藏药臭蚤草中 11个倍半萜化学成分研究
黄圣卓,蒋思萍,朱华结
1中国科学院昆明植物研究所植物化学与植物资源持续利用国家重点实验室,昆明 650204;2西藏高原生物研究所,拉萨 850001;3中国科学院研究生院,北京 100049
藏药臭蚤草中 11个倍半萜化学成分研究
黄圣卓1,3,蒋思萍2,朱华结1*
1中国科学院昆明植物研究所植物化学与植物资源持续利用国家重点实验室,昆明 650204;2西藏高原生物研究所,拉萨 850001;3中国科学院研究生院,北京 100049
首次进行中国特有菊科藏药臭蚤草 (Pulicaria insignisDrumm.ex Dunn)的化学成分研究,从全草中分离得到 11个倍半萜类化合物,通过波谱数据分析分别鉴定为 Xanthanolide(1),(3αR,4αR,5R,7αS,9αS)-5-Hydroxy-5-methyl-3,8-dimethylenedecahydroazuleno[6,5-β]furan-2(3H)-one(2)10α-Hydroxy-14H-inuviscolide(3), (3R,3αR,4αS,7αS,8S,9αS)-3,4α,8-Tr imethyldecahydroazuleno[6,5-β]furan-2,5-dione(4)8-Epi-conferti(5), (3αR,5S,11αS,E)-5-Hydroxy-10-methyl-3,6-dimethylene-3α,4,5,6,7,8,11,11α-octahydrocyclodeca[α]furan-2 (3H)-one(6),(3αR,8αR,9αR)-5,8α-Dimethyl-3-methylene-3α,4,8,8α,9,9α-hexahydronaphtho[2,3-β]furan-2,6 (3H,7H)-dione(7),Pterodonoic acid(8),8-Epi-isovangustin(9),(3αR,4αS,8R,8αR,9αR)-8-Hydroxy-5,8α-dimethyl-3-methylene-3,3α,4,4α,8,8α,9,9α-octahydronaphtho[2,3-β]furan-2(7H)-one(10),(5S,8αR,9αR)-3-(Hydroxymethyl)-5,8α-dimethyl-6,7,8,8α,9,9α-hexahydronaphtho[2,3-β]furan-2(5H)-one(11)。所有化合物均首次从该植物中分离得到,同时补充了文献中化合物 6和 9所缺少的碳谱数据。
藏药;臭蚤草;倍半萜
臭蚤草Pulicaria insignisDrumm.ex Dunn是菊科 Compositea(Asteraceae)旋复花族Inluneae蚤草属Pulicaria的多年生草本。仅产西藏南部江孜、拉萨、察日坝、沙伽、尼木、日喀则、南太林等处。生于山脊岩石上,常见于石砾坡地和草丛中,海拔 4000~4310 m,根状茎粗壮、多分枝,“成片丛生,植物有粘液,具恶臭”[1],为西藏特有的药用植物。臭蚤草的花在西藏供药用,藏名“Ming·chen·serpo”;全草味苦性凉,具有镇咳舒肝,清血热,透骨蒸。用于肺痨咳嗽,两肋疼痛,劳热骨蒸,花序有清热、止痛的功效,还有驱跳蚤的作用[2]。目前人们从该属植物中分离到的化合物主要为倍半萜、二萜和黄酮等化合物[3-10],未见臭蚤草的化学成分报道。为了了解臭蚤草的活性成分,我们对该植物全草进行化学成分研究,从中分离得到 11化合物,所有化合物均首次从该植物中分离得到,同时补充了文献中化合物6和 9所缺少的碳谱数据。
1 仪器和材料
VGA Autospec-3000型质谱仪;Bruker AM-400和DRX-500超导核磁共振仪,T MS(四甲基硅烷)为内标;JASCO D IP-370和 OA AA-55型数字旋光仪,单位为 c单位为 g/mL;薄层色谱和正相柱色谱硅胶(青岛海洋化工);Sephadex LH-20(Phar macia公司);ODS(Y MC公司);MCI树脂(三菱公司)。
植物样品于 2007年 10月采于西藏拉萨,植物标本由西藏高原生物所蒋思萍研究员鉴定为Pulicaria insignisDrumm.exDunn,标本保存于西藏高原生物所标本室。
2 提取和分离
90 Kg干燥臭蚤草全草,粉碎后用甲醇提取浓缩获得浸膏,石油醚脱脂后用乙酸乙酯萃取 10次浓缩获得浸膏 780 g。乙酸乙酯部石油醚 /乙酸乙酯(20∶1-0∶1)分为 5个部分 (20∶1,10∶1,5∶1,2∶1, 0∶1),其中石油醚/乙酸乙酯 (10∶1)部分经MCI树脂脱色(收集甲醇洗脱部分),再经石油醚/丙酮 (50∶1-10∶1)和氯仿/丙酮 (100∶1-20∶1)多次反复梯度洗脱,经ODS柱(甲醇/水 1∶4-4∶1)梯度洗脱后,再分别经 SephadexLH-20凝胶(氯仿/甲醇 1∶1洗脱)纯化,分别获得化合物 1(256 mg),2(11.1 mg),3(851 mg),4(16.2 mg),5(382 mg),6(8.8 mg),7(20 mg),8 (8.8 mg),9(171 mg),10(4 mg),11(18.8 mg)。
3 结构鉴定
Xanthanolide(1) 白色块状晶体,C15H20O3; mp.128~129℃,+26.6(c0.0064 g/mL,下同,MeOH);1H NMR(CDCl3,500 MHz)δ(ppm): 6.12(d,J=3.2 Hz,1H),5.43(d,J=3.2 Hz,1 H),5.33(m,1H),4.54(td,J=8.7,2.7 Hz,1H), 3.22(m,1H),2.39(m,1H),2.30(m,3H),2.10 (m,3 H),2.08(s,3H),1.87(m,1H),1.80(dd, 1H),1.02(d,J=7.0 Hz,3H),ESI-MS(positive)m/z:249[M+1]+,271[M+Na]+,228;碳谱数据见表 1。其波谱数据与文献[11]报道一致。
(3αR,4αR,5R,7αS,9αS)-5-Hydroxy-5-methyl-3,8-d imethylenedecahydroazuleno[6,5-β]furan-2(3H)-one(2) 白色块状晶体,C15H20O3;mp.132~133℃;+62.3(c0.0014,CHCl3);1H NMR (CDCl3,400 MHz)δ(ppm):2.69(1H,d,J=10.5 Hz),1.96(1H,m),1.65(1H,m),1.85(m,1 H), 1.65(m,1H),1.71(1H,m),2.28(1H,t,J= 13.3,3.7 Hz),1.24(1H,m),2.66(1H,m),4.31 (1H,ddd,J=10.6,9.3,1.6 Hz),2.55(1H,dd,J= 15.5,10.6 Hz),6.22(1H,d,J=3.5 Hz),5.54 (1H,d,J=3.5 Hz),5.09(1H,s),4.97(1H,s), 1.20(3H,s),EI-MSm/z:248[M]+,230[MH2O]+,215,190,145;碳谱数据见表 1。其波谱数据与文献[12]报道一致。
10α-Hydroxy-14H-inuviscolide(3) 无色油状物质,C15H22O4;+56.7(c0.00335 g/mL, CHCl3);1H NMR(CDCl3,500 MHz)δ(ppm):6.00 (1H,d,J=3.5 Hz),5.40(1H,d,J=3.5 Hz),4.25 (1H,dd,J=12.5,10.0 Hz),2.43(1H,ddd,J= 11.0,10.5,3.5 Hz),2.33(1H,d,J=14.5 Hz), 2.20(1H,ddd,J=12.5,4.0,2.0 Hz),1.89(1H, dd,J=14.5,12.0 Hz),1.83(1H,m),1.67(1H, m),1.54(2H,m),1.48(1H,ddd,J=11.5,11.0, 2.0 Hz),1.67(3H,s),1.05(3H,s),EI-MSm/z: 267[M+1]+,230[M-H2O]+,191,190,95,81;碳谱数据见表 1。其波谱数据与文献[12]报道一致。
(3R,3αR,4αS,7αS,8S,9αS)-3,4α,8-Tr imethyldecahydroazuleno[6,5-β]furan-2,5-dione(4)无色油状物质,C15H22O3;+59.2(c0.0071 g/mL,CHCl3),1H-NMR (CDCl3,400 MHz)δ (ppm):1.81(1H,m),2.01(1H,m),2.05(1H, m),2.20(1H,ddd,J=19.0,9.0,9.0 Hz),2.49 (1H,ddd,J=19.0,9.0,2.0 Hz),2.57(1H,dd,J= 15.0,5.0 Hz),1.30(1H,dd,J=15.0,11.5 Hz), 1.85(1H,ddd,J=11.5,11.0,9.0 Hz),4.29(1H, ddd,J=12.0,9.0,3.0 Hz),2.29(1H,m),1.74 (1H,ddd,J=12.0,12.0,6.0 Hz),2.31(1H,m), 2.33(1H,m),1.22(3H,d,J=7.0 Hz),1.18(3H, d,J=7.0 Hz),1.08(3H,s),FAB-MS(positive)m/ z:251[M+1]+,233,111;碳谱数据见表 1。其波谱数据与文献[13]报道一致。
8-Epi-confertin(5) 白色块状晶体,C15H20O3;mp.125~126℃,+163.5(c0.00315 g/mL,CHCl3),1H NMR(CDCl3,400 MHz)δ(ppm): 6.18(1H,d,J=3.5 Hz),5.53(1H,d,J=3.5 Hz), 4.28(1H,td,J=8.8,2.4 Hz),2.73(1H,m),2.67 (1H,dd,J=14.8,5.3 Hz),2.50(1H,m),2.30 (2H,m)2.17(1H,dd,J=19.5,9.7 Hz),2.15 (1H,m),2.08(1H,m),1.85(2H,m),1.43(1H, t,J=11.8 Hz),1.16(3H,d,J=7.6 Hz,),1.07 (3H,s),EI-MSm/z:248[M]+,228,230,215,204, 189,136,81;碳谱数据见表 1。其波谱数据与文献[12]报道一致。
(3αR,5S,11αS,E)-5-Hydroxy-10-methyl-3,6-d imethylene-3α,4,5,6,7,8,11,11α-octahydrocyclodeca[β]furan-2(3H)-one(6) 淡黄色油状物质,C15H20O3;-52.63 (c0.00152 g/mL, MeOH),1H NMR(CDCl3,400 MHz)δ(ppm):6.30 (1H,d,J=3.0 Hz),6.18(1H,d,J=3.0 Hz),5.50 (1H,dd,J=7.7,7.5 Hz),5.33(1H,s),5.16(1H, s),4.14(1H,m),4.08(1H,d,J=6.3 Hz),2.75 (2H,m),2.38(2H,m),2.04(1H,t,J=11.6 Hz), 1.96(1H,dd,J=7.0,14.5 Hz),1.85(1H,m), 1.73(2H,m),1.70(3H,s),ESI-MS(positive)m/ z:249[M+1]+,271[M+Na]+;碳谱数据见表 1。其波谱数据与文献[14]报道一致。
(3αR,8αR,9αR)-5,8α-D imethyl-3-methylene-3α,4,8,8α,9,9α-hexahydronaphtho[2,3-β]furan-2,6(3H,7H)-dione(7) 白色粉末,C15H18O3;+17.3(c0.00075 g/mL,MeOH),1H NMR (CDCl3,500 MHz)δ(ppm):6.38(1H,d,J=3.2 Hz),5.72(1H,d,J=2.6 Hz),4.61(1H,ddd,J= 11.5,8.5,4.7 Hz),3.22(1H,m),3.04(1H,dd,J=13.1,7.3 Hz),2.60(1H,td,J=14.1,5.5 Hz), 2.46(1H,dt,J=14.1,3.1 Hz),2.23(1H,t,J= 14.5 Hz),2.05(1H,m),1.94(1H,dd,J=13.8, 4.7 Hz),1.83(3H,s),1.60-1.79(2H,m),1.24 (3H,s),FAB-MS(positive)m/z:247[M+1],154, 136,107,83;碳谱数据见表 1。其波谱数据与文献[15]报道一致。
Pterodonoic acid(8) 白色粉末,C15H20O3;+17.3(c0.00075 g/mL,CH3OH)1H NMR (CDCl3,500 MHz)δ (ppm):6.41(1H,s),5.76 (1H,s),2.88(1H,d,J=14.5 Hz),2.41-2.84 (3H,m),2.06(1H,t,J=13.6 Hz),1.70-1.85 (5H,m),1.79(s,3H),1.51(2H,dt,J=4.1,12.7 Hz),1.25(3 H,s),ESI-MS(positive)m/z:249[M +1]+,271[M+K]+,519[2 M+Na]+;碳谱数据见表 1。其波谱数据与文献[16]报道一致。
8-Epi-isovangustin(9) 白色块状晶体,C15H24O3;mp.138~140℃,+17.3(c0.0035 g/ mL,CH3OH),1H NMR(CDCl3,400MHz)δ(ppm): 6.13(1H,d,J=1.1 Hz),5.63(1H,d,J=1.1 Hz), 2.46(1H,tt,J=8.5,3.5 Hz),1.94(1H,d,J=12.4 Hz),1.70(1H,dd,J=12.4 Hz),1.70~1.15 (11H,m),1.07(3H,s),0.93(3H,s),ESI-MS (positive)m/z:251[M+Na]+,503[2M+1]+;碳谱数据见表 1。其波谱数据与文献[17]报道一致。
(3αR,4αS,8R,8αR,9αR)-8-Hydroxy-5,8αd imethyl-3-methylene-3,3α,4,4α,8,8α,9,9α-octahydronaphtho[2,3-β]furan-2(7H)-one(10) 无色油状物质,C15H20O3;[α]21D-31.5(c0.0016 g/mL, MeOH),1H NMR(CDCl3,400 MHz)δ(ppm):3.7 (1H,dd,J=10.0,7.0 Hz),4.05(1H,ddd,J= 12.5,12.5,4.0 Hz),5.34(1H,s),6.09(1H,d,J= 3.0 Hz),5.45(1H,d,J=3 Hz),2.51(1H,dd,J= 12.5,4.0 Hz),2.30-2.40(2H,m),2.25(1H,ddd,J=12.5,12.5,3.0 Hz),2.17(1H,d,J=3.0 Hz), 2.01(1H,dddq,J=12.0,10.0,2.15,2.15 Hz), 1.69(3H,s),1.42(2H,m),0.89(3H,s),ESI-MS (positive)m/z:249[M+1]+,271[M+Na]+,519 [2M+Na]+,131,185,157,119,105;碳谱数据见表1。其波谱数据与文献报道一致[18].
(5S,8αR,9αR)-3-(Hydroxymethyl)-5,8αd imethyl-6,7,8,8α,9,9α-hexahydronaphtho[2,3-β]furan-2(5H)-one(11) 淡黄色油状物质,C15H20O3;+249.2(c0.00915 g/mL,CHCl3);1H NMR(CDCl3,400 MHz)δ(ppm):1.60(1H,m), 1.68(1H,dd,J=13.0,4.0 Hz),1.56(1H,m), 1.95(1H,m),1.56(1H,m),1.75(1H,m),2.75 (1H,ddq,J=7.5,6.5,2.0 Hz),6.36(1H,s),4.78 (1H,dd,J=13.0,6.0 Hz),2.14(1H,dd,J=13.0, 6.0 Hz),1.54(1H,dd,J=13.0,13.0 Hz),4.40 (2H,s),1.32(3H,s),1.29(3H,d,J=7.5 Hz), 2.68(br),EI-MSm/z:248[M]+,230[M-H2O]+, 215,159,146;HR-ES IMS(positive)[M+Na]+271.1314,Calcd for[C15H20O3+Na]+m/z: 271.1310;碳谱数据见表 1。其波谱数据与文献报道一致[19].
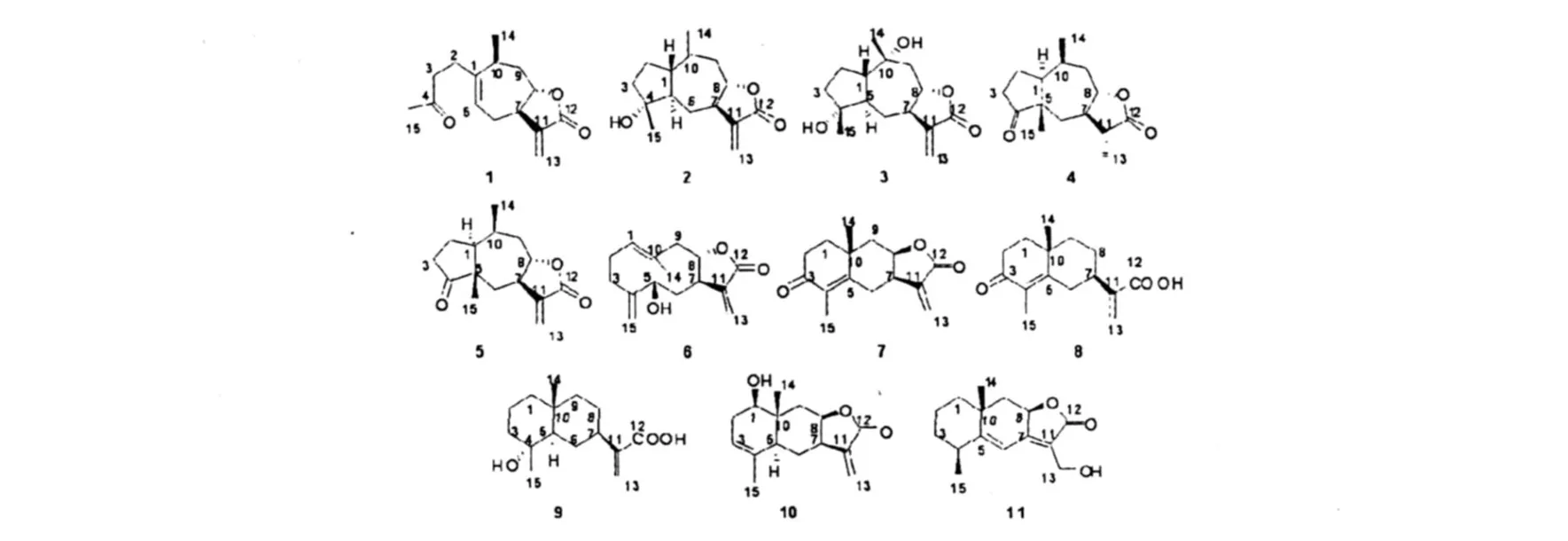
图 1 臭蚤草中分离到的化合物 1~11Fig.1 Compounds 1-11 isolated fromPulicaria insignis

表 1 化合物 1~11的13C NMR数据 (13C,100 MHz in CDCl3)δ(ppm)Table 113C NMR of compounds 1-11(13C,100 MHz in CDCl3)δ(ppm).
1 Editorial board of Flora of China.Flora of China.Beijing:Science Press,1997,Tums 75:286-293.
2 China National Group Corp.Traditional&HerbalMedicinel, Chinese Medicine Resources Books-Flora of Chinese Medicine Resources,Beijing:Science Press,1994.1325-1326.
3 Pares JO,Oksuz S,et al.6-Hydroxyflavonoids from Pulicaria dysenterica(Compositae).Phytochem istry,1981,20:2057.
4 Singh P,Sharma MC,et al.Diterpenes derived from clerodanes fromPulicaria angustifolia.Phytochem istry,1985,24: 190-192.
5 Triana J,Lopez M,et al.Sesquiterpenoids fromPulicaria anariensisand theircytotoxic activities.Joural of Natural Products,2005,68:523-531.
6 El-Negoumy SI,Mansour RMA,et al.Flavonols ofPulicaria arabica.Phytochem istry,1982,21:953-954.
7 Bohlmann F,AhmedM,et al.Naturally occurring terpene derivatives,Part 430.Caryophyllane derivatives fromPulicaria scabra.Phytochem istry,1982,21:1659-1661.
8 Marco JA,Sanz JF,et al.Caryophyllene derivatives fromPulicaria dysenterica.Phytochem istry,1992,31:2409-2413.
9 Mossa JS,Muhammad I,et al.Bisabolene and guaiane sesquiterpenes fromPulicaria glutinosa.Phytochem istry,1992, 31:575-578.
10 Hafez S,Sarg T M,et al.Caryophyllene derivatives fromPulicaria arabica.Phytochem istry,1987,26:3356-3358.
11 Dendougui H,Benayache S,et al.Sesquiterpene lactones fromPulicaria crispa.Fitoterapia,2000,71:373-378.
12 Jaber S,Mossa,Farouk S,et al.Sesquiterpene lactone and thymol esters fromV icoa Pentanem a.Joural of Natural Products,1997,60:550-555.
13 Rustaiyan A,Jakupovic J,Chau-Thi YV,et al.Further sesquiterpene lactones from the genusD ittrichia.Phytochem istry,1987,26:2603-2606.
14 Bohlmann F,GranzM.Ein Neues Ger macranolid AusM unnozia m aronii.Phytochem istry,1979,18:334-335.
15 Greger H,Zdero C,Bohlmann F.Eudes man-12,8β-olides and other Terpenes fromA rtem isia Species.Phytochem istry,1986, 25:891-897.
16 Cui YX,Xiong Z M,Zhou G.What is the structure of pterodonoic acid?Chinese Chem ical Letters,1998,9:389-391.
17 Werner H,Hiroaki C,TetherLR.Constituents ofAm brosia ilicifolia(Gray)Payne1,2.Journal of O rganic Chem istry, 966,31:632-1634.
18 Bolmannn FPradip K M,Jasmin J,et al.New sesquiterpene lactones fromInula specieces.Phytochem istry,1987,17:1165-1172.
19 Yang C,Zhu QX,YongW,et al.A new eudenmanolide and a new aromatic derivative fromCarpesiumCernum.Chinese Chem ical Letters,2001,12:597-600.
Sesquiterpenoids from Tibetan Folk DrugPulicaria insignis
HUANG Sheng-zhuo1,3,J IANG Si-ping2,ZHU Hua-jie1*
1State key laboratory of Phytochem istry and Plant Rescources of W est of China,Kunm ing Institute of Botany,Academy of Sciences Kunm ing 650204,Yunnan China;2Plateau Institute of B iology Lhasa 850001,Tibet China;3Graduate University of Chinese Academy of Sciences Beijing 100049,China
Eleven known sesquiterpenoids were isolated from the Tibetan Folk DrugPulicaria insignis.On the basis of MS and NMR data,theywere identified as Xanthanolide(1),(3αR,4αR,5R,7αS,9αS)-5-Hydroxy-5-methyl-3,8-d imethylenedecahydroazuleno[6,5-β]furan-2(3H)-one(2),10α-hydroxy-14H-inuviscolide(3),(3R,3αR,4αS,7αS,8S,9αS)-3,4α,8-Trimethyldecahydroazuleno[6,5-β]furan-2,5-dione(4),8-Epi-conferti(5),(3αR,5S,11αS,E)-5-Hydroxy-10-methyl-3,6-dimethylene-3α,4,5,6,7,8,11,11α,-octahydrocyclodeca[α]furan-2(3H)-one(6),(3αR,8αR,9αR)-5,8α-Dimethyl-3-methylene-3α,4,8,8α,9,9α-hexahydronaphtho[2,3-β]furan-2,6(3H,7H)-dione(7), Pterodonoic acid(8),8-Epi-isovangustin(9),(3αR,4αS,8R,8αR,9αR)-8-Hydroxy-5,8α-d imethyl-3-methylene-3, 3α,4,4α,8,8α,9,9α-octahydronaphtho[2,3-β]furan-2(7H)-one(10)(5S,8αR,9αR)-3-(Hydroxymethyl)-5,8αd imethyl-6,7,8,8α,9,9α-hexahydronaphtho[2,3-β]furan-2(5H)one(11).All compounds were isolated from this plant for the first t ime,and the13C NMR data of compounds 6 and 9 were shawed in this paper.
Tibetan drug;Pulicaria insignis;chemical constituents;sesquiterpenoids
R284.2;Q946
A
1001-6880(2010)05-0736-05
2009-04-30 接受日期:2009-10-20
国家自然科学基金(NSFC#30770235);中国科学院院地合作基金(YZ-06-1)
*通讯作者 Tel:86-871-5216179;E-mail:hjzhu@mail.kib.ac.cn
——青蒿素