Effects of Sintering Atmosphere on the Microstructure and Surface Properties of Symmetric TiO2Membranes*
ZHOU Shouyong (周守勇), ZHONG Zhaoxiang (仲兆祥), FAN Yiqun (范益群),** XU Nanping (徐南平) and HE Yuehui (贺跃辉)
Effects of Sintering Atmosphere on the Microstructure and Surface Properties of Symmetric TiO2Membranes*
ZHOU Shouyong (周守勇)1, ZHONG Zhaoxiang (仲兆祥)1, FAN Yiqun (范益群)1,** XU Nanping (徐南平)1and HE Yuehui (贺跃辉)2
1State Key Laboratory of Materials-Oriented Chemical Engineering, College of Chemistry and Chemical Engineering, Nanjing University of Technology, Nanjing 210009, China2State Key Laboratory for Powder Metallurgy, Central South University, Changsha 410083, China
The effects of sintering atmosphere on the properties of symmetric TiO2membranes are studied with regard to sintering behavior, porosity, mean pore size, surface composition, and surface charge properties. The experimental results show that the symmetric TiO2membranes display better sintering activity in the air than in argon, and the mean pore diameters and porosities of the membrane sintered in argon are higher than those of the membrane sintered in the air at the same temperature. The surface compositions of the symmetric TiO2membrane sintered in the air and in argon at different temperatures, as studied by X-ray photoelectron spectroscopy, are discussed in terms of their chemical composition, with particular emphasis on the valence state of the titanium ions. The correlation between the valence state of the titanium ions at the surface and the surface charge properties is examined. It is found that the presence of Ti3+, introduced at the surface of the symmetric TiO2membranes by sintering in a lower partial pressure of oxygen, is related to a significant decrease in the isoelectric point. TiO2with Ti4+at the interface has an isoelectric point of 5.1, but the non-stoichiometric TiO2-xwith Ti3+at the interface has a lower isoelectric point of 3.6.
TiO2membrane, sintering atmosphere, sintering behavior, surface, microstructure
1 Introduction
Composite membranes consisting of a ceramic layer on a porous metal support advantageously combine the properties of the porous metal and the ceramic membrane. Metal supports are easily fixed,.., by welding, crimping, or brazing, to a module. Ceramic membrane layers enable the elaboration of finer microstructures because submicron ceramic powders are easier to be handled than metal powders of the same particle size [1]. Furthermore, ceramic membrane layers possess some advantages, such as stability at high temperatures, resistance to high pressure, good chemical stability, high mechanical resistance, long life, good defouling properties, and well-defined stable pore structure [2]. Titania can be sintered at a lower temperature compared with Al2O3and ZrO2, it shows good adhesion to metal supports, and it has a narrow particle size distribution. Thus, titania is usually selected as the ceramic membrane layer material to prepare composite membranes supported on porous metal supports [3, 4].
以水资源消耗 (LNTW)为自变量,人口城镇化 (LNPOP)、经济城镇化 (LNINC)、产业城镇化 (LNIND)为因变量,利用式 (2)设立的模型对4个区域的面板数据进行固定效应面板模型的估计,得到的估计结果如表6所示。根据面板固定效应估计结果,四大区域的面板回归模型的R2与F值都很高,说明各方程的拟合效果很好。
Sintering is a key step in the preparation of composite membranes consisting of a ceramic layer on a porous metal support. In order to protect metallic supports from oxidation, sintering has to be carried out in a reducing or inert atmosphere, or in vacuo. However, as TiO2is an oxygen-deficient compound, the temperature and the oxygen partial pressure can greatly affect its crystal structure, chemical composition, and properties. Its properties are determined by the degree of non-stoichiometry and the concentrations of various defects that may exist in its lattice. In addition, the properties of the TiO2surface may be greatly affected by the bulk structure and defects [5, 6]. At high temperatures, Ti interstitials are regarded as the major defects [7].
这个效果并不一定要求使用绝对的纯黑和纯白色,只要足够深的黑和足够浅的白放在一起就能得到不错的效果。阈值调整图层只有一个控制选项——阈值色阶。使用这个滑块我们可以直接更改画面的黑白分界点。这个效果最适合本例中这种在纯色背景下拍摄的肖像,效果非常像装饰画。
Many efforts have been made to investigate the formation of non-stoichiometric TiO2-x[8-11] and the nature of its defects [12-14], as well as its electronic properties [15], photoelectrochemical properties [16], and wettability [17]. Kuscer. [18] showed that the presence of Ti3+, introduced at the titanium-oxygen ceramic surface by sintering at a lower partial pressure of oxygen, significantly improved the wettability of the sintered ceramics. TiO2ceramics with exclusive Ti4+at the interface showed a water-contact angle of 67°, but non-stoichiometric TiO2-xwith Ti3+at the interface showed a lower water-contact angle of 26°. In the preparation of composite membranes consisting of a titania layer on a porous nickel alloy support, it was found that a new non-stoichiometric titanium oxide phase of type TiO2-x,.. Ti4O7, was formed [19]. In the preparation of the supported TiO2/Ti-Al composite membrane, we also found that the reduction of TiO2led to the formation of the non-stoichiometric titanium oxide phase of type TiO2-x,.. Ti4O7, during sintering of the functional layer in argon atmosphere [20].
消费心理是指消费观。从经济学角度来对年轻人不正确的消费心理进行认识,不仅能更好地了解消费心理的内涵,也可以为年轻人正确判断自身消费行为提供相关依据。当然,消费心理作为衡量人们消费习惯的标准,集中展现了人们在消费过程中个人心理行为的具体表现,人们的消费观念会根据人们的收入水平以及社会风气等发生变化。所以在研究年轻人的消费心理时,需要从多个方面出发,比如:要从当前社会氛围、对购买活动的态度等多个方面,进行综合考虑。就目前年轻人在成长过程中的诸多现象看,存在很多不健康的消费心理。归纳起来,主要表现在以下几方面:
However, little attention has been paid to the detailed effects of the sintering atmosphere on the sintering behavior, microstructure, and surface properties of titania membranes. In fact, this knowledge may eventually help us to produce composite membranes consisting of a TiO2layer on a porous metal support with reliable and desirable properties for industrial applications.
The variation in the porosity of the symmetric TiO2membranes with temperature is shown in Fig. 3. The porosities of the membranes sintered in the air and in argon decreased as the sintering temperature increased from 1025 to 1075°C. However, at the same sintering temperature, the porosity of the symmetric TiO2membrane sintered in argon was higher than that of the membrane sintered in the air. This is consistent with the results concerning the sintering behavior of TiO2in the air and in argon. Kuscer. found that a TiO2ceramic sintered in Ar/H2(7%) was more porous than that sintered in the air at 1400°C for 2 h. The variation in mean pore diameter with temperature for the membranes is shown in Fig. 4. The results indicate that the mean pore diameter of a symmetric TiO2membrane sintered in argon is larger than that of a membrane sintered in the air at the same temperature. The differences in porosities and mean pore diameters stem from the fact that the shrinkage of the TiO2membrane in argon is smaller than that in the air at the same temperature.
Therefore, the objective of this work is to apply different sintering atmosphere, and investigate their effects on the properties of the resulting symmetric titania membranes. The effect on the microstructure of the symmetric titania membranes is investigated through the changes in porosity and mean pore size. Other surface properties of symmetric titania membranes sintered in the air or in argon are also investigated. The chemical compositions of the titania membranes are measured by XRD and XPS. The effect of sintering in the air or argon on the surface charge properties of titania are also investigated.
经过实际的“计算机网络”课程的教学使用,这种基于Moodle平台下的探究式学习模式比较适合计算机应用类高等教育课程,通过这种学习模式的构建和使用使学生的主体意识有了明显的提升,使原先的被动学习转化为主动学习、自主探究学习,提高了探究学习和自主学习的能力,学生之间合作学习的氛围也逐渐形成,学习效率有了大幅提高.
2 Experimental
2.1 Materials and fabrication of symmetric membranes
Rutile titania powder with an average particle size of 0.3mm was used in this work. The average particle size and the particle size distribution were determined using a Zetersizer 3000HSA (Malvern Instruments Ltd., England). All powders were used without further treatment. Polyethylenimine with an average molecular weight of 10000 (Aldrich, USA) was used as a dispersant. Methylcellulose was used as a binder.
2.2 Fabrication of symmetric membranes
Sintering behavior is an important property of a precursor material, which determines the microstructure and performance of the final material. Thus, we investigated the sintering behavior of symmetric titania membranes in the air and argon atmosphere. Dilatometry is an effective means of measuring the sintering behavior of a material. Sintering curves for the TiO2membranes in the air and argon up to 1125°C are shown in Fig. 1. The TiO2membranes show similar sintering curves in the temperature range 25-1125°C in the air and argon atmosphere. The onset temperature for both cases was 960°C. However, Fig. 1 (a) also shows that the shrinkage of the TiO2membrane sintered in argon is less than that in the air at the same temperature, and the difference between the two curves becomes more pronounced with increasing temperature. This can be seen clearly in Fig. 1 (b), in which the sintering rates of the TiO2membranes in the air and argon rapidly increase with temperature from 960 to 1070°C. However, compared to the shrinkage rate for the TiO2in the air, the shrinkage rate for the TiO2in argon is lower in the temperature range from about 1000 to 1125°C. The sintering rate of the TiO2membrane in argon at 1070°C is about 84.5% of that in the air at the same temperature, indicating that the TiO2membranes sintered in the air have a better sintering activity, which can be explained as follows. Sintering process is a diffusion process of atoms and/or ions, which happens when an atom/or ion gains sufficient energy to leave its mooring and migrate. As for the sintering of TiO2, the oxygen grain boundary diffusion is fast and therefore the cationic diffusion is the rate limiting step [22]. That is, the titanium bulk diffusion controls the kinetics of the sintering of TiO2. At high temperature and in argon (low oxygen partial pressure), the material will lose part of oxygen and form oxygen vacancies to maintain the electroneutrality, at the same time high valence Ti in TiO2is reduced to lower valence states. Thus, the diffusion rates of metal ions are suppressed in argon atmosphere. In addition, the lower valence cations increase the ion radius, and then reduce the cation diffusion rate. Furthermore, this result can be used to constitute proper sintering process for titania composite membranes.
2.3 Characterization
The surfaces of the TiO2symmetric membranes were analyzed by means of X-ray photoelectron spectroscopy (XPS, ESCALAB 250, Thermo-VG Scientific). All such spectra were recorded using monochromated Al-Kαradiation (1486.8 eV), with a pass energy of 20 eV. The shift in binding energy due to relative surface charging was corrected for by using the C1s level at 285.0 eV as an internal standard. The surface composition was calculated from the XPS intensities using the relative sensitivity factors provided by the instrument manufacturer.
The average pore sizes and porosities of the symmetric membranes were estimated by mercury porosimetry (Poremaster GT-60, Quantachrome, USA). The crystal structure of the sintered membranes was studied by X-ray diffraction analysis (XRD, Bruker D8 Advance) using Cu-Kαradiation, with 2from 20°to 80°. The microstructure of the synthesized membranes was observed by scanning electron microscopy (SEM, LEO 1530VP).
Samples used to study the sintering behavior of TiO2membranes were of dimensions 6 mm×6 mm× 2 mm. Dilatometric investigations were performed with a dilatometer (model DIL 402C, Netzsch, Germany) in either air or argon, as in the sintering studies, with a constant heating rate of 1°C·min-1up to 1125°C. The flow rate of sintering atmosphere was kept at 30 ml·min-1(STP).
The zeta potentials of the TiO2powders were determined using a Zetersizer 3000HSA. For these measurements, samples were prepared at solid contents of 0.1 g·L-1in a 1×10-3mol·L-1solution of NaCl to maintain constant ionic strength. The pH values were adjusted from 2 to 12 using either 0.1 mol·L-1HCl or 0.1 mol·L-1NaOH solutions. Prior to the zeta potential measurements, the suspensions were aged for 24 h at room temperature to reach surface equilibrium, using an orbital shaker. All suspensions were prepared using an ultrasound probe for homogenization.

Figure 1 Sintering behavior of titania membranes in the air and argon atmosphere from 25 to 1125°C at a heating rate of 1°C·min-1

3 Results and Discussion
3.1 Sintering behavior of symmetric titania membranes
The titania powder was dispersed in pure water, along with different amount of polyethylenimine as a dispersant, and milled in a ball mill for 12 h. The grinding media were high-purity corundum balls. The binder methylcellulose was then added and the suspension was stirred for 50 min at 16.66 Hz (1000 r·min-1). Symmetric membranes were prepared on plaster supports and then separated from these supports before sintering [21]. The membranes were dried for 12 h at room temperature, 70 or 110°C, and were then sintered in either air or argon for 3 h at 1025, 1050 or 1075°C in an electric furnace with a heating and cooling rate of 1°C·min-1.
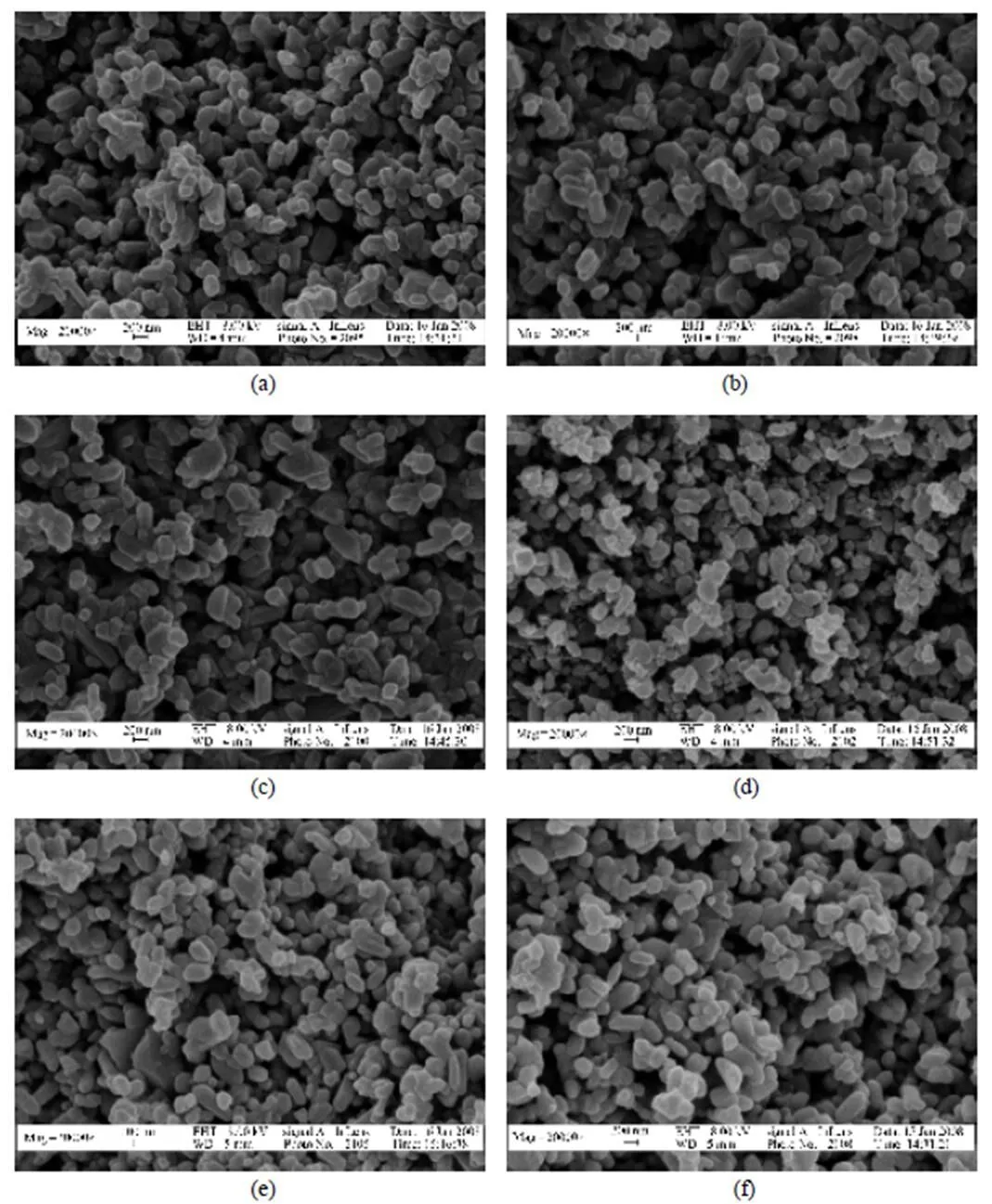
Figure 2 SEM image of the surface of titania membranes
[(a), (b) and (c) sintered in the air for 3 h at 1025, 1050 and 1075°C, respectively; (d), (e) and (f) sintered in argon for 3 h at 1025, 1050 and 1075°C, respectively]
3.2 Microstructure of symmetric titania membranes
如何解决这个问题?我觉得,要先从调结构做起。关于结构调整,首先必须改变机关化的倾向。调研中,武汉市关于新招录的人员都要到基层先干两年再回机关的做法,十分符合实际。没有基层经验的人,可以把组织关系、行政关系放在机关,那没问题,但是人你得下基层,到基层实践、接受锻炼,这样不仅会改变我们整个司法行政系统的队伍结构,也会增加基层力量。机关干部扎扎实实在基层呆两年,来了新的同志再下去呆两年。这样的做法,首先确保基层两年的力量有了保证,其次把这种做法形成一种制度,基层力量不足的情况就会不断得到改善。
翠姨的妹妹有一张,翠姨有一张,我的所有的同学,几乎每人有一张。就连素不考究的外祖母的肩上也披着一张,只不过披的是蓝色的,没有敢用那最流行的枣红色的就是了。因为她总算年纪大了一点,对年轻人让了一步。
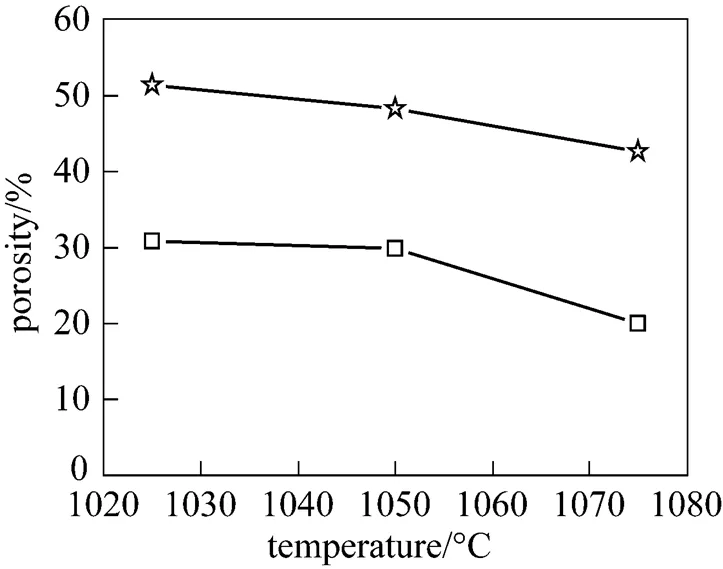
Figure 3 Porosity as a function of sintering temperature of titania membranes
□ TiO2membrane in air;☆ TiO2membrane in argon
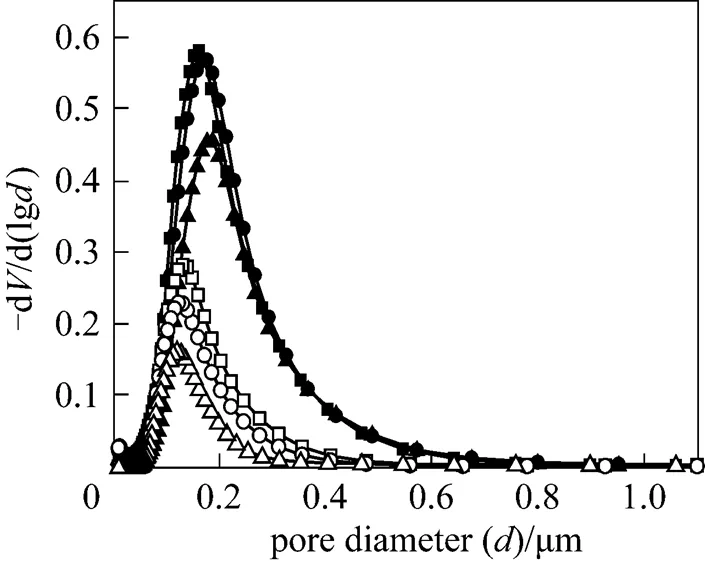
Figure 4 Pore size distribution of titania membranes
□ TiO2membrane in air, 1025°C;○ TiO2membrane in air, 1050°C;△ TiO2membrane in air, 1075°C;■ TiO2membrane in argon, 1025°C;● TiO2membrane in argon, 1050°C;▲ TiO2membrane in argon, 1075°C
3.3 Surface compositions of symmetric titania membranes
It is well known that the sintering atmosphere may appreciably affect the densification process and the final microstructure in the fabrication of ceramics. SEM images of the surfaces of symmetric TiO2membranes sintered in the air and in argon for 3 h are shown in Fig. 2. It can be observed that the TiO2particles fuse together with increasing sintering temperature, and all of the sintered TiO2membranes are porous. In addition, the TiO2membrane obtained in the air is seen to be more compact than that obtained in argon at the same temperature.
The compositions of the titania membranes were measured by XPS. XPS survey spectra of the TiO2membranes sintered in argon at 1025, 1050, and 1075°C, along with that of TiO2, are shown in Fig. 6. Ti and O are detected at the surface for all of the samples.
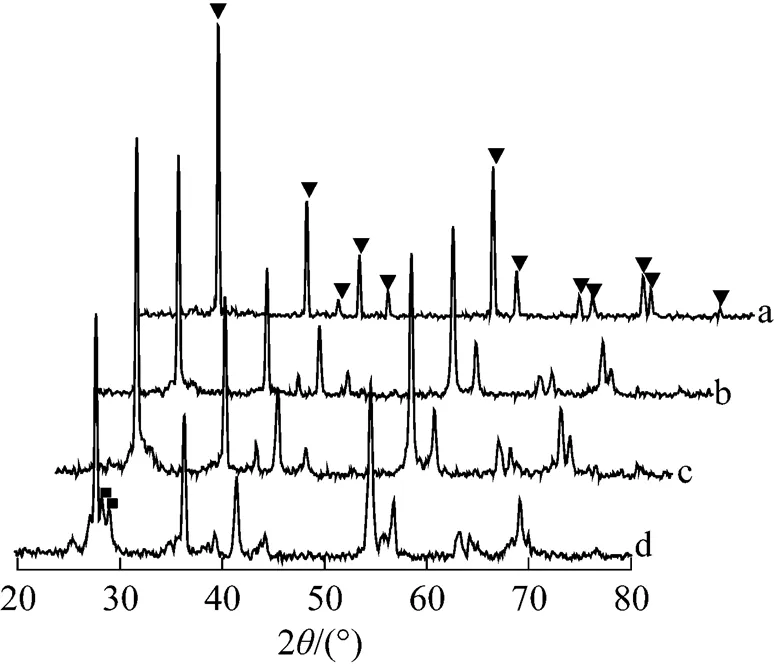
Figure 5 XRD patterns of TiO2and TiO2membranes sintered in argon for 3 h
(a) TiO2; (b) 1025°C; (c) 1050°C; (d) 1075°C;▼ rutile peak; ■ TiO2-xpeak
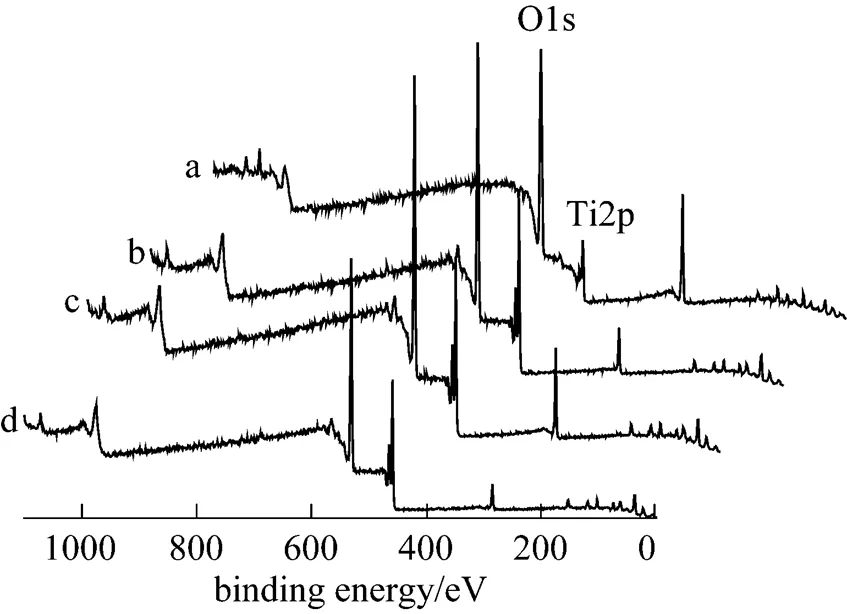
Figure 6 XPS survey spectra from TiO2and the TiO2membranes sintered in argon
(a) TiO2; (b) 1025°C; (c) 1050°C; (d) 1075°C
In order to investigate the precise nature of the TiO2structure, we examined the XPS spectra of Ti2p3/2species obtained by deconvolution of the TiO2peak, as shown in Fig. 7. If TiO2has perfect stoichiometry, then the Ti2p3/2peak should be fitted with only TiO2species with a binding energy of about 458.8 eV. Fig. 7 (a) shows the Ti2p region of the stoichiometric TiO2surface. In the spectra with a carefully calibrated energy scale, the Ti2p3/2peak, attributed to (formally) Ti4+, is located at 458.39 eV. However, curve-fitting of Ti2p3/2of the surface of the TiO2membranes sintered in argon at 1025, 1050, and 1075°C using only TiO2species did not give a satisfying result. Some deviations were evident in the range of 457.63-457.81 eV. According to the XPS database showing binding energies of titanium compounds [23], the binding energies for TiO1.5, TiO, and Ti are 456.1, 455.1, and 454 eV, respectively. The lower the number of oxygen atoms bound to Ti in a given species, the lower the binding energy of this species. Therefore, in the case of the TiO2membranes sintered in argon, the Ti2p3/2peak positions located at 459, 458.9, and 458.81 eV are typical of Ti4+in TiO2, in accordance with the literature data [24]. However, the Ti2p3/2peaks at 457.63, 457.8, and 457.81 eV,.. lower than 458.8 eV of TiO2species, could best be deconvoluted by invoking the presence of another species. The newly added species is considered to be Ti3+, namely TiO2-xas a non-stoichiometric oxide. Table 1 shows the areas and binding energies of the deconvoluted peaks of Ti2p3/2for the TiO2samples, along with the TiO2-x/TiO2ratios calculated from the areas, which give an indication of the degree of non-stoichiometry. The TiO2-x/TiO2ratios of the surfaces of the TiO2membranes sintered in argon at 1025, 1050, and 1075°C were around 10%.
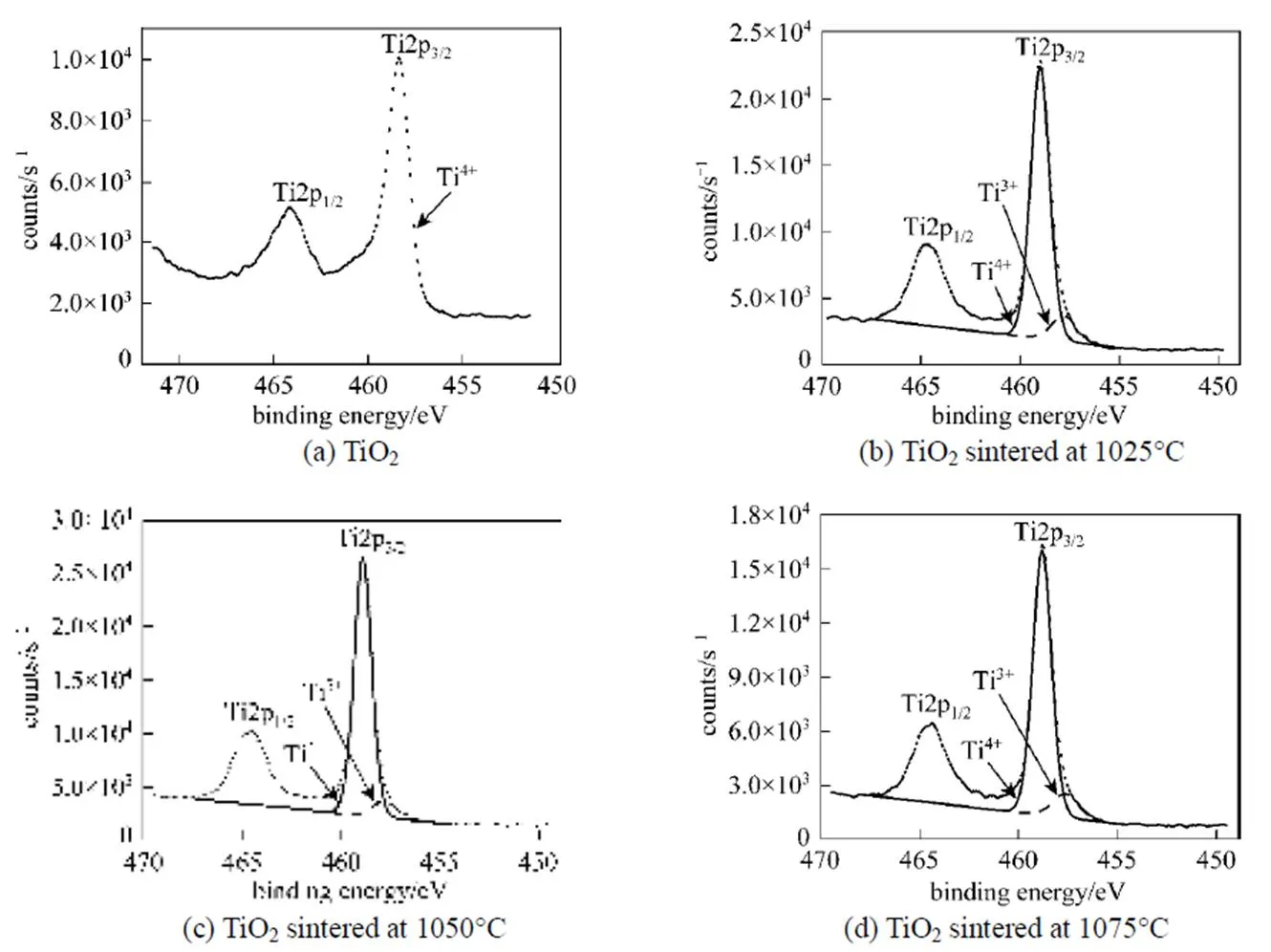
Figure 7 Deconvolute XPS Ti 2p3/2peaks of TiO2and the TiO2membranes sintered in argon


Table 1 Areas and binding energies of deconvoluted peaks of Ti2p3/2 of XPS of TiO2 samples, and calculated TiO2-x/TiO2 and relative atomic mole ratios of TiO2
3.4 Surface charge properties of titania
It is generally recognized that the surface charge characteristics of membranes is an important factor in determining their separation performances and fouling tendencies [25, 26]. The zeta potential is an important and reliable indicator of the surface charge of membranes [27-29]. The effects of pH on the zeta potential of the non-stoichiometric TiO2-xpowders sintered in argon at 1025, 1050, and 1075°C, as well as that of as-purchased rutile TiO2, are illustrated in Fig. 8. The isoelectric points (IEP, the pH value at which the zeta potential equals zero) of the non-stoichiometric TiO2-xpowders obtained at different temperatures are almost the same, at pH 3.6, but the IEP of the rutile TiO2is 5.1. Furthermore, the zeta potentials of the non-stoichiometric TiO2-xare larger than that of the as-purchased rutile TiO2. The significant IEP and zeta potential shifts are attributable to different chemical compositions at the surfaces. The zeta potential is very sensitive to the surface density of OH groups because their dissociation is the underlying mechanism for surface charge creation. The defect structure due to oxygen vacancies on non-stoichiometric TiO2affects the adsorption of water at the surface and the rate of dissociation of the water into hydroxyl groups and protons [30]. TiO2incorporating some TiO2-xresulting from the defect structure may have more OH groups on its surface. XPS results show that only Ti4+ions are present at the TiO2surface. Without Ti3+ions present at the surface, the formation of oxygen vacancies is not expected. However, for the non-stoichiometric TiO2-xsurface, Ti3+states have been detected by XPS analyses. In order to achieve electroneutral conditions in the non-stoichiometric TiO2-x, in addition to Ti3+, the formation of oxygen vacancies is then expected. As reported in the literature [31, 32], water molecules can be easily incorporated at an oxygen-vacancy- containing surface and cause an increase in the adsorbed hydroxyl group density. The surface charge properties of membranes can exert profound influence on the nature and magnitude of the interactions between the membrane and the solution, thus affecting its filtration properties such as permeability and permselectivity. As the non-stoichiometric TiO2-xmembrane has more surface charges than TiO2membrane, it can produce greater electrostatic repulsion during filtration of the same type of charge species and inhibit the formation of a fouling layer on the membrane surface, leading to a higher permeate flux. Certainly, this result also can be used to predict surface charge properties of titania composite membranes and filtration properties such as permeability and permselectivity.

Figure 8 Zeta potential of TiO2particles in 10-3mol·L-1NaCl solution as a function of pH
□ TiO2; ○ TiO2, Ar, 1025°C, 3 h; △ TiO2, Ar, 1050°C, 3 h;▽ TiO2, Ar, 1075°C, 3 h
4 Conclusions
The effects of sintering atmosphere on the properties of TiO2membranes were studied with regard to sintering behavior, porosity, mean pore size, surface composition, and surface charge properties.
The shrinkage of a TiO2membrane sintered in argon was found to be smaller than that in the air at the same temperature, and this disparity became more pronounced with increasing temperature. Compared to the shrinkage rate of the TiO2membrane sintered in the air, the corresponding shrinkage rate with argon is smaller between about 1000 and 1125°C. That is to say, the TiO2membrane sintered in the air showed better sintering activity.
The mean pore diameters and porosities of TiO2membranes sintered in argon were larger than those of membranes sintered in the air.
The presence of Ti3+, introduced at the surface of the TiO2membranes by sintering at a lower partial pressure of oxygen, was found to be related to a significant decrease in the isoelectric point. TiO2with exclusive Ti4+at the interface showed an isoelectric point of 5.1, but the non-stoichiometric TiO2-xwith Ti3+at the interface showed a lower isoelectric point of about 3.6.
1 Meulenberg, W.A., Mertens, J., Bram, M., Buchkremer, H.P., Stöver, D., “Graded porous titania membranes for microfiltration”,...., 26, 449-454 (2006).
2Benfer, S., Árki, P., Tomandl, G., “Ceramic membranes for filtration applications—Preparation and characterization”,..., 6, 495-500 (2004).
3Zhao, L., Bram, M., Buchkremer, H.P., Stöver, D., Li, Z., “Preparation of TiO2composite microfiltration membranes by the wet powder spraying method”,..., 244, 107-115 (2004).
4Bram, M., Zhao, L., Buchkremer, H.P., Stover, D., “Graded hybrid membrane for microfiltration”,.., 29, 295-300 (2005).
5Diebold, U., “The surface science of titania dioxide”,..., 48, 53-229 (2003).
6Millot, F., Blanchin, M.G., Tetot, R., Marucco, J.F., Poumellec, B., Picard, C., Touzelin, B., “High temperature nonstoichiometric rutile TiO2-x”,.., 17, 263-293 (1987).
7Marucco, J.F., Gautron, S., Lemasson, P., “Thermogravimetric and electrical study on non-stoichiometric titanium oxide TiO2−xbetween 800 and 1100°C”,..., 42, 363-367 (1981).
8Luca, D., Macovei, D., Teodorescu, C.M., “Characterization of titania thin films prepared by reactive pulsed-laser ablation”,.., 600, 4342-4346 (2006).
9Kumar, D., Chen, M.S., Goodman, D.W., “Characterization of ultra-thin TiO2films grown on Mo(112)”,, 515, 1475-1479 (2006).
10Matsumoto, T., Batzill, M., Hsieh, S., Koel, B.E., “Fundamental studies of titanium oxide-Pt(100) interfaces I. Stable high temperature structures formed by annealing TiOfilms on Pt(100)”,.., 572, 127-145 (2004).
11Tsuyumoto, I., Hosono, T., Murata, M., “Thermoelectric power in nonstoichiometric orthorhombic titanium oxides”,...., 89, 2301-2303 (2006).
12Bowker, M., “The surface structure of titania and the effect of reduction”,......, 10, 153-162 (2006).
13Li, M., Hebenstreit, W., Diebold, U., Tyryshkin, A.M., Bowman, M.K., Dunham, G.G., Henderson, M.A., “The influence of the bulk reduction state on the surface structure and morphology of rutile TiO2(110) single crystals”,...., 104, 4944-4950 (2000).
14Nowotny, J., Bak, T., Nowotny, M.K., Sheppard, L.R., “Defect chemistry and electrical properties of titanium dioxide (1) Defect diagrams”,...., 112, 590-601(2008).
15Hazra, S.K., Tripathy, S.R., Alessandri, I., Depero, L.E., Basu, S., “Characterizations of porous titania thin films produced by electrochemical etching”,...., 131, 135-141 (2006).
16Nowotny, M.K., Sheppard, L.R., Bak, T., Nowotny, J., “Defect chemistry of titanium dioxide. Application of defect engineering in processing of TiO2-based photocatalysts”,...., 112, 5275-5300 (2008).
17Feng, A., McCoy, B.J., Munir, Z.A., Cagliostro, D., “Wettability of transition metal oxide surfaces”,...., 242, 50-56 (1998).
18Kuscer, D., Kovač, J., Kosec, M., Andriesen, R., “The effect of the valence state of titanium ions on the hydrophilicity of ceramics in the titanium-oxygen system”,...., 28, 577-584 (2008).
19Gill, J.A., “Filter element with porous nickel based alloy substrate and metal oxide membrane”, W.O. Pat., 0226366 (2002).
20Zhou, S.Y., Fan, Y.Q., He, Y.H., Xu, N.P., “Preparation of titania microfiltration membranes supported on porous Ti-Al alloys”,..., 325, 546-552 (2008).
21Feng, J., Fan, Y.Q., Qi, H., Xu, N.P., “Co-sintering synthesis of tubular bilayer α-alumina membrane”,..., 288, 20-27 (2007).
22Odier, P., Lecomte, J., Loup, J.P., “Effect of non-stoichiometry on the sintering of doped TiO2”,..., 23, 4547-4553 (1988).
23Mouler, J.F., Stickle, W.F., Sobol, P.E., Bomben, K.D., Handbook of X-ray Photoelectron Spectroscopy, Physical Electronics Inc (1995).
24Mayer, J.T., Diebold, U., Madey, T.E., Garfunkel, E., “Titanium and reduced titania overlayers on titanium dioxide (110)”,....., 73, 1-11 (1995).
25Nyström, M., Lindstrom, M., Matthiasson, E., “Streaming potential as a tool in the characterization of ultrafiltration membranes”,., 36, 297-312 (1989).
26Clark, W.M., Bansal, A., Sontakke, M., Ma, Y.H., “Protein adsorption and fouling in ceramic ultrafiltration membranes”,..., 55, 21-38 (1991).
27Zhao, Y.J., Xing, W.H., Xu, N.P., Wong, F.S., “Effects of inorganic electrolytes on zeta potentials of ceramic microfiltration membranes”,..., 42, 117-121 (2005).
28Zhang, Q., Jin, W.Q., Fan, Y.Q., Xu, N.P., “An improved Parks equation for prediction of surface charge properties of composite ceramic membranes”,..., 318, 100-106 (2008).
29Wang, Z., Zhao Y.Y., Ye, N., Wang, J.X., Zhao, Z.P., Wang, S.C., “Evaluation of four measurement operation modes of streaming potential for microfiltration and ultrafiltration Membranes”,...., 14, 456-463 (2006).
30Kim, W.W., Lee, E.H., Kim, Y.J., Lee, M.H., Kim, K.H., Shin, D.W., “A relation between the non-stoichiometry and hydroxyl radical generated at photocatalytic TiO2on 4CP decomposition”,...:, 159, 301-310 (2003).
31Fujishima, A., Rao, T.N., Tryk, D.A., “Titanium dioxide photocatalysis”,...:.., 1, 1-21 (2000).
32Carp, O., Huisman, C.L., Reller, A., “Photoinduced reactivity of titania dioxide”,.., 32, 33-177 (2004).
2008-11-20,
2009-04-09.
the National Basic Research Program of China (2003CB615707) and the National Natural Science Foundation of China (20636020).
** To whom correspondence should be addressed. E-mail: yiqunfan@njut.edu.cn
 Chinese Journal of Chemical Engineering2009年5期
Chinese Journal of Chemical Engineering2009年5期
- Chinese Journal of Chemical Engineering的其它文章
- Molecular Simulation of CO2/H2 Mixture Separation in Metal-organic Frameworks: Effect of Catenation and Electrostatic Interactions*
- Deactivation Kinetics of Nitrile Hydratase in Free Resting Cells*
- Corrosion Behavior of TP316L of Superheater in Biomass Boiler with Simulated Atmosphere and Deposit
- Influence of A-type Zeolite on Methane Hydrate Formation*
- Improvement of Isomerization Process of Crude Isoamylene with Tertiary-amyl-alcohol Addition
- Prediction of Thermophoretic Deposition Efficiency of Particles in a Laminar Gas Flow along a Concentric Annulus: A Comparison of Developing and Fully Developed Flows
