Porous ZnO Sheets Transformed from Zinc Sulfate Hydroxide Hydrate and Their Photoluminescence Performance
HE Peng GAO Xiang-Dong WU Li-Bin JIANG Zheng-Wu WANG Cai-Lu LI Xiao-Min
(1State Key Laboratory of High Performance Ceramics and Superfine Microstructures,Shanghai Institute of Ceramics,Chinese Academy of Sciences,Shanghai 200050,P.R.China; 2University of Chinese Academy of Sciences,Beijing 100049,P.R.China;3Shanghai Research Institute of Petrochemical Technology,Shanghai 200137,P.R.China; 4Key Laboratory of Advanced Civil Engineering Materials of Ministry of Education,Tongji University,Shanghai 201804,P.R.China)
1 lntroduction
As one of the few dominant nanomaterials for nanotechnology,ZnO has been a subject of intensive research driven by its wide applications in optoelectronics,sensors,actuators,energy,biomedical sciences and spintronics.1While ZnO nanostructures with a broad range of configurations have been demonstrated,recently,a special class of ZnO nanostructures begins to come into view,i.e.,those nanostructures possessing welldefined shape and porous feature at micron/nanoscale.2-5
Owing to their radically-different microstructures from the traditional single crystal-like nanostructures(e.g.,nanowires),these nanostructures are expected to possess much higher surface area due to the porous structure,and to exhibit much improved photo and/or electrical properties or exhibit some new physical/chemical phenomena related to either the quantum confinement or the surface/interface process,which is greatly advantageous for their applications in gas sensor,6photodetector,7biosensor,8photovoltaic cell,9and nanocatalysis10etc.However,due to the complexity of the microstructure in these materials,their controllable synthesis is still a tough task.
Several methods have been developed toward porous ZnO nanostructures,including the bottom-up assembly of nanocrystals driven by dipolar field,11and the molecule building blockdirected assembly of coordination-polymer templates,12and the transformation technology from zinc-based compounds such as ZnSe,Zn(NO3)2·6H2O,Zn3(OH)2V2O7·H2O,zinc hydroxide carbonate,and zinc fluorohydroxide etc.13-18However,the core difficulty on the morphological control of zinc-based compounds,which determines the overall morphology of ZnO nanostructures,has not been overcome up to now.More comprehensive and in-depth studies in the area are required.
Here we report the synthesis of sheet-like structures of a rarely-studied zinc-based compound zinc sulfate hydroxide hydrate(3Zn(OH)2·ZnSO4·xH2O,briefed as ZSH)viachemical bath deposition in a well-known Zn2+-hexamethyltetramine(HMT)precursor,19and transform them into porous ZnO sheets by sintering at 1000°C.The crystallinity,microstructure,and photoluminescence behavior of ZnO porous sheets were characterized.The formation mechanism of ZnO porous sheet from ZSH was analyzed.We believe that both the formation mechaism of ZSH from Zn2+-HMT alcohol system and its transformation into highly crystalline porous ZnO sheets will broaden our knowledge on the controllable synthesis of ZnO porous nanostructures.
2 Experimental
2.1 Chemicals
Sulfate heptahydrate zinc(ZnSO4·7H2O,≥99.5%(w))was purchased from Shanghai Jinshan Chemical Plan.Hexamethyltetramine(C6H12N4,briefed as HMT,≥99.0%(w)),sodium sulfate(Na2SO4,≥99.0%(w)),ammonia(NH3·H2O,28%(w))and sulfuric acid(H2SO4,98%(w))were supplied by Sinopharm Chemical reagent Co.Ltd.(China).Ethanol(AR,≥99.8%(w))was obtained from Shanghai Zhenxing Chemicals Reagent Co.Ltd.
2.2 Chemical bath deposition of ZSH sheets
ZSH sheet powder was synthesizedviaa simple chemical bath deposition method(CBD).In a typical synthesis,equal volume(5 mL)and molar concentration(0.2 mol·L-1)of ZnSO4·7H2O and HMT solutions were mixed and blended with 10 mL ethanol,forming the precursor solution.The pH value was adjusted to 5-6 by adding diluted sulfuric acid.The precursor container was covered by a soft plastic film and heated to 70°C in an oven for 2 h.The white precipitate was collected,washed with water and dried at 70°C for further characterization.
2.3 Transformation of ZSH to ZnO sheets
The as-prepared ZSH sheet powder was annealed in an airtight tube furnace,with the annealing temperature of 1000°C for 2 h.Fig.1 depicted the detail synthesis of ZSH sheets and their transformation into ZnO porous sheets.
2.4 Characterization
X-ray diffraction(XRD)patterns were recorded on a D/max 2550V X-ray diffractometer(Rigaku,Japan)using CuKα(λ=0.15406 nm)radiation at 40 kV and 60 mA.Scanning electron microscope(SEM)images were recorded on a JSM-6700F scanning electron microscope(JEOL,Japan)operating at 10.0 kV.Transmission electron microscope(TEM)images and energy dispersive X-ray(EDX)spectra were acquired on a 2010 transmission electron microscope(JEOL,Japan)operated at 200 kV,and the nanostructures were first dispersed in ethanol and then spread on the copper grid with carbon film.The photoluminescence measurements were carried out on a LabRAM HR 800HR UV micro-Raman spectrometer(HORIBA Jobin Yvon,USA)using 325 nm line He-Cd Laser as the excitation source.Thermogravimetry(TG)and differential scanning calorimetry(DSC)spectra of ZSH sheets in 30-1000°C were recorded from STA429C thermal analyzer(Netzsch Co.,Germany),with the heating rate of 10 °C·min-1.

Fig.1 Schematic illustration of the synthesis of ZnO porous sheets
3 Results and discussion
It is known that various ZnO nanostructures can be deposited from Zn2+-HMT aqueous system since the pioneer work of Vayssieres.20However,a few studies have shown that H2O-ethanol component solvent will lead to some special zinc-based compounds including zinc chloride hydroxide,21basic zinc carbonate,6and zinc fluorohydroxide,18which are strongly dependent on the counter ions of Zn2+.In our case,wherewas used as the counter ions of Zn2+,a special zinc based compound zinc sulfate hydroxide hydrate(ZSH)other than ZnO was formed.We believe the higher affinity ofwith Zn2+than that ofor OH-may account for the underlying reason.The Gibbs free energies for aqueous Zn(NO3)2,Zn(OH)2,and ZnSO4are-369.6,-461.62,and-891.6 kJ·mol-1,respectively.21As the formation of Zn(OH)2is the prerequisite for the deposition of ZnO in any aqueous system,the Gibbs energy of the anion with Zn2+(i.e.,the affinity with Zn2+)will determine the type of precipitates.While ZnO is developed in Zn(NO3)2-HMT H2O-ethanol precursor due to the lower affinity ofwith Zn2+than OH-,ZSH is finally formed in ZnSO4-HMT H2O-ethanol system due to the higher affinity ofwith Zn2+than OH-.
To provide further insight into the chemical deposition process of ZSH in the EtOH/H2O solvent system,we investigated the effects of the mole ratio of/Zn2+,volume ratio of EtOH/H2O,pH value of the precursor,and the reaction temperature on the stability of the precursor and the morphologies of obtained ZSH.Results show that these parameters have no obvious effects on the morphology of the hexagon ZSH sheets,but strongly affect the formation rate and the yield.(1)/Zn2+molar ratio(SZMR):Our previous studies22found that the addition of other zinc salts in the EtOH/H2O hybrid system would result in the formation of other zinc compounds(e.g.,ZnCl2for ZnCl2·4Zn(OH)2·H2O).So it is impossible to increase the concentration of Zn2+in this work without affecting the formation of ZSH.Therefore,we added Na2SO4in the precursor to adjust the concentration of,and found that the SZMR value of 1 to 2 was suitable to form ZSH with no obvious variation in the morphology,while higherwould lead to the formation of floccose sediment in the precursor before the CBD process.(2)EtOH/H2O volume ratio(EHVR):In the pure water system or the hybrid solvent with lower EHVR,the growth rate and the yield of ZSH were decreased greatly,and the deposited ZSH exhibited irregular shape,without the regular hexagonal shape.In the pure ethanol system,ZnSO4can not dissolve in the precursor.In the system with higher EHVR(>1.5),white floccose sediments were formed in the precursor before CBD.So we chose the EHVR value of 1 as the best solvent system.(3)pH value:We adjusted the pH value of the precursor by adding diluted sulfuric acid(1 mol·L-1)or ammonia water(1 mol·L-1),and found that higher pH value(>6.5)would result in the floccose sediment in the precursor,and lower pH value(2-3)would dramatically decrease the growth rate and the yield of ZSH.So the moderate pH value of 5-6 was selected.(4)Reaction temperature:For the EtOH/H2O hybrid solvent system,the reaction temperature could be changed from 50 to 80°C,and the higher the temperature was,the faster the ZSH formed.To cut down the evaporation of ethanol during the CBD process and maintain a reasonable growth rate of ZSH,we fixed the temperature at 70°C in the experiment.
Fig.2 illustrated XRD patterns of as-prepared ZSH powder and ZnO porous sheets by sintering ZSH at 1000°C.Results showed that,while ZSH sheets exhibited intensive diffraction peaks and good crystallinity,its pattern could not be indexed to any known phase.According to the EDS analysis(xZn:xS=4:1,atomic ratio)and the nature of ZSH(i.e.,the XRD pattern varies with the value of‘x’in the formula of 3Zn(OH)2·ZnSO4·xH2O,we assumed that the product was ZSH.After annealing at 1000°C,all of the diffraction peaks related to ZSH disappeared,and the pattern could be ascribed to wurtzite ZnO(PCPDF No.36-1451)with excellent crystallinity.This result indicated that the nanostructures of zinc related compound ZSH could be effectively converted to ZnO nanostructures by a simple sintering procedure.
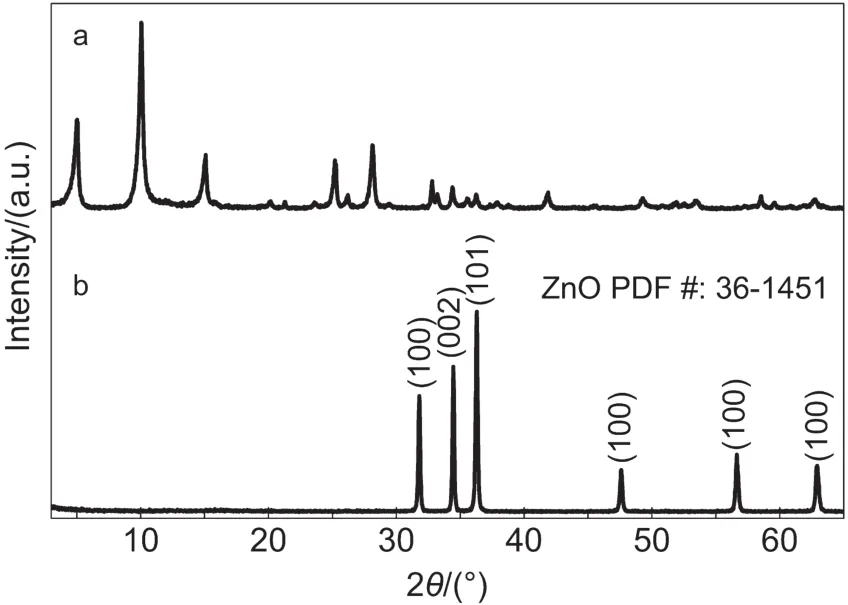
Fig.2 XRD patterns of(a)as-prepared ZSH sheet powder and(b)ZnO porous sheets
Fig.3 gave SEM images of ZSH sheets with 2 h of growth and ZnO porous sheets after sintering.As shown in Fig.3a,obtained ZSH sheets in general exhibited well-defined hexagonal shape but with large size distribution.The thickness of each hexagonal sheet was 200-500 nm.The agglomeration of neighboring sheets was significant due to the centrifuging and drying process.After annealing,though part of the hexagonal shape was damaged,the overall sheet structure of original ZSH was maintained.The agglomeration degree of neighboring sheets was reduced possibly due to the release of gaseous products in the sintering process(Fig.3c).The crystallites of ZnO were in size of 200-500 nm in average,with good connectivity in neighboring particles(Fig.3d).Throughout the whole sheet(Fig.3b),numerous pores in submicron size were clearly observed,an interesting feature that would increase greatly the surface area and be beneficial to the preparation of ZnO based hybrid nanostructures with inorganic nanoparticles or organic materials due to the much increased interfacial bondings.These porous ZnO sheets can be used as the photoanodes of dye-sensitized solar cells with highly dye loading23or as highly sensitive gas-sensing materials.24
To obtain ZSH with controllable size and more uniform size distribution,we prepared ZSH sheets with different concentrations of Zn2+ions([Zn2+])and deposition time.Results showed that the hexagonal size of ZSH sheet changed little when[Zn2+]varied from 0.01 to 0.1 mol·L-1and the reaction time was fixed at 2 h.On the other hand,the deposition time affected the sheet size significantly.As shown in Fig.4,the size of ZSH sheets growing for 30 min was on average 20 μm,which was nearly one-half of those grown for 1 h.Then we assumed that the wide size distribution of ZSH sheets for 2 h growth(Fig.3a)may result from the continuous precipitation of the larger ZSH sheets formed at the early stage and the smaller sheets formed at the later stage.
TEM analysis was performed to obtain a clear picture on the microstructure and the micro-area composition of ZnO porous structures,as shown in Fig.5.The particle size of primary ZnO crystallites can be down to tens of nanometers and up to several hundred nanometers at the same time.The pores between crystallites also exhibited a broad size distribution,which in fact indicated its capability to host other inorganic semiconductor nanoparticles and/or organic molecules or assembly in different sizes.An interesting phenomenon was that ZnO crystallites obtainedviasintering at 1000°C showed a distinctive polygon(e.g.,pentagon)or irregular shape(Fig.5a),different from the traditional hexagonal shape.This phenomenon could be ascribed to the constraint of mass transfer during the formation of ZnO,which was a key factor affecting the growth of crystallites during sintering especially for the solid phase systems.The good crystallinity of ZnO particles can be confirmed by selected area electron diffraction(SAED)analysis(inset of Fig.5b).We supposed this special microstructure of ZnO porous sheet was closely related to the thermal decomposition process of ZSH.

Fig.3 SEM images of(a)ZSH sheets and(b-d)porous ZnO sheet,with(b)overall view,(c)side view,and(d)magnified view of a typical sheet
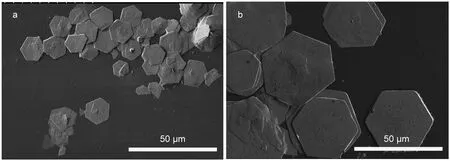
Fig.4 SEM images of ZSH sheets with different growth time

Fig.5 TEM images of(a)typical ZnO porous sheet and(b)magnified view of ZnO crystallites and nanopore,with the inset showing the selective area electron diffraction pattern
Fig.6 gave the thermogravimetry(TG)and differential scanning calorimetry(DSC)spectra of ZSH sheet powder from room temperature to 1000°C.Fig.7 presented the X-ray diffraction patterns of decomposition products of ZSH sheets annealed at different temperatures for 1 h.As seen in Fig.7a,crystalline ZnO was formed in annealed ZSH under 200°C,indicating that the mass loss and endothermic peak at around 107°C(Fig.6)were resulted from the loss of adsorbed water,as well as the decomposition of Zn(OH)2part of ZSH.When the annealing temperature increased to 300°C,zinc oxysulfate(Zn3O(SO4)2)was found in the products(Fig.7b).The emergence of zinc oxysulfate might be ascribed to the decomposition of ZnSO4part of ZSH by losing the combined water,which brought about the mass loss and endothermic peak at about 254 °C.At the annealing temperature of 400 °C,the XRD pattern was much similar to that at 300°C,but the fullwidth at half-maximum(FWHM)value of ZnO(002)peak changes obviously from 0.513°to 0.462°,indicating the crystal growth of ZnO particles.So the mass loss and the endothermic peak at 368.7°C came from the crystal growth of ZnO.The final endothermic peak at 882.3°C was originated primarily from the decomposition of zinc oxysulfate because of the full disappearance of zinc oxysulfate phase in sample with 1000°C annealing(see Fig.2b).21

Fig.6 Thermogravmetry and differential scanning calorimetry spectra of ZSH sheets
Based on the above discussion,the formation mechanism of ZnO transformed from ZSH can be depicted as follows.ZSH in chemical form of 3Zn(OH)2·ZnSO4·xH2O first looses the adsorbed water and the combined water at 50-200°C,and simultaneously decomposes Zn(OH)2part to form crystalline ZnO.ZnSO4part of ZSH is firstly converted to zinc oxysulfate(Zn3O(SO4)2)at around 254.3°C by losing the combined water,and finally changes into ZnO at 800-1000°C due to the decomposition of zinc oxysulfate.
To further examine the formation mechanism of porous ZnO sheets transformed from dense ZSH sheets,we investigated morphologies of ZSH sheets sintered at 400°C for 2 h,as shown in Fig.8.Different from as-prepared samples,ZSH sheets sintered at 400°C possessed a rather rough surface with an obviously disordered nature,which may be resulted from the gas release during the decomposition of ZSH and the formation of zinc oxysulfate.Crystalline ZnO particles decomposed from Zn(OH)2part of ZSH at 100-200°C were detected(Fig.8c)on the sheet surface.Nanopores that were resulted from the gas releasing during the decomposition of ZnSO4part can also be found on the surface of sheets,but in a very small quantity.Therefore,it could be concluded that the majority of the nanopores were formed during the decomposition of zinc oxysulfate at around 800-900°C,in which more gas products(SO3)would form and release from the sheets.The high decomposition temperature may significantly improve the crystallinity of porous ZnO and inhibit the formation of oxygen defects,which is beneficial for its application in many photoelectrical fields.

Fig.7 XRD patterns of decomposition products of ZSH sheets annealed for 1 h at(a)200 °C,(b)300 °C,and(c)400 °C

Fig.8 SEM images of ZSH sheet deposited on Si substrate after sintering at 400°C for 2 h
Fig.9 presented the photoluminescence(PL)spectrum of ZnO porous sheet excited by a 325 nm He-Cd laser.A single emission in the ultraviolet band centered at 388 nm(corresponding to a band gap of 3.19 eV)was observed,with a narrow FWHM value of 22 nm,which originated from the intrinsic photoluminescence of ZnO.No emission in the visible band related to the oxygen defects or interstitial zinc atoms was detected,indicating the high optical quality of ZnO sheets.Compared with ZnO thin films/nanostructures preparedviaboth the traditional solution routes such as the chemical bath deposition25or the electrochemical deposition26and the physical vapor deposition techniques including sputtering27and pulsed laser deposition,28the obtained ZnO porous sheet exhibited better optical quality by its strong UV emission and absence of defect-related emissions,similar to that of ZnO films grown by radio frequency sputtering enhanced chemical vapor deposition.29

Fig.9 Room temperature photoluminescence spectrum of ZnO porous sheet excited from a 325 nm He-Cd laser(100 mW)
When compared with the photoluminescence behavior of ZnO single crystal film or nanostructures,30,31the UV emission in our work shows relatively lower emission intensity and larger FWHM value.We suppose that it may be related to the minor doping of sulfur atoms in the lattice of ZnO crystals,while the sulfur may originate from both the residue sulfur at the grain boundaries and from the gas SO3in the sintering atmosphere,and the high temperature may possibly promote the doping of sulfur in ZnO.It has been reported that S-doped ZnO films exhibited red-shift due to the band gap shift to the lower energy induced by S doping.32,33Also some researchers found that S-doping in ZnO can obviously inhibit the UV emission and enhance the visible emission.34Taking these factors into consideration,we suppose that the unintentional doping of minor sulfur atoms in ZnO may be the underlying reason for the reduced UV emission in our case.Further work to testify the S-doping in ZnO porous sheets,to modulate its UV emission,and to explore their application in some photoelectric devices is on-going.
4 Conclusions
In summary,we have prepared sheets of a rarely-studied zinc-compound ZSH from a well-known Zn2+-HMT precursor system,and transformed them into porous ZnO sheetsviaa simple calcination process.The obtained ZnO porous sheets exhibited well-defined hexagonal sheet-like morphology,excellent crystallinity,and single UV emission when excited at room temperature.The formation mechanism of ZnO from ZSH indicated the significant influences of the thermolysis on the morphologies and compositions of ZnO sheets.Further work may direct to the controllable synthesis of ZnO sheets with smaller size and thickness,in-depth investigation of the formation mechanism,and exploration of their applications in UV-related detectors.
(1) Wang,Z.L.Mat.Sci.Eng.R2009,64,33.doi:10.1016/j.mser.2009.02.001
(2) Yoshida,T.;Terada,K.;Schlettwein,D.;Oekermann,T.;Sugiura,T.;Minoura,H.Adv.Mater.2000,12,1214.
(3)Li,S.;Li,Z.W.;Tay,Y.Y.;Armellin,J.;Gao,W.Cryst.Growth Des.2008,8,1623.doi:10.1021/cg701056q
(4) Liu,Z.;Wen,X.D.;Wu,X.L.;Gao,Y.J.;Chen,H.T.;Zhu,J.;Chu,P.K.J.Am.Chem.Soc.2009,131,9405.doi:10.1021/ja9039136
(5) Shen,J.B.;Zhuang,H.Z.;Wang,D.X.;Xue,C.S.;Liu,H.Cryst.Growth Des.2009,9,2187.doi:10.1021/cg800847d
(6)Zhang,J.;Wang,S.R.;Xu,M.J.;Wang,Y.;Zhu,B.L.;Zhang,S.M.;Huang,W.P.;Wu,S.H.Cryst.Growth Des.2009,9,3532.doi:10.1021/cg900269a
(7) Chivukula,V.;Ciplys,D.;Shur,M.;Dutta,P.Appl.Phys.Lett.2010,96,233512.doi:10.1063/1.3447932
(8)Park,H.Y.;Go,H.Y.;Kalme,S.;Mane,R.S.;Han,S.H.;Yoon,M.Y.Anal.Chem.2009,81,4280.doi:10.1021/ac900632n
(9) Zhang,Q.F.;Chou,T.R.;Russo,B.;Jenekhe,S.A.;Cao,G.Z.Angew.Chem.Int.Edit.2008,47,2402.
(10)Wang,Q.;Geng,B.Y.;Wang,S.Z.Environ.Sci.Technol.2009,43,8968.doi:10.1021/es902568h
(11)Yang,M.;Sun,K.;Kotov,N.A.J.Am.Chem.Soc.2010,132,1860.doi:10.1021/ja906868h
(12) Jung,S.;Cho,W.;Lee,H.J.;Oh,M.Angew.Chem.Int.Edit.2009,48,1459.doi:10.1002/anie.v48:8
(13) Shan,C.X.;Liu,Z.;Zhang,Z.Z.;Shen,D.Z.;Hark,S.K.J.Phys.Chem.B2006,110,11176.doi:10.1021/jp0600514
(14)Wu,J.H.;Varghese,B.;Zhou,X.D.;Teo,S.Y.;Sow,C.H.;Ang,S.G.;Xu,G.Q.Chem.Mater.2010,22,1533.doi:10.1021/cm902490g
(15) Qian,H.S.;Yu,S.H.;Gong,J.Y.;Luo,L.B.;Wen,L.L.Cryst.Growth Des.2005,5,935.doi:10.1021/cg049666l
(16)Kong,Q.S.;Wu,X.L.;Guo,Y.G.;Wang,Y.Q.;Xia,Y.Z.;Yu,J.;Liu,H.H.;Duan,X.F.Acta Phys.-Chim.Sin.2008,24,2179.[孔庆山,吴兴隆,郭玉国,王乙潜,夏延致,于 建,刘海华,段晓峰.物理化学学报,2008,24,2179.]doi:10.3866/PKU.WHXB20081206
(17)Zhou,X.F.;Hu,Z.L.;Fan,Y.Q.;Chen,S.;Ding,W.P.;Xu,N.P.J.Phys.Chem.C2008,112,11722.doi:10.1021/jp802619j
(18)Gao,X.D.;Li,X.M.;Gao,W.;Qiu,J.J.;Gan,X.Y.;Wang,C.L.;Leng,X.Crystengcomm2011,13,4741.doi:10.1039/c1ce05125c
(19)Gao,X.D.;Li,X.M.;Yu,W.D.;Peng,F.;Zhang,C.Y.Materials Research Bulletin2006,41,608.doi:10.1016/j.materresbull.2005.09.001
(20) Vayssieres,L.Adv.Mater.2003,15,464.doi:10.1002/adma.200390108
(21) Tagawa,H.;Saijo,H.Thermochimica Acta1985,91,67.doi:10.1016/0040-6031(85)85202-3
(22)Gao,X.D.;Gao,W.;Yan,X.D.;Zhuge,F.W.;Bian,J.M.;Li,X.M.Funct.Mater.Lett.2009,2,27.doi:10.1142/S1793604709000508
(23)Qiu,Y.C.;Chen,W.;Yang,S.H.J.Mater.Chem.2010,20,1001.doi:10.1039/b917305f
(24) Hsueh,T.J.;Hsu,C.L.Sens.Actuator B-Chem.2008,131,572.doi:10.1016/j.snb.2007.12.045
(25)Gao,M.D.;Li,M.M.;Yu,W.D.J.Phys.Chem.B2005,109,1155.doi:10.1021/jp046267s
(26)Wang,D.L.;Ruan,Y.F.;Zhang,L.C.;Yang,H.B.Acta Phys.-Chim.Sin.2010,26,3369.[王丹丽,阮永丰,张灵翠,杨红波.物理化学学报,2010,26,3369.]doi:10.3866/PKU.WHXB201210082
(27)Zhao,J.L.;Li,X.M.;Bian,J.M.;Yu,W.D.;Zhang,C.Y.Thin Solid Films2006,515,1763.doi:10.1016/j.tsf.2006.06.032
(28)Kim,D.;Shimomura,T.;Wakaiki,S.;Terashita,T.;Nakayama,M.Physica B2006,376,741.doi:10.1016/j.physb.2005.12.185
(29)Al-Salman,H.S.;Abdullah,M.J.J.Alloy.Compd.2013,547,132.doi:10.1016/j.jallcom.2012.08.119
(30)Gu,Y.F.;Li,X.M.;Yu,W.D.;Gao,X.D.;Zhao,J.L.;Yang,C.J.Cryst.Growth2007,305,36.doi:10.1016/j.jcrysgro.2007.03.050
(31)Li,C.;Fang,G.J.;Fu,Q.;Su,F.H.;Li,G.H.;Wu,X.G.;Zhao,X.Z.J.Cryst.Growth2006,292,19.doi:10.1016/j.jcrysgro.2006.03.061
(32) Zhang,X.H.;Yan,X.Q.;Zhao,J.;Qin,Z.;Zhang,Y.Mater.Lett.2009,63,444.doi:10.1016/j.matlet.2008.11.006
(33)Yoo,Y.Z.;Jin,Z.W.;Chikyow,T.;Fukumura,T.;Kawasaki,M.;Koinuma,H.Appl.Phys.Lett.2002,81,3798.doi:10.1063/1.1521577
(34) Yousefi,R.;Kamaluddin,B.Solid State Sci.2010,12,252.doi:10.1016/j.solidstatesciences.2009.11.002

