads to increased levels of pro-inflammatory cytokines, and the imbalance between pro-inflammatory and anti-inflammatory cytokines can significantly lead to colonic tissue injury and mucosal dysfunction.Our previous studies have shown that the severity of UC is closely related to the production of pro-inflammatory cytokines.
Activated neutrophils produce excess ROS in the intestinal mucosa.Overproduction of ROS or its low efficiency clearance can induce tissue oxygenation reaction, and subsequently lipid peroxygenation reaction and hypoinflammatory reaction, which play an important role in the pathogenesis of UC[21].Oxidative stress and its consequent lipid peroxidation can aggravate the free radical chain reaction, damage the integrity of intestinal mucosal barrier,and activate pro-inflammatory reaction.When cells are exposed to excess ROS, apoptosis becomes the main mode of cell death.Under normal physiological conditions, the colon mucosa contains relatively low levels of endogenous antioxidants (e.g., SOD, GSHPx).They are responsible for scavenging free radicals directly,thus providing protection against oxidative damage in biological tissues.When the accumulation of ROS in the body increases, the endogenous antioxidant defense mechanism in the body cannot be cleared in time, which will cause oxidative stress and cytoplasmic diseases, such as increased lipid peroxygenation reaction and cytoplasmic death.SOD activity indirectly indicates the ability of the body to remove oxygen free radicals[22].GSH-Px is a nonenzymatic antioxidant that is considered to be the first oxygenation defense system against free radicals.When the lipid content in the cell membrane is attacked by the free radicals, the process of lipid peroxygenation is activated, which leads to cell damage.GSHPx can protect the structure and function of the protective film by removing MDA, the product of lipid peroxidation[23].MDA is a product of oxidative stress reaction, and the level of MDA can reflect the severity of dysfunctional oxidative stress[24], and can reflect the degree of cell damage.MDA water level is a common indicator of lipid peroxygenation and oxidative stress.In this study, we found that ROS and MDA contents in colon tissue of rats in UC model group were significantly increased, indicating that colon tissue exposed to DSS damaged the antioxidant power of colon tissue, produced large amounts of ROS, induced oxygenation stress, inflammatory cell infiltration, and caused lipid peroxidation.The MDA content is increased, leading to colitis, and the ROS and MDA content in colon tissue can be significantly inhibited after intervention with medium and high concentrations of WSP and Sulfa.In addition, the level of non-enzyme and enzyme defense system in colon tissue of rats in UC model group decreased significantly, indicating the existence of oxygenation damage in the upper intestinal epithelium.After dry preconditioning with medium and high concentration WSP and Sulfa, the results showed that The level rise of SOD and GSH-Px water in the rat’s nodal tissue showed significant statistical difference.The results showed that WSP could slow down the oxidative damage of colon induced by DSS by enhancing the antioxidant capacity and inhibiting lipid peroxylation.
Dietary WSP at a certain concentration has beneficial effects on DSS induced rat jejunitis, which can reduce lipid peroxygenation damage and its final product by preventing and stopping the deterioration of endogenous antioxidant oxidase activities such as SOD and GSH-Px in the jejunal tissue.Such as MDA, reduce the oxidative stress during UC; Inhibit the immune response of abnormal activation of neutrophils, play a role in protecting the intestinal mucosal barrier, and relieve intestinal inflammation in experimental UC rat models.WSP is a promising natural product for the treatment of UC.
 Journal of Hainan Medical College2023年18期
Journal of Hainan Medical College2023年18期
- Journal of Hainan Medical College的其它文章
- Study on the mechanism of action of Feng-Liao-Chang-Wei-Kang combined with 5-fluorouracil in the treatment of colitis-associated colon cancer
- Isoliquiritigenin regulated ox-LDL through activating the PPAR-γ signaling pathway to stabilize atherosclerosis plaques
- Effect of hepatocyte growth factor on inflammatory factors associated with CCL4-induced hepatocyte injury
- The expression of TUSC3 in Preeclampsia and the function in trophoblast cell
- Effects of intravenous infusion of esketamine on analgesia and postpartum antidepressant after cesarean section
- Expression and significance of ADAM12 in bladder cancer
