银含量对二氧化硅固载磷钨酸银催化四氢呋喃聚合反应性能的影响
张 坡 黄 明 储 伟 罗仕忠 李 通
(四川大学化学工程学院,成都610065)
1 Introduction
Polytetramethylene glycol(PTMEG)is an important kind of polymer material,which could be utilized in many fields of chemical industries due to its high polarisability and flexibility.1-3It is commonly synthesized by the ring-opening polymerization of tetrahydrofuran with acid catalysts,4,5such as HCl,H2SO4,HClO4,HF,and other homogeneous acid.However,these acids are noxious,corrosive,and also caused difficulties on product separation.6For increasing environmental concerns,many solid acids,such as zeolite,clay,mixed metal oxides,7and heteropoly compounds,8-10have been implemented in the synthesis of PTMEG.
The heteropolyacid was widely used in many organic reactions because of its strong acidity and redox property.11,12But its low specific surface and good solubility in polar solvent had made the heteropolyacid leaching and deactivation in further recycles.13Through the synthesis of insoluble salt of heteropolyacid containing large ionic radius cation(K+,Cs+,NH+4,Ag+)by the partial or total neutralization,heteropolyacidʹs leaching could be prevented14-17and these salts also showed high activity in the reactions which were catalyzed by heteropolyacid.In our previous report,18the supported Cs salt of phosphotungstic acid Cs2.5H0.5PW12O40/SiO2also displayed high catalytic activity and excellent reusability in the polymerization of tetrahydrofuran.On the other hand,the different partial substitution amount of the heteropolyacidʹs protons by cation could have significant influences on catalystsʹmorphological property and acid strength distribution.19When the substitution amount of Cs cation was different,the Cs salt of 12-phosphotungstic acid revealed different micropore or mesoporous structure,and this would also make a difference on its catalytic activity.20However,so many reports about insoluble salts of H3PW12O40have usually focused on K+,Cs+,NH+4salts of heteropolyacid,the catalytic properties of supported silver salts have been received few attentions.
In this work,we prepared a series of silica supported silver salts of phosphotungstic acid AgxH3-xPW/SiO2(x=0.5,1.0,1.5,2.0,2.5,3.0).The activity and catalytic performance of these salts,including the effect of Ag substitution amount and loadings(10%to 50%,mass fraction)on reaction yield and average molecular weight of PTMEG,were investigated in the polymerization of tetrahydrofuran.For contrast,the catalyst bulk 12-tungstophosphoric acid supported on silica was also prepared.
2 Experimental
2.1 Preparation and characterization of the catalysts
Supported silver salts of tungstophoric acid were prepared in a procedure similar to the previous literature21by impregnation method.The phosphotungstic acid(HPW,AR)aqueous solution was added by a certain amount of support silica(SBET=282.4 m2·g-1,Vg=0.4875 cm3·g-1,Dp=6.932 nm,SBET:surface area calculated by BET model;Vg:total pore volume estimated at a relative pressure of 0.97,assuming full surface saturation;Dp:pore diameter calculated from BJH model,Qingdao Haiyang Chemical Company)and the mixture was stirred constantly,after that the stoichiometric solution of AgNO3(AR)was dripped slowly with stirring.A white colloidal solution was formed and evaporated to dryness at 60 °C.By drying at 110 °C overnight,the white power supported silver salts of heteropolyacid with different Ag amounts,AgxH3-xPW12O40/SiO2(x=0.5,1.0,1.5,2.0,2.5,3.0),were obtained.The supported heteropolyacid HPW/SiO2was synthesized directly similar to the preparation procedure of the supported silver salts.
The catalystʹs infrared spectra were performed on a Bruker TENSOR 27 Fourier transform infrared(FTIR)spectrometer with the solution of 4 cm-1at the range of 4000-400 cm-1.X-ray diffraction(XRD)patterns of the samples were recorded on the X-ray diffractometer XʹPRO MPO using CuKαradiation at the range of 5°-80°.Volume-averaged particle sizes of some special samples were calculated by the Scherer equation to the reflections at ca 26°.The acidity measurements of catalysts were implemented by then-butylamine potentiometric titration according to the reference.2250 mg of solid suspended in 45 mL acetonitrile was stirred for 1 h.Then,the titration was carried out with a solution ofn-butylamine in acetonitrile(0.05 mol·L-1)at a flow rate of 0.025 cm3·min-1.The potential variation of solution was recorded with a PHS-2C pH meter with the E-201C double-junction electrode.Morphology of sample was studied by means of Field Emission Scanning Electron Microscope XL30 ESEM.
2.2 Synthesis of polytetrahydrofuran
The synthesis of polytetrahydrofuran was catalyzed by the supported sliver salts of heteropolyacid with chloroepoxy propane(ECH,AR)as initiator.The reaction was carried out at 50°C for 4 h.The details of the reaction were the same as our previous reported work.18The yield of reaction was measured with the following formula:Yield=m(PTMEG)/[m(THF)+m(ECH)]×100%,THF is tetrahydrofuran.The viscosity-averaged molecular weight(Mη)of polytetrahydrofuran was calculated according to the formulas in the reference:23[η]=2.36×10-4×Mη0.77,[η]=3(ηγ1/3-1)/c,[η]:intrinsic viscosity of PTHF;ηγ:the retention time ratio between polytetrahydrofuran toluene solution and toluene at Ubbelohde viscometer on 30°C,andcmeant the concentration of polymer product in toluene solution(10 g·L-1).The polymer polytetrahydrofuran was confirmed by1H NMR spectrum and infrared spectrum according to our previous work.24
3 Results and discussion
3.1 Characterizations of catalysts AgxH3-xPW/SiO2
The XRD powder diffraction patterns of supported silver salts with different Ag contents are shown in Fig.1.All six supported silver salts show the characteristics diffraction(Fig.1(a)),which is different from that of anhydrous phosphotungstic acid H3PW12O40.21Along with the increase of substituted silverion,the XRD diffraction peaks of the samples(Fig.1(b)at 2θ=21.2°,25.9°,23.5°),shift to higher diffraction angle in comparison with the pure heteropolyacid.It is due to the acidic proton exchange between the Keggin ion of heteropolyacid and H5O+2causing the contraction of silver saltsʹunit-cell parameters.25Whenx≥2.0,the silver salts display the single crystalline phase diffraction,indicating that the active component on silica surface is only the phosphotungstic acid silver.And whenx<2.0,the diffractions on 2θ=25.9°show two crystalline phases containing the silver tungstophosphate and the bulk phosphotungstic acid,which is consistent with the characterization result of literature.21It showed that the silver salts with low silver ion substitution content(x<2.0)were the mixture of silver salts and their bulk heteropolyacid.

Fig.1 XRD patterns of 30%Ag salts of H3PW12O40(HPW)(a)and magnification in the range of 20°-28°(b)
The FTIR spectra of H3PW12O40and AgxH3-xPW12O40/SiO2supported on silica are presented in Fig.2.All samples clearly show Keggin structureʹs characteristic bands at 1080,982,890,and 800 cm-1,21and the weak absorption peaks at 595 and 523 cm-1are attributable to P-O25and W-O-P bend vibrations,26respectively.The peaks at 1631 and 470 cm-1appearing vibration peak,are assigned to the vibration of water molecules27and the bend vibration of Si-O-Si,26respectively.It indicates that the introduction of Ag ion and supporting on silica did not destroy the Keggin structure of phosphotungstic acid.The absorption peaks at 800 and 982 cm-1of catalyst H3PW12O40/SiO2shift to 804 and 894 cm-1,respectively,which is due to the interaction between Keggin anion and carrier silica.The silver salts Ag0.5-Ag3.0showed wide peaks in the regions 1000-1300 and 800 cm-1,which were because of the partial overlap or screen of silicaʹs Si-O-Si symmetric and asymmetric vibrations by the characteristic peaks of phosphotungstic acid at 1080 and 800 cm-1.28After the introduction of Ag ion,the IR spectra of supported Ag salt were changed little compared with that of bulk phosphotungstic acid.

Fig.2 IR spectra of supported H3PW12O40andAg salts with variousAg contents
The special samplesʹacidity was determined by potentiometric titration with the solution ofn-butylamine in acetonitrile(Fig.3).It was suggested that the initial electrode potential(Ei)indicates maximum acid strength of catalystʹs acid sites,and the value(unit in mmol·g-1(the mole mass ofn-butylamine per gram catalyst consumes))where the plateau was reached indicates the total amount of acid sites.The acid strength of acid sites could be classified to the following scale:Ei>100 mV(very strong sites),0<Ei<100 mV(strong sites),-100 mV<Ei<0(weak sites),andEi<-100 mV(very weak sites).22
The maximum acid strength and acid sites of the special catalysts are displayed in Table 1.Silica supported H3PW12O40catalyst HPW/SiO2obtains very strong acid sites(Ei>100 mV),while the supported Ag salts reveal lower acid strength sites.The supported saltʹs acidic strength and amount of acid sites also decline along with the content of Ag anion increase.The maximum acid strength and amount of acid sites of supported catalyst Ag2HPW/SiO2are 300 mV and 0.28 mmol·g-1,whichare lower than those of the HPW/SiO2catalyst.When the protons of H3PW12O40are replaced completely,the acidity of supportedAg saltAg3PW/SiO2is the lowest.

Fig.3 Potentiometric titration curves with n-butylamine of special catalysts

Table 1 Acid strength by potentiometric titration and catalytic performance of different heteropolyacidAg salts
3.2 Catalytic performances of catalysts AgxH3-xPW/SiO2
The catalytic performances of supported Ag salts with different Ag substitution contents in the polymerization of tetrahydrofuran are shown in Fig.4.The Ag content of supported silver salts affects their catalytic activity.Whenx=1.0,the reaction yield of the catalyst AgH2PW/SiO2is 48.0%,which is lower than the HPW/SiO2catalystʹs yield(64.3%).Whenx=3.0,the heteropolyacidʹs protons are all replaced,its catalytic activity drops dramatically and the reaction yield is only 3.0%,which is consistent with the result reported in the literature.29It is because acidities of the supported Ag salts are weaker than that of HPW/SiO2(in Fig.3 and Table 1).30In the series of supported silver salts of heteropolyacid,the catalyst Ag2HPW12O40/SiO2shows the highest catalytic activity,and the reaction yield achieved 57.0%,which is just a little lower than that of HPW/SiO2catalyst.

Fig.4 Catalytic performance ofAgxH3-xPW12O40/SiO2(x=0.5,1.0,1.5,2.0,2.5,3.0)
3.3 Characterizations of catalysts Ag2HPW/SiO2with different loadings
XRD patterns of pure Ag2HPW and Ag2HPW deposited on silica with different loadings are shown in Fig.5.The XRD pattern of bulk Ag2HPW sample(Fig.5(a))corresponds to a cubic structure,which is similar to the anhydrous HPW(ICCD card No.50-0657).But the reflexes assigning to the Keggin salt are shifted to higher diffraction degree,in comparison with the pure heteropolyacid.The diffractions of hydrate heteropolyacid are attributed to the fact that the H3PW12O40·nH2O and became stabilized at the surface of Ag salt.25In the samples(b-e),when the loading was lower than 20%,it does not show the obvious diffraction of bulk Ag2HPW,which indicates that the active component Ag2HPW existed with high dispersion degree.The samples 30%Ag2HPW/SiO2and 50%Ag2HPW/SiO2,showed the characteristic diffractions at 2θ=10.6°,21.2°,23.7°,26.0°,30.1°,35.4°.The volume-averaged particle sizes of some special samples were calculated by the Scherer equation to the reflections at ca 26°,and the average particle sizes of the supported samples 30%Ag2HPW/SiO2and 50%Ag2HPW/SiO2are 32.0 and 38.5 nm,bigger than those of 10%Ag2HPW/SiO2and 20%Ag2HPW/SiO2,which are 14.7 and 19.5 nm,respectively.It indicates that with the increase of loadings,the Ag2HPW particle aggregated and grew.It seems that the optimal Ag2HPW loading is 30%,and the catalyst 30%Ag2HPW/SiO2could get highest dispersion degree and most availably active sites.
IR spectra of Ag2HPW/SiO2catalysts with different loadings supported on silica are presented in Fig.6.All samples show the four characteristic bands of heteropolyacid,1080 cm-1(POa),982 cm-1(W=Od),890 cm-1(W-Ob-W),and 800 cm-1(W-Oc-W).31It had been reported that the exact locations of above four O atom diffraction peaks,are related to the hydration of heteropoly compound and the type of counter cation.32After the introduction of Ag cation and supported on silica,the characteristic peaks of tungstophosphoric acid 890 cm-1(WOb-W)and 800 cm-1(W-Oc-W)are shifted to 888 and 804 cm-1,respectively.It is due to the strong interaction of Ag cation with terminal oxygen atom or silica support,29which causes the structure distortion of Keggin anion and the shift of vibra-tion peaks.Along with the increase of Ag2HPW loading,the intensity of Keggin structure peaks at 888 and 892 cm-1become stronger,and when the loading is 30%,a weak W-O-P bending vibration of heteropolyacid is observed at 524 cm-1.With the loading increasing to 50%,this bending vibration at 524 cm-1also enhances.26This suggestes that,with the increase of active component amount,the Ag2HPW particle will become agglomeration and show some properties of bulk Ag2HPW,which are consistent with the result of XRD characterization in Fig.5.

Fig.5 XRD patterns of supportedAg2HPW catalysts with different loadings
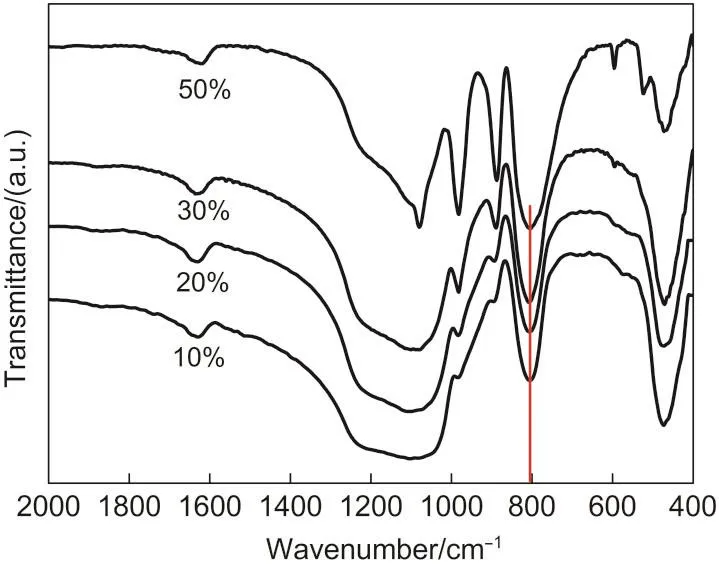
Fig.6 IR spectra ofAg2HPW/SiO2catalysts with different loadings
The micrographs of as-prepared bulk Ag2HPW12O40and 30%Ag2HPW/SiO2are displayed in Fig.7.It can be seen that the bulk Ag salt Ag2HPW12O40is in the form of well-shaped and highly ordered crystalline particle.This characterization result is in accord with that of literature.21Through the right micrograph,it is obvious that the particle size of Ag2HPW12O40decreases.And this characterization result is identical with that of the result of XRD.
3.4 Catalytic performances of the catalysts Ag2HPW/SiO2with different loadings
In Fig.8 the reaction performance of catalysts Ag2HPW/SiO2with different loadings in the polymerization of tetrahydrofuran is reported.Along with the active component Ag2HPW loadingʹs increase,the catalytic reaction yield and productʹs average molecular weight increase,and the sample with 30%loading gets the best catalytic performance.However,when the loading is more than 30%,the reaction yield declines slightly,and the average molecular weight remains unchanged.It may be due to the fact that the silver salt Ag2HPW could get high dispersion on silica surface when catalystʹs loading is 30%.When its loading is higher than 30%,the Ag salt particles will aggregate and obtain poor dispersion degree,which is consistent with the result of XRD characterization in Fig.5.And these samples get less available surface active sites,their reaction activities are also lower than that of 30%Ag2HPW/SiO2.It indicates that 30%loading is the best for silver salt Ag2HPW supported on silica.
3.5 Catalytic reusability of the catalyst 30%Ag2HPW/SiO2
The reusability of catalysts 30%Ag2HPW/SiO2and HPW/SiO2are shown in Fig.9.The supported heteropolyacid catalyst HPW/SiO2gets the reaction yield of 43.2%at the fourth recy-cle,which is declined 33%compared with its initial activity of 64.3%.And the catalyst 30%Ag2HPW/SiO2shows excellent performance in repeat reactions,whose yield is only slightly reduced after four reuses,and its reaction yield is still 53.3%,which is higher than the yield 30%reported in the literature.10The productʹs average molecular weight also keeps stable;there is not any decline at the fourth reuse.Therefore,through the introduction of silver icon to supported silver salt Ag2HPW/SiO2,it is in insoluble in polar solvent THF and was easy separated after reaction.Moreover,the reusability of supported heteropolyacid is greatly improved both on polytetrahydrofuranʹs yield and average molecular weight.
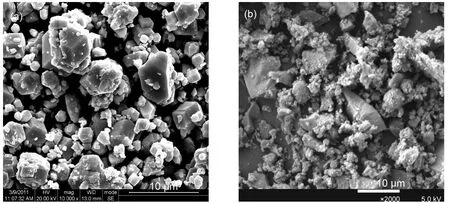
Fig.7 SEM pictures of bulkAg saltAg2HPW12O40(a)and 30%Ag2HPW12O40/SiO2(b)

Fig.8 Catalytic performance of catalystAg2HPW/SiO2with different loadings

Fig.9 Reusability of 30%Ag2HPW/SiO2and HPW/SiO2samples
4 Conclusions
The silver salts of 12-tungstophoric acid supported on silica were prepared.Their catalytic performance was affected remarkably by different silver substitution amount and different loadings.The supported silver salts AgxH3-xPW/SiO2show different crystalline phase structure due to different substitution content of silver.Whenx<2.0,the active components are the mixture of silver salt and bulk heteropolyacid,and whenx≥2.0,it shows a single crystalline phase structure of silver salt.Different content will replace Ag influence in the silver bear type acid strength.When the substitution amountx=2.0,the catalyst Ag2HPW/SiO2gets the highest catalytic activity,which is close to the catalyst HPW/SiO2.And whenx=3.0,all hydrogen protons of heteropolyacid are replaced with silver ion,so its acidity and catalytic acidity both achieve the minimum.The Ag2HPW/SiO2catalystʹs optimal loading is 30%,and the 30%Ag2HPW/SiO2could get the highest catalytic yield and average molecule weight due to its best dispersion degree of bulk silver salt Ag2HPW.The novel supported phosphotungstic acid silver salt 30%Ag2HPW/SiO2shows slightly lower catalytic activity than HPW/SiO2but better reusable performance,as a novel effective and stable catalyst in the synthesis of polytetrahydrofuran.
Acknowledgment:The authors thank the Analytical&Testing Center of Sichuan University in P.R.China for providing the1H NMR characterization.
(1) Nakazono,K.;Takashima,T.;Arai,T.;Koyama,Y.;Takata,T.Macromolecules2010,43,691.doi:10.1021/ma902161d
(2) Macit,H.;Hazer,B.;Arslan,H.;Noda,I.J.Appl.Polym.Sci.2009,111,2308.doi:10.1002/app.v111:5
(3)Tasdelen,M.A.;Camp,W.V.;Goethals,E.;Dubois,P.;Prez,F.D.;Yagci,Y.Macromolecules2008,41,6035.doi:10.1021/ma801149x
(4) Suzuki,Y.;Ohya,K.Polymerization Catalysts of Tetrahydrofuran for Relatively Low Molecular Weight Polytetramethylene Glycol.JP.Pat.Appl.48000999,1973.
(5) Murai,N.;Shirato,M.;Takeo,H.;Tanaka,H.Method for the Production of Polytetramethylene Ether Glycol.US.Pat.Appl.5393866,1995.
(6) Chu,W.;Hu,J.P.;Xie,Z.K.;Chen,Q.L.Catal.Today2004,90,349.doi:10.1016/j.cattod.2004.04.046
(7)Setoyama,T.;Kobayashi,M.;Kabata,Y.;Kawai,T.;Nakanishi,A.Catal.Today2002,73,29.doi:10.1016/S0920-5861(01)00515-6
(8) Aouissi,A.;Al-Deyab,S.S.;Al-Shahri,H.Molecules2010,15,1398.doi:10.3390/molecules15031398
(9)Zhang,Y.;Pan,L.;Gao,C.G.;Wang,Y.Z.;Zhao,Y.X.J.Sol-Gel Sci.Technol.2010,56,27.doi:10.1007/s10971-010-2268-8
(10) Zhu,Q.;Liang,L.P.;Jia,Z.Q.;Gao,C.G.;Zhao,Y.X.Acta Phys.-Chim.Sin.2011,27,491.[朱 晴,梁丽萍,贾志奇,高春光,赵永祥.物理化学学报,2011,27,491.]doi:10.3866/PKU.WHXB20110212
(11)Hossein,A.O.;Heravi,M.A.;Majid,M.;Fatemeh,F.B.Chin.J.Chem.2010,28,299.doi:10.1002/cjoc.v28:2
(12) Majid,M.H.;Yahya,S.B.;Maliheh,K.;Bita,B.B.;Fatemeh,F.Chin.J.Chem.2009,27,569.doi:10.1002/cjoc.v27:3
(13)Li,Y.;Chu,W.;Chen,M.H.;Hu,J.Y.J.Wuhan Univ.Technol.2008,23,234.doi:10.1007/s11595-006-2234-z
(14) Zieba,A.;Matachowski,L.;Lalik,E.;Drelinkiewicz,A.Catal.Lett.2009,127,183.doi:10.1007/s10562-008-9669-0
(15) Matachowski,L.;Zieba,A.;Zembala,M.;Drelinkiewicz,A.Catal.Lett.2009,133,49.doi:10.1007/s10562-009-0149-y
(16) Gong,S.W.;Liu,L.J.;Cui,Q.X.;Ding,J.H.J.Hazard.Mat.2010,178,404.doi:10.1016/j.jhazmat.2010.01.095
(17) Gao,S.Q.;Rhodes,C.;Moffat,J.B.Catal.Lett.1998,55,183.doi:10.1023/A:1019091130576
(18) Liao,X.M.;Chu,W.;Li,Y.;Zhou,F.D.;Luo,S.Z.Chin.Chem.Lett.2009,20,344.doi:10.1016/j.cclet.2008.11.032
(19) Parent,M.A.;Moffat,J.B.Catal.Lett.1997,48,135.doi:10.1023/A:1019012128507
(20) Park,H.W.;Park,S.Y.;Park,D.R.;Choi,J.H.;Song,I.K.Catal.Commun.2010,12,1.doi:10.1016/j.catcom.2010.08.002
(21) Zieba,A.;Matachowski,L.;Gurgul,J.;Bielańska,E.;Drelinkiewicz,A.J.Mol.Catal.A2010,316,30.doi:10.1016/j.molcata.2009.09.019
(22) Mansilla,D.S.;Torviso,M.R.;Alesso,E.N.;Vázquez,P.G.;Cáceres,C.V.Appl.Catal.A-Gen.2010,375,196.doi:10.1016/j.apcata.2009.12.029
(23) Huang,M.;Chu,W.;Liao,X.M.Catal.Lett.2011,141,1670.doi:10.1007/s10562-011-0679-y
(24) Huang,M.;Chu,W.;Liao,X.M.;Dai,X.Y.Chin.Sci.Bull.2010,55,2652.doi:10.1007/s11434-010-3266-5
(25) Haber,J.;Pamin,K.;Matachowski,L.;Napruszewska,B.;Poltowicz,J.J.Catal.2002,207,296.doi:10.1006/jcat.2002.3514
(26)Wang,G.J.;Liu,G.Q.;Xu,M.X.;Yang,Z.X.;Liu,Z.W.;Liu,Y.W.;Chen,S.F.Appl.Surf.Sci.2008,255,2632.doi:10.1016/j.apsusc.2008.07.186
(27) Kasza,T.;Bielański,A.Catal.Lett.2009,128,307.doi:10.1007/s10562-008-9728-6
(28) Palcheva,R.;Spojakina,A.;Dimitrov,L.;Jiratova,K.Microporous Mesoporous Mat.2009,122,128.doi:10.1016/j.micromeso.2009.02.026
(29) Aouissi,A.;Al-Deyab,S.S.;Al-Shehri,H.Chin.J.Polym.Sci.2010,28,305.doi:10.1007/s10118-010-9007-z
(30) Wei,Y.X.;Ma,G.M.;Li,S.Y.;Li,K.L.Chin.J.Inorg.Chem.2012,28,1909.[魏云霞,马明广,李生英,李康兰.无机化学学报,2012,28,1909.]
(31) Yadav,J.S.;Reddy,B.V.S.;Purnima,K.V.;Jhansi,S.;Nagaiah,K.;Lingaiah,N.Catal.Commun.2008,9,2361.doi:10.1016/j.catcom.2008.05.032
(32) Dias,J.A.;Caliman,E.;Dias,S.C.L.Microporous Mesoporous Mat.2004,76,221.doi:10.1016/j.micromeso.2004.08.021

