Improvement of Isomerization Process of Crude Isoamylene with Tertiary-amyl-alcohol Addition
Xu Zehui (徐泽辉)*, Xia Ronghui (夏蓉晖), Guo Shizhuo (郭世卓), Fan Cunliang (范存良) and FANG Dingye (房鼎业)
Improvement of Isomerization Process of Crude Isoamylene with Tertiary-amyl-alcohol Addition
Xu Zehui (徐泽辉)1,2,*, Xia Ronghui (夏蓉晖)1, Guo Shizhuo (郭世卓)1, Fan Cunliang (范存良)1and FANG Dingye (房鼎业)2
1SINOPEC Shanghai Petrochemical Company Limited, Chemical Research Institute, Shanghai 200540, China2Department of Chemical Engineering, East China University of Science and Technology, Shanghai 200237, China
The present work contributed to a new developed production method for enhancing the quality of isoamylene (IA) by adding a small amount of tertiary amyl alcohol (TAA) to the catalyst of strong acid cation exchange resin. TAA improved the selectivity of 2-methyl-2-butene (2M2B) at a high conversion level for the isomerization of IA. Compared with the other results from the current IA units, the conversion of 2-methyl-1-butene (2M1B), the mass ratio of 2M2B to 2M1B and the selectivity of 2M2B were increased from 0.5474, 7.32 and 0.6864 to 0.72, 12 and 0.95, respectively, while the dimers content in the products decreased from 4.38% to below 1.0%. Optimized conditions for IA isomerization consisted of temperature between 28 and 33°C and system pressure of 0.5 MPa, weight hourly space velocity of 8.0 h-1with TAA mass fraction of 0.7%-0.9% in raw material. The results in lab supported bases for the developed process in industrial application which was later proved to be successful. In addition, a possible mechanism of the isomerization process was speculated to propose a key step of water formation in the TAA-added isomerization process and a verified experiment was conducted to support this speculation.
isoamylene, 2-methyl-1-butene, 2-methyl-2-butene, isomerization process, tertiary amyl alcohol
1 INTRODUCTION
Isoamylene (abbreviated as IA) is an important fine chemical material that mainly applied in the production of perfume and intermediate of pesticide [1]. Generally IA might term as a mixture of 2-methyl-2-butene (abbreviated as 2M2B) and 2-methyl-1-butene (abbreviated as 2M1B), and the former determines the reaction activity and application of the mixture. Therefore, the key technique to produce high quality IA is to increase its 2M2B content.
IA mainly exists in the C5of fluid catalytic cracked (FCC) gasoline or C5fractions from naphtha cracking for producing ethylene. Due to similar boiling points, conventional distillation could not separate IA from the other compounds with high purity. Thus, the present method usually takes C5fractions separated from diolefin as raw materials to react with methanol by etherification over acid catalyze to form-amyl methyl ether (abbreviated as TAME) [2-11], which goes through cracking at high temperature followed by water-washing and distillation orderly, and finally produce IA with high quality [12]. However, the content of 2M2B in such produced IA is relatively low with usual mass ratio of 2M2B/2M1B to be 1-5. To improve the quality of IA product, a gentle isomerization reaction can be employed to convert 2M1B to 2M2B of higher stability [13, 14].
During the isomerization process, exothermic dimerization reaction of 2M1B is the main side reaction which reduces the yield of objective product of 2M2B and increases the temperature of catalyst bed, consequently initiates the dimerization reaction of 2M2B, and thus makes the reaction process out of control. In order to restrain the dimerization reaction and enhance the yield, the present work developed a novel isomerization reaction process in which a small amount of-amyl alcohol (abbreviated as TAA) was added into the raw materials to limit the reaction rate of dimerization and thus enhance the yield with controllability of the reaction.
2 Experimental
2.1 Raw material and catalyst
The crude IA raw material was obtained from an annual 3kt IA plant in Chemical Research Institute of SINOPEC Shanghai Petrochemical Company Limited and the composition is listed in Table 1. TAA was from Shanghai Chemical Reagent Company of Shanghai Medical Group(CP reagent) with TAA of 99.5%, and water of 0.08%. Strongly acidic ion exchange resin catalyst was from Qilu Petrochemical Research Institute and its properties are shown in Table 2.
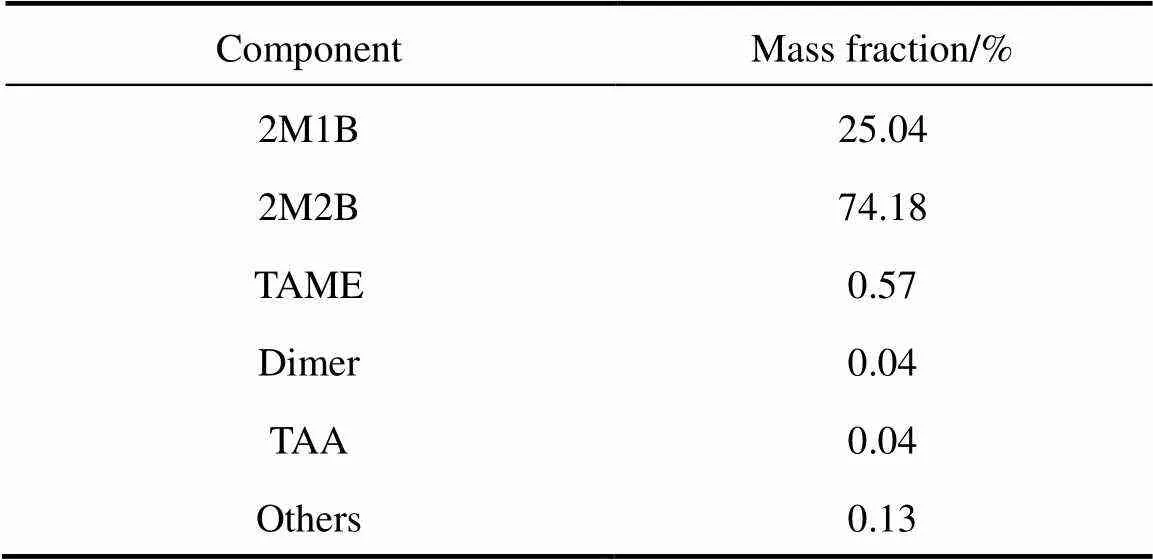
Table 1 The composition of crude isoamylene

Table 2 The specification of resin catalysts
2.2 Experimental
Isomerization reaction was performed in a tube fixed-bed reactor (25 mm×1500 mm) made of stainlesssteel. A 60 ml spherical catalyst (0.32 mm-1.25 mm, 37.56 g) was loaded into the reactor, the outside of which was equipped with a temperature-controlling jacket with hot water circulating. Platinum sensors were employed to measure the temperature from the upper, middle and lower layers in the catalyst bed. The feed rate of crude IA raw material was controlled by a pump and the system pressure was regulated by a back pressure valve.
2.3 Analysis
An HP4890 Chromatograph with a 50 m Hp-1 type capillary column was used for quantitative analysis. Column temperature was programmed to increase from 50°C to 240°C. The calculation method was shown as follows:
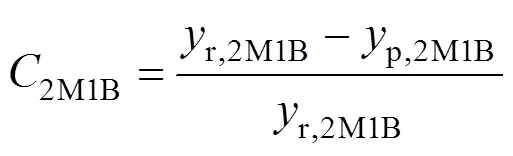

where2M1Bdenotes the conversion of 2M1B,r,2M1Bis the concentration of 2M1B in the raw material,p,2M1Bmeans the concentration of 2M1B in the products,2M2Brepresents the selectivity of 2M2B,p,2M2Bis the concentration of 2M2B in the products, andr,2M2Bis the content of 2M2B in the raw material. Note that dimerization reaction of 2M2B was regarded not to occur under such mild reaction conditions.
3 Results and discussion
3.1 Analysis of operation in production plant
Figure 1 displays the operation results of IA plant of Chemical Research Institute of Shanghai Petrochemical Company Limited in the period of 29th September to 31st December of 2007. The temperature of feedstock fluctuated between 20.3°C and 46.8°C and the system pressure between 0.51MPa and 0.52MPa with a weight hourly space velocity (WHSV) of 8.0 h-1.
The average concentrations of 2M1B, 2M2B and dimer consisted of 11.43%, 82.90% and 4.38% in the liquid products, respectively. As seen in Fig. 1, the mean conversion of 2M1B arrived at 0.5474 and the selectivity of 2M2B fluctuated around 0.6864 with a 2M2B/2M1B mass ratio of 7.32. During the operation period, the fluctuation of conditions was likely to overheat the catalyst bed, which consequently enhanced the dimerization side reaction of 2M2B, thus lowered its selectivity and facilitated drop of sulfonic acid group from the catalyst surface, and then decreased the lifetime of catalyst. Therefore, seeking a suitable assisted catalyst to prevent the side reaction of 2M2B dimerization is of high importance to improve the yield of objective product.
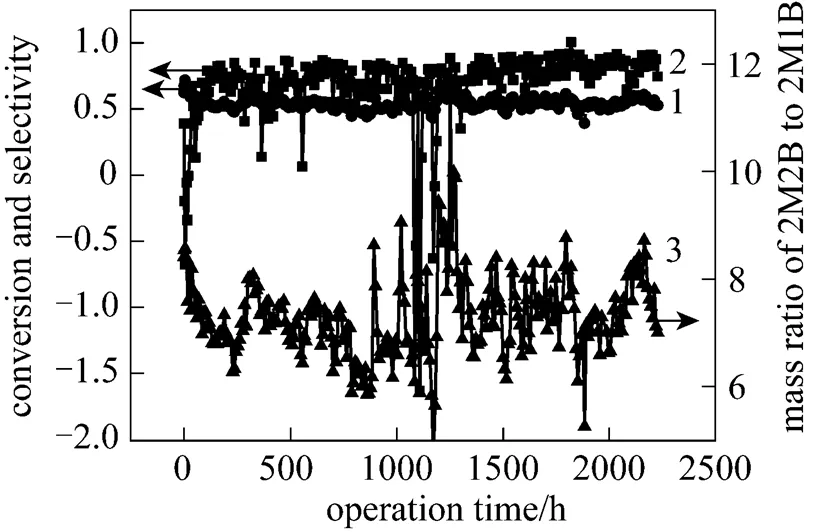
Figure 1 Operation results of isomerization process in isoamylene unit
1—conversion of 2M1B; 2—selectivity of 2M2B; 3—mass ratio of 2M2B to 2M1B
3.2 Idea for improving the isomerization of crude IA
The initial work indicated that the reaction kinetics of isobutene dimerization depends on the acidity of strong acidic ion exchange resin catalyst, and addition of-butyl alcohol could reduce the acid strength by being adsorbed on the catalyst surface, which lowered the reaction rate of isobutene dimerization but improved the selectivity of di-isobutylene. Based on this point, isomerisation of crude IA can be improved by adding TAA that has similar properties with-butylene and does not trouble the separation and purification of IA in the industrial plants.
A verified test was conducted in a 500 ml stirred tank reactor to investigate the effect of TAA on isomerisation of crude IA. 100 g of crude IA was added into the reactor with temperature up to 30°C and 2 g catalyst was employed to accelerate the reaction with continuous stirring. Fig. 2 shows the results of adding 0.6% TAA in the reactant.
As can be seen, addition of TAA decreased both the conversion of 2M1B slightly and the content of dimer significantly. Although TAA addition lowered the reaction rate, it enhanced the selectivity of 2M1B. The quality of products therefore could be obviously improved by adding TAA at a similar conversion of 2M1B.

Figure 2 Isomerization of crude isoamylene in absence/ presence of TAA
1—conversion of 2M1B in absence of TAA; 2—selectivity of 2M2B in absence of TAA; 3—conversion of 2M1B in presence of TAA; 4—selectivity of 2M2B in presence of TAA
3.3 Investigation on technological conditions of improved isomerization process for crude IA
The tank reactor displayed an excellent improvement effect for adding TAA into the raw materials. To further investigate the technological parameters, a fixed-bed reactor was used for supporting its application in industrial plants.
3.3.1
It was found that the interaction between the sulfonic group of the strongly acidic ion exchange resin catalyst and-butyl alcohol could reduce the acid strength of the sulfonic group and improve the surface environment, which benefits selectivity of objective product in initial work. The effect of TAA addition amount on the isomerization reaction was investigated under the conditions of feeding temperature of 33°C and system pressure of 0.5 MPa with WHSV of 8.0 h-1.
The results in Fig. 3 shows that both conversion of 2M1B and 2M2B/2M1B mass ratio decrease slowly with increasing TAA, while the selectivity of 2M2B enhances. It was in agreement with the results from the previous tank reactor. Coupled with the consideration of industrial cost and limitation of dimer content <1%, an optimized amount of TAA is located between 0.7% and 0.9%.
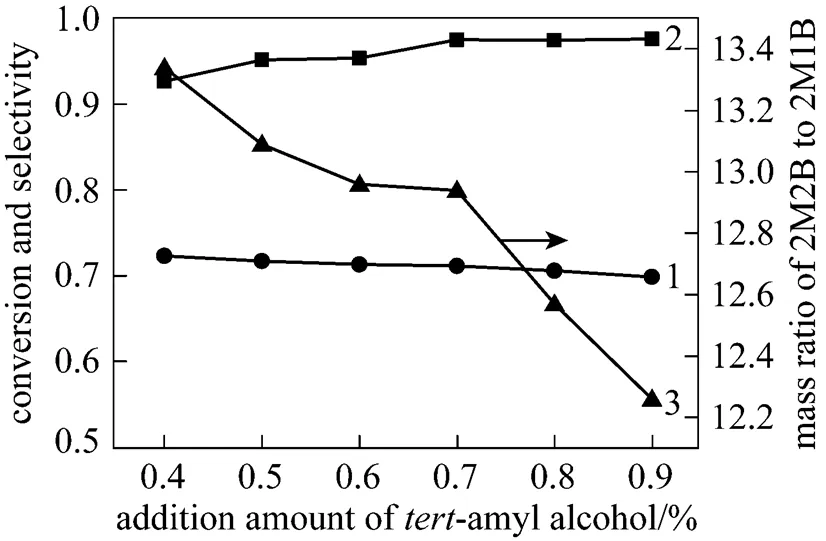
Figure 3 Effect of addition amount of-amyl alcohol on isomerization of crude isoamylene
1—conversion of 2M1B; 2—selectivity of 2M2B; 3—mass ratio of 2M2B to 2M1B
3.3.2
当前,社区大学教师专业发展支持较为缺乏,主要是社区提供的资源有限,因而学习实践社群是一种专业发展支持网络。社区大学要定期组织提升教师专业发展能力的活动,综合考虑社区大学的整体发展,结合教师实际需求,提供教学支持。并通过协同管理模式邀请社区大学教师、专家学者、社区工作者形成互助学习网络,以增加所关注内容的广度与深度,提供分享与支持的机会。由社区大学成员与教师共同规划相关活动,将有助于激发教师热情、提升参与度,提高教师对专业发展的积极性。
Reaction temperature was one of the key factors during the isomerization process in the operation of the isomerization unit of IA plant. Double-bond isomerization, and dimerization of IA and its polymerization could occur at certain temperature. The effect of temperature on the isomerization reaction was investigated under the conditions of 8.0 h-1and 0.5MPa with 0.5%TAA addition in the raw materials.
The results shown in Fig. 4 demonstrate that isomerization and dimerization can be suppressed at low temperatures. The content of dimer is significantly influenced by reaction temperature. The higher temperature facilitates to improve the dimer content without effect on the conversion of 2M1B. Hence, choosing lower temperature would improve the quality of products. However, in the industrial operation, the feedstock was ranged between 25-35°C due to environmental effect. Under the optimized temperature of 30°C, dimer consisted of 0.4%-0.7% in the products and conversion of 2M1B fluctuated between 0.72-0.74 with selectivity of 2M2B >0.95 and 2M2B/2M1B mass ratio >12. Additionally, temperature higher than 33°C would lower the 2M2B/2M1B ratio, implying that 2M2B suffered from dimerization.

Figure 4 Effect of reaction temperature on isomerization of crude isoamylene
1—conversion of 2M1B; 2—selectivity of 2M2B; 3—mass ratio of 2M2B to 2M1B
3.3.3
Since the acidity is related to the quantity of sulfonic acid group in the surface of strong acidic ion exchange resin catalyst, addition of TAA would theoretically reduce the acidity of catalyst surface and consequently lower the reaction rate of isomerisation. To match with the industrial operation parameters, conditions of 33°C and 0.5MPa with 0.5% TAA addition were employed to observe the effect of space velocity on the reaction and the results are shown in Fig. 5.
Figure 5 demonstrated that the WHSVs of 7.0 h-1to 9.0 h-1have little effect on the conversion of 2M1B and selectivity of 2M2B, both of which could meet the requirements of production. Thus, the present industrial plant (8.0 h-1) can be improved without significant change. Although the acidity of catalyst is reduced by adding TAA, it is still enough to catalyze the isomerization and has ability of suppressing dimerization of 2M1B. Therefore, it can be said that the macroporous strongly acidic ion exchange resin catalyst coupled with TAA is effective to catalyze isomerization of 2M1B.
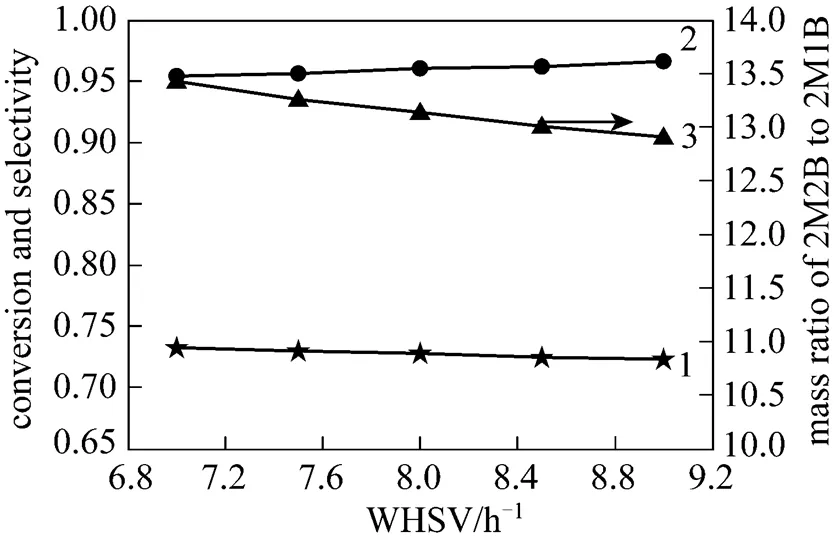
Figure 5 Effect of WHSV on isomerization of crude isoamylene
1—conversion of 2M1B; 2—selectivity of 2M2B; 3—mass ratio of 2M2B to 2M1B
3.3.4
The addition of TAA could eliminate the temperature runaway and lower the possibility of exfoliation of sulfonic group from the catalyst surface. However, since it is a new method, it is necessary to test the stability of the developed resin-TAA catalyst system for the purpose of its coming industrial application. The experiment was carried out under the conditions of 30°C for jacket water bath temperature, system pressure of 0.5 MPa, WHSV of 8.0 h-1and 0.6% TAA addition. The result is shown in Fig. 6.
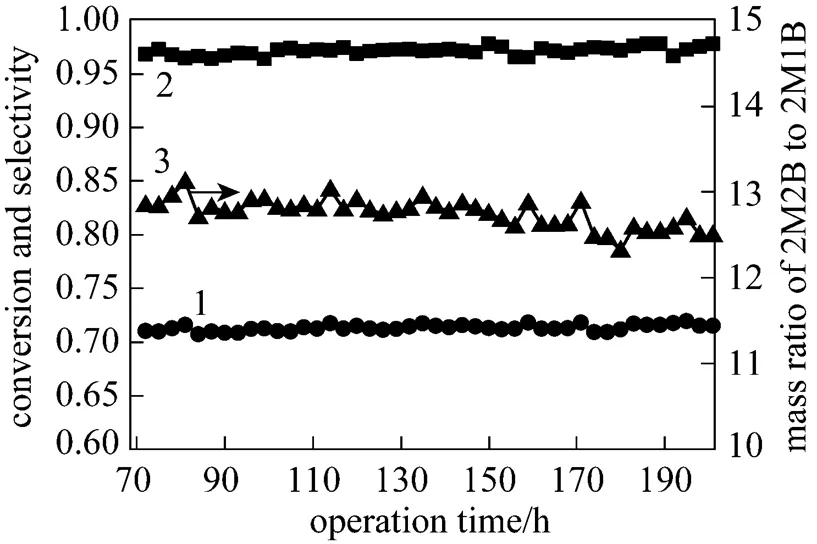
Figure 6 The stability experiment of catalyst for isomerization of 2M1B
1—conversion of 2M1B; 2—selectivity of 2M2B; 3—mass ratio of 2M2B to 2M1B
Figure 6 shows that during a period of 120 h, the catalyst possesses excellent activity and stability with high selectivity of 2M2B and less than 0.7% of dimers in the products. The temperatures are stabilized at 33°C, 37-38°C and 34°C for the start, middle and end of the catalyst bed, respectively.
3.4 Reaction mechanism

The reaction mechanism shown in Fig. 7 consists of dehydration of TAA and 2M2B formation by hydrogenating 2M1B with protonated TAA by water and its sequent dehydrogenation. An important reaction in the process is water formation from TAA dehydration, which enhances 2M2B selectivity and inhibits dimers production. To test the speculated mechanism, a verified experiment was conducted with addition of water instead of TAA under the conditions of 30°C and 5 g of catalyst as well as 100 g of IA raw materials with 0.1 g water addition at a stirring speed of 300 r·min-1. The results are shown in Fig. 8.
The results in Fig. 8 indicate that during the initial 90 min the content of 2M1B decreases as TAA increases consequently. After TAA reaches the maximum at 120 min, 2M2B is produced with constant TAA content. This phenomenon proves that the hydration of 2M1B is prior to isomerisation and the latter occurs after the former reaches almost equilibrium state. Therefore, the addition of water has a similar influence with that of TAA addition and more details are still under further study.
3.5 Primary industrial application
Based on results from the lab experiments, the improvement measurement was applied in industrial IA plant in Chemical Research Institute of SINOPEC Shanghai Petrochemical Company Limited. The operation period started from 11th June of 2008 to 6th August of 2008. 140 liter catalysts were loaded in the tubular reactors and the exothermic heat was removed by low temperature water flowed between the tubes to control the reaction temperature. The temperature difference between the head and the end of the catalyst bed was kept in 1.3-2.1°C under stable status. The average temperature in the head, middle and end of catalyst bed was 22.3-26.0°C, 23.4-27.5°C and 24.5-27.9°C, respectively, and the average system pressure was 0.47 MPa with average WHSV of 5.0-8.0 h-1and TAA addition amount of 0.5%-1.2%. The results are shown in Fig. 9 with mean 2M1B conversion of 0.6633, 2M2B selectivity of 0.9756 and 2M2B to 2M1B ratio of 12.40.
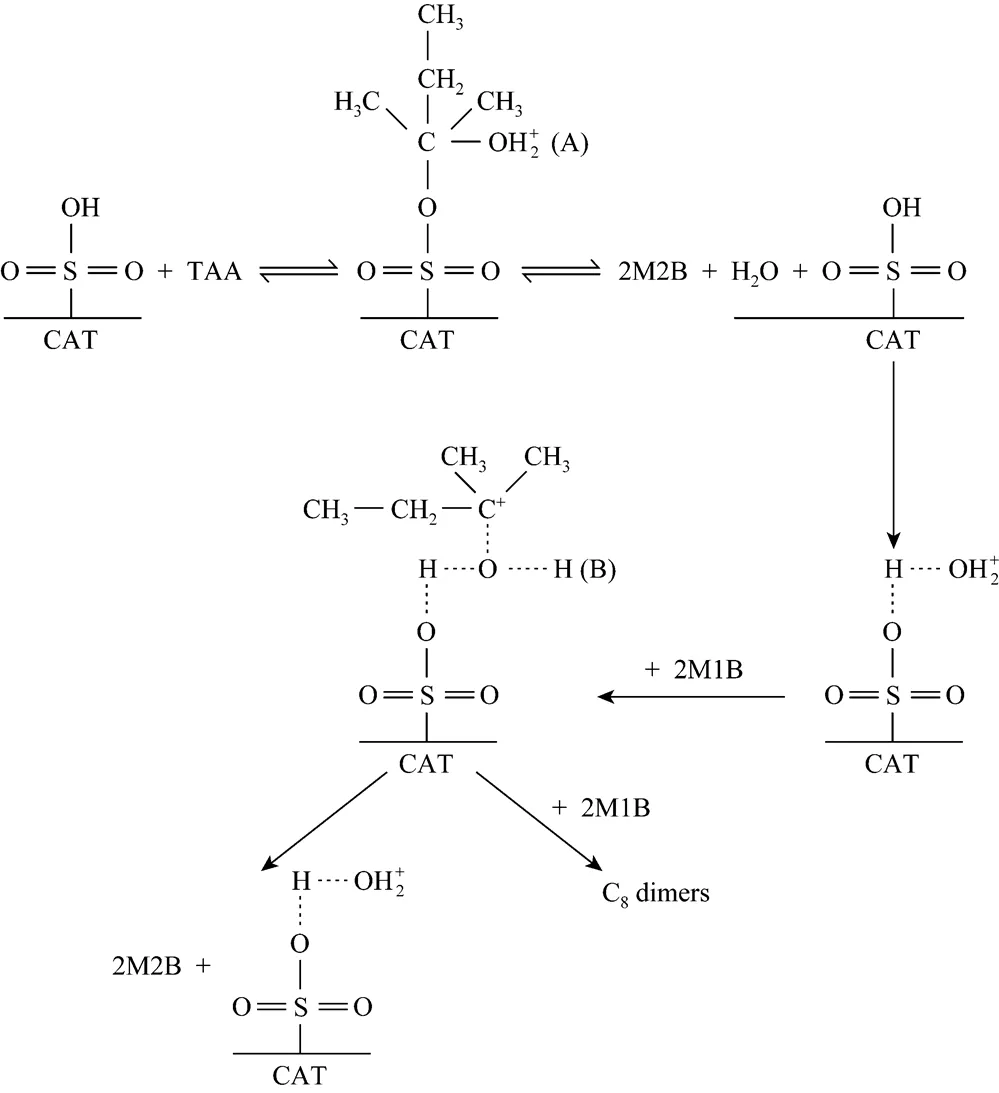
Figure 7 The isomerization mechanisms of 2M1B to 2M2B
Figure 8 Relation between different compound contents and reaction times
1—2M2B; 2—2M1B; 3—TAA
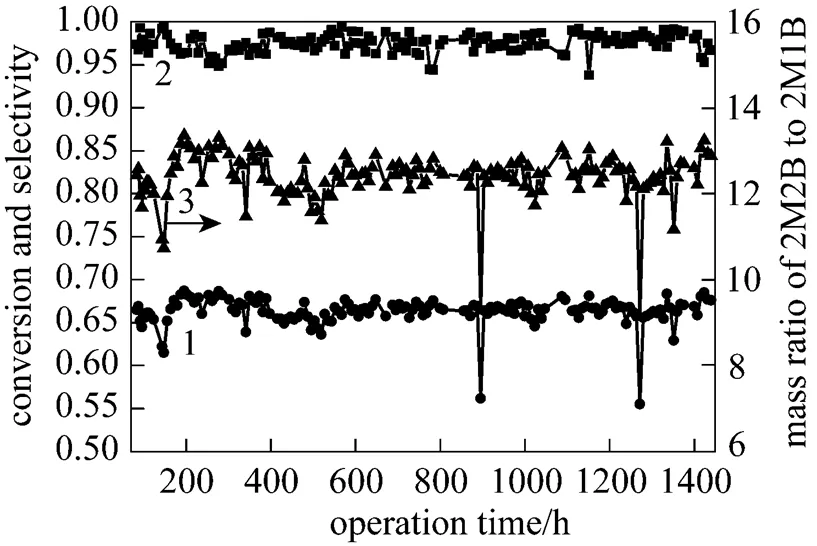
Figure 9 Operating results of new process
1—conversion of 2M1B; 2—selectivity of 2M2B; 3—mass ratio of 2M2B to 2M1B
It is found from Fig. 9 that 2M1B, 2M2B and dimer are accounted for 7.19%, 89.41% and 0.62% in the liquid products (dimer accounted for 0.23% in the raw materials), and the mean conversion of 2M1B is 0.6651 with 2M2B selectivity of 0.9727 as well as 2M2B/2M1B ratio of 12.45. Compared with Fig. 1, the results here indicate that the addition of TAA improves the activity of catalyst and selectivity of objective product, and also enhances the 2M2B/2M1B mass ratio. The content of 2M2B is increased from 89.36% to 92.21% after performing same distillation operation. Note that the side reaction of dimerization was suppressed and the temperature of catalyst bed was kept stable during the operation period. The industrial results proved the success of improvement measurements in the isomerization process. However, due to low content of 2M1B in the raw materials and thermodynamic limitation, the conversion is lower than that from lab experiment despite of 2M2B/2M1B up to 12.45.
4 Conclusions
According to the aforesaid work, addition of TAA was regarded to decrease the surface acidity of the strongly acidic ion exchange resin catalyst, thus improved its adsorption ability for 2M1B and effectively suppressed the dimerization. Therefore, the yield of objective product was enhanced and the reaction process was apt to stabilization. The annually developed 3.5kt plant produced a similar result with the lab experiment. In addition, a possible reaction mechanism was speculated.
1 Li, Y., “View on development of integrated utilization of C5resource from isopentene united plant”,—, 23 (1), 57-61 (2007).
2 Mao, W., Wang, X.L., Wang, H., Chang, H.Y., Zhang, X.W., Han, J.Y., “Thermodynamic and kinetic study of-amyl methyl ether (TAME) synthesis”,:, 47 ( 5), 761-769 (2008).
3 Katariya, A.M., Kamath, R.S., Mahajani, S.M., Moudgalya, K.M., “Study of non-linear dynamics in reactive distillation for TAME synthesis using equilibrium and non-equilibrium models”,, 21, 469-474 (2006).
4 Boz, N., Dogu, T., Murtezaoglu, K., Dogu, G., “Mechanism of TAME and TAEE synthesis from diffuse-reflectance FTIR analysis”,, 100 ( 3/4), 419-424 (2005).
5 Kołodziej, A., Jaroszyński, M., Sałacki, W., Orlikowski, W., Frączek, K., Klöker, M., Kenig, E.Y., Górak, A., “Catalytic distillation for TAME synthesis with structured catalytic packings”,, 82 ( 2), 175-184 (2004).
6 Muhammad, A.A., “Design of extraction column methanol recovery system for the TAME reactive distillation process”,, 18, 319-324 (2004).
7 Pääkkönen, P.K., Krause, A.O.I., “Comparative study of TAME synthesis on ion-exchange resin beads and a fibrous ion-exchange catalyst”,, 55 (2), 139-150 (2003).
8 Baur, R., Taylor, R., Krishna, R., “Bifurcation analysis for TAME synthesis in a reactive distillation column: Comparison of pseudo- homogeneous and heterogeneous reaction kinetics models”,, 42 (3), 211-221 (2003).
9 Bozga, G., Bumbac, G., Plesu, V., Muja, I., Popescu, C.D., “Modelling and simulation of kinetics and operation for the TAME synthesis by catalytic distillation”,, 14, 575-580 (2003).
10 Klöker, M., Kenig, E., Górak, A., Fraczek, K., Salacki, W., Orlikowski, W., “Experimental and theoretical studies of the TAME synthesis by reactive distillation”,, 14, 713-718 (2003).
11 Oost, C., Kai, S., Hoffmann, M.U., “Synthesis of tertiary amyl methyl ether (TAME): Equilibrium of the multiple reactions”,, 18 (2), 110-117 (2004).
12 Xie, J.M., Xu, Z.H., “Performance of catalyst for-amylmethyl ether decomposition to produce isoamylene”,, 26 (1), 51-54 (2006).
13 Gustavo, C., Robert, C.M., “Increasing the level of 2-methyl-2-butene in isoamylene”, U.S.Pat., 5073663 (1991).
14 An, Y., Zhu, Z.Z., Liu, H.T., “Process for preparing isopentane containing high content of 2-methyl-2-butene from methyl tertiary amyl ether”, C.N.Pat.,1371894 (2002).
15 Xia, R.H., Tang, Y.J., “Study on isomerization process for crude isoamylene”,—, 24 (6), 29-34 (2008).
16 Xu, Z.H., Fang, D.Y., “Dimerization reaction of isobutene”,, 19 (9), 1413-1418 (2007).
17 Honkela, M.L., “Comparison of ion-exchange resin catalysts in the dimerisation of isobutene”,:, 295 (2), 216-223 (2005).
18 Honkela, M.L., Tuomas, O., Kraus, A.O.I., “Thermodynamics and kinetics of the dehydration of-butyl alcohol”,...., 43, 4060-4065 (2004).
19 Talwalkar, S., Chauhan, M., Aghalayam, P., “Kinetic studies on the dimerization of isobutene with ion-exchange resin in the presence of water as a selectivity enhancer”,...., 45 (4), 1312-1323 (2006).
20 Haag, W.O., “Oligomerization of isobutylene on cation exchange resins”,...., 63, 140-147 (1967).
21 Honkela, M.L., Krause, A.O.I., “Kinetic modeling of the dimerization of isobutene”,...., 43, 3251-3260 (2004).
2008-12-02,
2009-08-09.
* To whom correspondence should be addressed. E-mail: spcxzh@163.com
 Chinese Journal of Chemical Engineering2009年5期
Chinese Journal of Chemical Engineering2009年5期
- Chinese Journal of Chemical Engineering的其它文章
- Molecular Simulation of CO2/H2 Mixture Separation in Metal-organic Frameworks: Effect of Catenation and Electrostatic Interactions*
- Deactivation Kinetics of Nitrile Hydratase in Free Resting Cells*
- Corrosion Behavior of TP316L of Superheater in Biomass Boiler with Simulated Atmosphere and Deposit
- Influence of A-type Zeolite on Methane Hydrate Formation*
- Effects of Sintering Atmosphere on the Microstructure and Surface Properties of Symmetric TiO2Membranes*
- Prediction of Thermophoretic Deposition Efficiency of Particles in a Laminar Gas Flow along a Concentric Annulus: A Comparison of Developing and Fully Developed Flows
