The Role of Jasmonates as Antibulbing Substances in the Bulb Formation of Onion
Noboru Takada Atsushi Saito Yuuki Matsuzuka Tatsushi Mochiduki Eriko Wakita Meng WANG Yasunori Koda
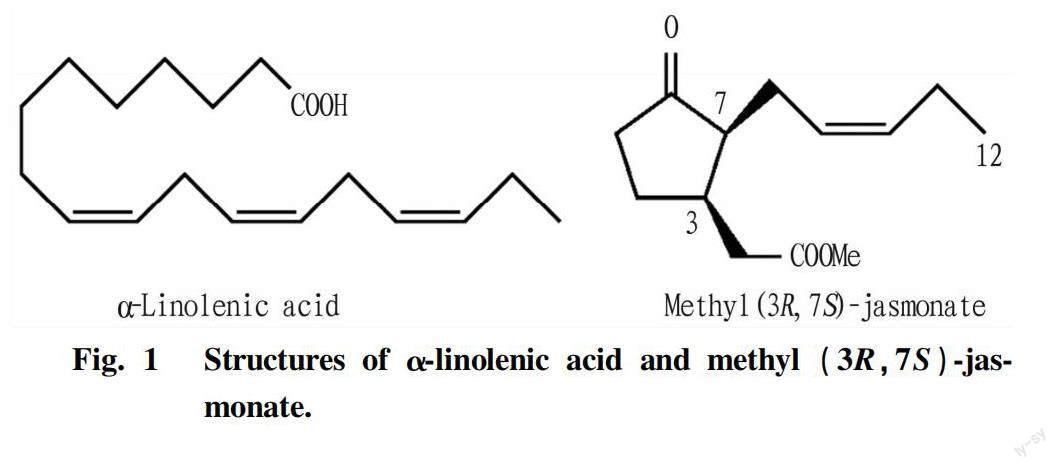

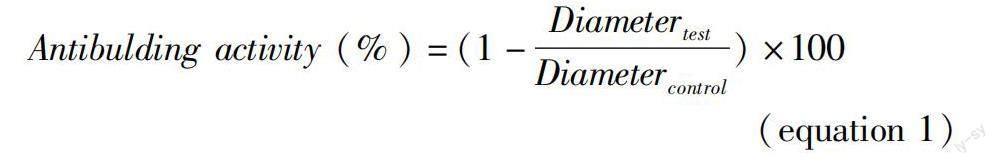
Abstract Onion plants form spherical bulbs under long-day conditions. Substances regulating bulb formation remain unknown. In the course of chemical studies on the bulb formation, α-linolenic acid was isolated from onion extracts as an antibulbing substance, the amount of which was synchronized with the bulb formation. Since allene oxide synthase inhibitor canceled the antibulbing activity of α-linolenic acid, it was disclosed that jasmonic acid concerns this regulation. Structure-activity-relationship study revealed that its (3R, 7S) stereochemistry is necessary for showing its antibulbing activity. It is concluded that (3R, 7S)-jasmonate derived from α-linolenic acid actually participates in the regulation of bulb formation.
Key words Onion (Allim cepa L. cv. Higuma); Isolation; Bulb formation; Antibulbing substance; α-Linolenic acid; Methyl jasmonate
Onion plants form spherical bulbs under long-day conditions[1-2]. The formation results from swelling of leaf sheath cells under the control of their cortical microtubules[3-5]. It has been proposed that gibberellins work as antibulbing substances and regulate the bulb formation[6-7], because gibberellins can stabilize cortical microtubules oriented transversely. However, quantitative analyses of gibberellins could not provide the direct evidence establishing this proposal, since its endogenous levels in long day-grown onion plants in which bulbs are formed were higher than those in short day-grown onion plants[8]. Therefore, none have achieved to a satisfactory conclusion.
We have succeeded in the isolation of α-linolenic acid from onion extract as an antibulbing substance, the amount of which synchronized with the bulb formation. Further, it was revealed that biosynthetic inhibitor of jasmonate suppressed the antibulbing activity of α-linolenic acid and methyl jasmonate exhibited the potent antibulbing activity. These results have led to the conclusion that jasmonates concern the bulb formation in onion plants as an antibulbing substance. We described the detail in this paper.
Experimental Methods
General procedures
The NMR spectra were recorded on a Bruker AMX500 spectrometer in Hokkaido University and a JEOL JNM-ECA500 spectrometer in Hirosaki University. The HREI/MS spectrum was obtained on a Jeol JMS-AX500 spectrometer in Hokkaido University. The IR spectra were measured on a HORIBA FT-720 spectrometer in Hirosaki University.
Linolenic acid, linoleic acid, oleic acid, stearic acid, and methyl jasmonate were purchased from Wako Pure Chemical Industries, Ltd. Sep-Pak C18cartridge and Inertsil ODS-3 column were obtained from Waters Corporation and GL Science Inc., respectively.
Assay for antibulbing activity
Onion seeds (Allium cepa L. cv. Higuma) were sterilized for 4 h with 1% sodium hypochlorite solution and rinsed thoroughly. Onion seeds were sown on in Murashige-Skoog (pH 5.8, including 0.2% of gellan gum) plate medium[9]. Seedlings were grown for 2 weeks under 16 h photoperiod at 25 ℃. Illumination (2.0 mW/cm2) was provided by white fluorescent lamps. After 2 weeks, 3 cm segments of seedlings were excised for the assay, while leaf tips and the roots were cut off. Subsequently, 20 μl of methanolic additive solution was added to a culture flask. After drying, the plate medium was prepared by pouring 20 ml of the medium containing 3×105M pacrobutorazole to the flask. Each five seedling segments was transplanted into the medium and grown under the same condition for 4 weeks. After incubation, the diameters of bulbs were measured. Results are presented as the mean obtained from ten plants±SD.
Identification of linolenic acid from onion leaves
Onion (Allium cepa L. cv. Higuma) seeds were sown in an experimental farm at Hokkaido University on May 10, 2006 and grown in the usual way.
Onion leaves (1 kg fresh weight) of June 22, 2006 were soaked in 70% EtOHaq(3 L) for one month. After removing EtOH in vacuo, the residual aqueous solution was partitioned between hexane (300 ml×3), EtOAc (300 ml×3) and n-BuOH (300 ml×3). The obtained fractions were concentrated in vacuo to give the hexane (5.8 g), EtOAc, n-BuOH, and aqueous fractions, respectively.
The hexane fraction was separated by silica gel (100 g) column chromatography with toluene-EtOAc [3:2 (0.2 L) → 1:4 (0.2 L)]. The fraction (1.2 g) eluted with toluene/EtOAc (3/2) was further fractionated with Sep-Pak?cartridge [10 g, MeOH:H2O:AcOH (90:10:0.1)]. The eluent (45 mg) was concentrated and subjected to HPLC [Inertsil ODS-3, 20 mm ?×250 mm, MeOH:H2O:AcOH (95:5:0.1), 5.0 ml/min flow, detected at 210 nm] to yield α-linolenic acid 4.0 mg. The retention time (tR=29.9 min) was identical to that of the authentic sample.
Quantification of α-linolenic acid
The hexane fractions equivalent to 10 g of onion leaves were prepared every week from June 15 to July 27. The fractions were loaded with Sep-Pak cartridge [10 g, MeOH:H2O:AcOH (90:10:0.1)]. The resulting eluents were concentrated in vacuo to give the crude materials, which were diluted again with methanol (100 ml). The resulting solution was injected into the HPLC instrument [Inertsil ODS-3, MeOH:H2O:AcOH (95:5:0.1), 1.0 ml/min flow, detected at 210 nm, 5 μl injection]. The amounts were quantified with peak area. Prior to the analyses, calibration curve was prepared with authentic samples. Recovery ratios of these samples through the above procedures were estimated to be 80%-95% by performing the same operations using authentic samples.
Results and Discussion
It was attempted to detect the antibulbing activity of onion leave extracts employing in vitro bioassay. The partition of alcoholic extract of onion leaves (1 kg fresh weight) provided hexane, EtOAc, n-BuOH, and H2O fractions. The hexane fraction exhibited the strongest activity at 1.5 mg/ml among them (Fig. 2). The hexane fraction was subjected to several types of chromatography, such as silica gel and ODS, to afford an antibulbing substance (4.0 mg from 1 kg of fresh weight onion leaves). The spectral data (NMR, IR and MS) and HPLC profile of active substance were in good accordance with these of commercially available α-linolenic acid, the antibulbing activity of which was detected at 0.29 mg/ml. Although α-linolenic acid has been known to show several bioactivities, such as induction of tendril coiling of Bryonia dioica[10]and inhibition of in vitro N-1-naphthylphthalmic acid binding[11], the present activity has not been reported.
It was focused on changes in the amount of α-linolenic acid over the course of bulb formation (Fig. 3). Antibulbing activity was first defined based on ratio of bulb diameter, which is given as equation 1.
In 2006, the bulb formation of onion plants commenced in our experimental field on late June and then swelling of bulbs dramatically proceeded. Onion plants (200 g fresh weight) sampled every week from June 15 to July 27 were extracted with 70% aqueous EtOH. The activities of obtained extracts (0.1 g fresh weight equivalent/ml) were examined employing in vitro bioassay. It was found that the extracts harvested before bulb formation (June 15 and 22) exhibited potent antibulbing activities (around 40%). In contrast, the activities hardly detected (less than 20%) from the extracts harvested during the process (June 29 to July 27). Quantitative analysis employing HPLC [Inertsil ODS-3, MeOH/H2O/AcOH (95/5/0.1)] disclosed that the amounts of α-linolenic acid until June 22 were sufficient (5.2 mmol/kg fresh weight) to suppress the bulb formation, while the amounts after June 29 kept low (around 0.7 mmol/kg fresh weight). Thus, the time-course change of amount of α-linolenic acid was resembled with that of the antibulbing activity in onion extract, suggesting that α-linolenic acid is responsible for the antibulbing activity in onion extract.
Gibberellins have been considered to be one of the most potential candidates for antibulbing substance[12]as described above. Then, it was investigated whether the activity of α-linolenic acid has a relation to that of gibberellins. Pactrobutrazole (a gibberellins biosynthetic inhibitor)[13]at more than 10 μM treatment suppressed the bulb formation (Fig. 4A). The amounts of α-linolenic acid in these grown seedlings were quantified with similar fashion as described above. The analyses revealed that its amount decreased from 50 to 15 μmol/g fresh weight as the applied amount of paclobutrazole increased. On the other hand, treatment of GA3, which inhibited the bulb formation at more than 1.0 μM, led to the increment of α-linolenic acid, the amount of which reached 50 μmol/g fresh weight when 10 μM of GA3 was applied. Thus, it was disclosed that the endogenous amount of α-linolenic acid synchronized with that of gibberellins in plant. Taking the activation of lipase by gibberellins into account[14-17], gibberellins might control the bulb formation through the regulation of lipase activity following the elimination of α-linolenic acid from cell membrane.
It was next focused on the structure-activity-relationship study of α-linolenic acid (Fig. 5A). 9,12-Linoleic acid showed as same strong activity (60%) as α-linolenic acid did (62%), whereas 9-oleic acid and stearic acid showed weak (28%) or no activities (8%), respectively. These results suggested that divinyl methylene moiety in α-linolenic acid is important for exhibiting the activity. This moiety has been known to suffer the oxidation easily during biosynthetic process. Various metabolites called oxylipins, such as jasmonic acid[19-20], traumatin[21-22], and 9-hydroxy-10-oxo-12Z,15Z-octadecadienoic acid[23-25], are derived from α-linolenic acid. Considering that divinyl methylene moiety in α-linolenic acid is important for showing the activity, it is supposed that some oxidative metabolite derived from α-linolenic acid actually participates in the bulb formation. Among them, we directed our attention to jasmonic acid, because tuberonic acid (12-hydroxyjasmonic acid) has been known to concern with the swelling of the potato cells as a tuber-inducing substance[26].
Then, it was examined whether miconazole, an allene oxide synthase inhibitor[27], affects on the bulb formation. The applied miconazole (10 μM) diminished the antibulbing activity of α-linolenic acid (1.0 mM, 62% to 20%), suggesting the participation of jasmonic acid in the process. Commercially available methyl jasmonate usually used in biological studies are equilibrium mixtures containing the major isomer (95%) with the trans side chains and the minor isomer (5%) with the cis side chains. The cis-isomer is believed to be the initial product formed in the biosynthesis of jasmonates in plants[28]. The trans-isomer is produced as a result of subsequent epimerization in plant or during isolation[29]. Furthermore, varying degrees of activity has been observed for the different isomers[30]. Then, it was examined the structure-activity-relationship study of methyl jasmonates. The all stereoisomers were prepared from commercially available methyl jasmonate employing HPLC [1st: Inertsil SIL-100A, hexane/iPrOH (98:2); 2nd: ChiralPak AS-H[31], hexane/iPrOH (9:1)]. The bioassay showed that naturally occurring (3R, 7S)-isomer inhibited the bulb formation strongly (67%) at 10 M, while other isomers hardly showed the activity (around 20%) at same concentrations. It was disclosed that the (3R, 7S) stereochemistry of it is necessary for showing its antibulbing activity. This is probably the reason why α-linolenic acid, not (3R, 7S)-jasmonate, was first isolated as an antibulbing substance in this study.
It has been known that jasmonic acid with the cis side chains is transformed to other jasmonates, such as tuberonic acid and cucurbic acid, through biosynthetic process. It remains unclear which (3R, 7R)-jasmonate among them actually works as the antibulbing substance in the onion plant. It is needed to obtain various (3R, 7S)-jasmonate derivatives in order to further understand the mechanism. Synthetic studies of (3R, 7S)-jasmonic acid derivatives are currently under way in our laboratory.
Conclusions
In the course of chemical studies on the bulb formation, α-linolenic acid was isolated from onion extracts as an antibulbing substance, the amount of which was synchronized with the bulb formation. Structure-activity-relationship study revealed that methyl (3R, 7S)-jasmonate with the cis side chains exhibited the most potent antibulbing activity. It is concluded that (3R, 7S)-jasmonate derived from α-linolenic acid actually participates in the regulation of bulb formation.
References
[1] MAGRUDER R, ALLARD HA. Bulb formation in some American and European varaieties of onions as affected by length of days[J]. J. Agr. Res., 1937(54): 719-752.
[2] SHIOMI N, BENKEBLIA N, ONODERA S, et al. Saccharide and fructooligosaccharide accumulation across leaf-bases during growth and bulb development of onion (Allium cepa L.)[J]. Acta Agro. Hung., 2008(56): 21-31.
[3] DHONUKSHE P, LAXALT AM, GOEDHART J, et al. Phospholipid D activation correlates with microtubule reorganization in living cells[J]. Plant Cell, 2003(15): 2666-2679.
[4] MITA T, SHIBAOKA H. Changes in microtubules in onion leaf sheath cells during bulb development[J]. Plant Cell Physiol., 1983(24): 109-117.
[5] SUSLOV D, VERBELEN JP. Cellulose orientation determines mechanical anisotropy in onion epidermis cell walls[J]. J. Exp. Bot., 2006(57): 2183-2192.
[6] MITA T, SHIBAOKA H. Gibberellin stabilizes microtubules in onion leaf sheath cells[J]. Protoplasma, 1984(119): 100-109.
[7] MITA T, SHIBAOKA H. Effects of S-3307, an inhibitor of gibberellin biosynthesis, on swelling of leaf sheath cells and on the arrangement of cortical microtubules in onion seedlings[J]. Plant Cell Physiol., 1984(25): 1531-1539.
[8] NOJIRI H, TOYOMASU T, YAMANE H, et al. Qualitative and quantitative analysis of endogenous gibberellins in onion plants and their effects on bulb development[J]. Biosci. Biotech. Biochem., 1993(57): 2031-2035.
[9] MURASHIGE T, SKOOG F. A revised medium for rapid growth and bio assays with tabacco cultures[J]. Physiol. Plant., 1962(15): 473-497.
[10] FALKENSTEIN E, GROTH B, MITHOEFER A, et al. Methyljasmonate and α-linolenic acid are potent inducers of tendril coiling[J]. Planta, 1991(185): 316-322.
[11] SUTTLE JC. Identification and characterization of linoleic acid as an endogenous modulator of in vitro N-1-naphthylphthalamic acid binding[J]. Plant Physiol., 1997(113): 519-525.
[12] SHIBAOKA H. The role of gibberellin in the formation of onion bulbs[M]. in: Takahashi, N., MacMillan, J., Phinney, B.O. (Eds.), Gibberellins. Springer-Verlag, New York, 1991.
[13] RUDEMACHER W. Growth retardants: Effects on gebberellin biosynthesis and metabolic pathways[J]. Ann. Rev. Plant Physiol. Plant Mol. Biol., 2000(51): 501-531.
[14] BLACK HS, ALTSCHUL AH. Gibberellic acid-induced lipase and alpha-amylase formation and their inhibition by aflatoxin[J]. Biochem. Biophys. Res. Commun., 1965(19): 661-664.
[15] FERNANDEZ DE, STAEHELIN LA. Does gibberellic acid induced the transfer of lipase from protein bodies to lipid bodies in barley aleurone cells? Plant Physiol. 85, 487-496.Heath, O.V.S., 1945. Formative effects of environmental factors as exemplified in the development of the onion plant[J]. Nature, 1987(155): 623-626.
[16] SHIMADA A, UEGUCHI-TANAKA M, NAKATSU T, et al. Structure basis for gibberellin recognition by its receptor GID1[J]. Nature, 2008(456): 520-523.
[17] UEGUCHI-TANAKA M, ASHIKARI M, NAKAJIMA M, et al. GIBBERELLIN INSENSITIVE DWARF1 Encodes a soluble receptor for gibberellin[J]. Nature, 2005(437): 693-698.
[18] MCCONN M, BROWSE J. The critical requirement for linolenic acid is pollen development, not photosynthesis, in an Arabidopsis mutant[J]. Plant Cell, 1996(8): 403-416.
[19] VICK BA, ZIMMERMAN DC. The biosynthesis of jasmonic acid: A physiological role for plant lipoxygenase[J]. Biochem. Biophys. Res. Commun., 1983(111): 470-477.
[20] VICK BA, ZIMMERMAN DC. Pathways of fatty acid hydroperoxide metabolism in spinach leaf chloroplasts[J]. Plant Physiol., 1987(85): 1073-1078.
[21] ENGLISH J, BONNER J, HAAGEN-SMIT AJ. The wound hormones of plants II. The isolation of a crystalline active substance[J]. Proc. Nat. Acad. Sci. USA, 1939(25): 323-329.
[22] ZIMMERMAN DC, COUDRON CA. Identification of traumatin, a wound hormone, as 12-oxo-trans-10-dodecenoic acid[J]. Plant Physiol., 1979(63): 536-541.
[23] SUZUKI M, YAMAGUCHI S, IIDA T, et al. Endogenous α-ketol linolenic acid levels in short day-induced cotyledons are closely related to flower induction in Pharbitis nil[J]. Plant Cell Physiol., 2003(44): 35-43.
[24] YAMAGUCHI S, YOKOYAMA M, IIDA T, et al. Identification of a component that induces flowering of Lemna among the reaction products of α-ketol linolenic acid (FIF) and norephinephrine[J]. Plant Cell Physiol., 2001(42): 1201-1209.
[25] YOKOYAMA M, YAMAGUCHI S, INOMATA S, et al. Stress-induced factor involved in flower formation of Lemna is an α-ketol derivative of linolenic acid[J]. Plant Cell Physiol., 2000(41): 110-113.
[26] KODA Y, KIKUTA Y, TAZAKI H, et al. Potato tuber-inducing activities of jasmonic acid and related compounds[J]. Phytochemistry, 1991(30): 1435-1438.
[27] OH K, SEKI Y, MUROFUSHI N, et al. Effect of miconazole, an antifungal agent, on allene oxide synthase: Inhibition, kinetics, and binding[J]. Pesticide Biochem. Physiol., 2009(94): 107-111.
[28] VICK BA, ZIMMERMAN DC. Biosynthesi of jasmonic acid by several plant species[J]. Plant Physiol., 1984(75): 458-461.
[29] MUELLER MJ, BRODSCHELM W. Quantification of jasmonic acid by capillary gas chromatography-negative chemical ionization-mass spectrometry[J]. Anal. Biochem., 1994(218): 425-435.
[30] KODA Y. The role of jasmonic acid and related compounds in the regulation of plant development[J]. Int. Rev. Cytol., 1992(135): 155-199.
[31] OKAMOTO M, NAKAZAWA H. Direct chromatographic separation of the enantiomers of methyl jasmonate and its derivatives[J]. Biosci. Biotech. Biochem., 1992(56): 1172-1173.
[32] FUJINO K, KODA Y, KIKUTA Y. Reorientation of cortical microtubules in the sub-apical region during tuberization in single-node stem segments of potato in culture[J]. Plant Cell Physiol., 1995(36): 891-895.
Editor: Yingzhi GUANG
Proofreader: Xinxiu ZHU
- 农业生物技术(英文版)的其它文章
- Study on the Photosynthetic Characteristics of Six Varieties (Strains) in Chinese Chestnut
- Effects of Abscisic Acid on Cold Resistance in Digitaria sanguinalis (L.) Scop.
- Mapping of Purple Gene in Spears of Asparagus (Asparagus officinalis L.)
- Evaluation of Trace Elements in the Soil of Typical Peach Orchards in Zunyi City
- Research on Soil Conservation and Improvement Technology in Zhaoyang District
- Analysis on Demonstration Application of Silicon Fertilizer in Field Cultivation of Rice

