Bromate formation in bromide-containing waters irradiated by gamma rays∗
ZHOU Yan(周艳),CAO Chang-Qing(曹长青),and WANG Min(王敏)
1Shanghai Institute of Applied Physics,Chinese Academy of Sciences,Shanghai 201800,China
2School of Environment and Architecture,University of Shanghai for Science and Technology,Shanghai 200093,China
Bromate formation in bromide-containing waters irradiated by gamma rays∗
ZHOU Yan(周艳),1,2CAO Chang-Qing(曹长青),1,†and WANG Min(王敏)1
1Shanghai Institute of Applied Physics,Chinese Academy of Sciences,Shanghai 201800,China
2School of Environment and Architecture,University of Shanghai for Science and Technology,Shanghai 200093,China
The formation of bromate,a classif i ed potential carcinogen,is of great concern when disinfection processes are used for treating high-bromide drinking waters.Bromide-containing aqueous solutions with various additives were irradiated by60Coγsource.With a 2.0kGy irradiation of N2O-saturated solutions at initial bromide concentrations of 180.2µgl−1,416.9µgl−1,663.1µgl−1and 823.9µgl−1.79.5%,84.0%,87.3%and 88.3% of bromide ions were transformed to bromate,respectively.Addomg CO32–/HCO3–or NO3–ions into N2O-saturated bromide solutions,the amount of bromate ions formed decreased with increasing concentrations of the additives.On the other hand,the bromate concentration was all below the detection limit of 1µgl−1whenever N2O was not added to quench eaq–and·H.The results indicated thatγ-rays irradiation could be used as a disinfection process,instead of ozonation,to comply with upcoming more stringent regulations,especially in waters containing high concentrations of bromide.
Bromide,Bromate,Gamma irradiation,Disinfection
I.INTRODUCTION
Bromide(Br–)is commonly found in water bodies,with concentrations varying from a fewµgl−1to several mgl−1. Through oxidative processes,Br–can be oxidized to bromate(BrO3–),which is classif i ed as a possibly carcinogen to humans by the International Agency for Research on Cancer(IARC).The World Health Organization(WHO)recommends a provisional guideline value of 10µgl−1for drinking water because of limitations in available analytical and treatment methods[1].The same value has recently been set as the maximum contaminant level(MCL)of bromate in drinking water in many countries,including China[2].
The main source of BrO3–in drinking water is the ozonation of bromide-containing waters,which may cause serious bromide levels of over 100µgl−1[3].Several authors reported that bromate formation during ozonation is due to three general pathways,the direct pathway,direct-indirect pathway and indirect-direct pathway.The direct pathway involves only molecular ozone(O3),and in the direct-indirect and indirectdirect pathways,both O3and·OH radicals(produced from O3decomposition)participate in bromate formation[4].Others reported that bromate was formed predominately through the free radical pathway[5,6].In a continuous f l ow reactor,the amount of BrO3–formation reduced by 90%in the presence oftert-butanol(acted as·OH scavenger)[6].
Therefore,it is important to investigate the factors affecting the BrO3–formation through the free radical pathway without involving any O3.In this study,aqueous solutions containing bromide and other additives were subjected toγ-rays irradiation,andfreeradicalsweregenerated.Theinf l uenceoffactors, such as atmospheres(namely N2O,O2,N2and natural air),absorbed dose,initial bromide concentration,pH,and commoninorganic ions(including nitrate,chloride,(bi)carbonate and sulfate)etc,were investigated.
II.EXPERIMENTAL
A.Materials
NaBr of extra pure grade was purchased from Acros Organics.Anion Standards of Br–and BrO3–and other anions were purchased from AccuStandard Inc.N2O,O2and N2gases were of high purity(99.99%).All other chemicals were of analytical grade and obtained through J&K Chemical Ltd and used as received.De-ionized water by Millipore Q system was used throughout the experiments.
B.Sample irradiation
The irradiation experiments were performed in a60Co gamma source at Shanghai Institute of Applied Physics,Chinese Academy of Sciences.Adsorbed doses were measured by a ceric sulfate dosimetry system.Prepared NaBr aqueous solutions under air-equilibration or saturated with N2,O2or N2O by bubbling for 20min of high-purity N2,O2or N2O gases in 80mL Pyrex glass tubes,were irradiated to 0.5~10kGy.Inorganic anions of nitrate,sulfate,chloride,and(bi)carbonate, were added in the form of their stock solutions of sodium salts. The solution pH was adjusted by adding perchloric acid or sodium hydroxide,and was adjusted to 7.0 unless otherwise stated.All experiments were carried out at ambient temperature.
C.Analysis methods
The concentrations of bromate and bromide and other anions were determined by a Dionex ICS-2000 reagent-freeion chromatograph,with an IonPac AS19 analytical colum (250mm×4mm ID),using 20mmoll−1potassium hydroxide eluent at a f l ow rate of 1mlmin−1.Sample volume loaded for all analysis was 200µl each.The detection limits of bromate and bromide were below 1µgl−1and 10µgl−1,respectively.The pH values were measured by a PHSJ-4A pH meter.
III.RESULTS AND DISCUSSION
A.Effect of atmosphere and initial concentration
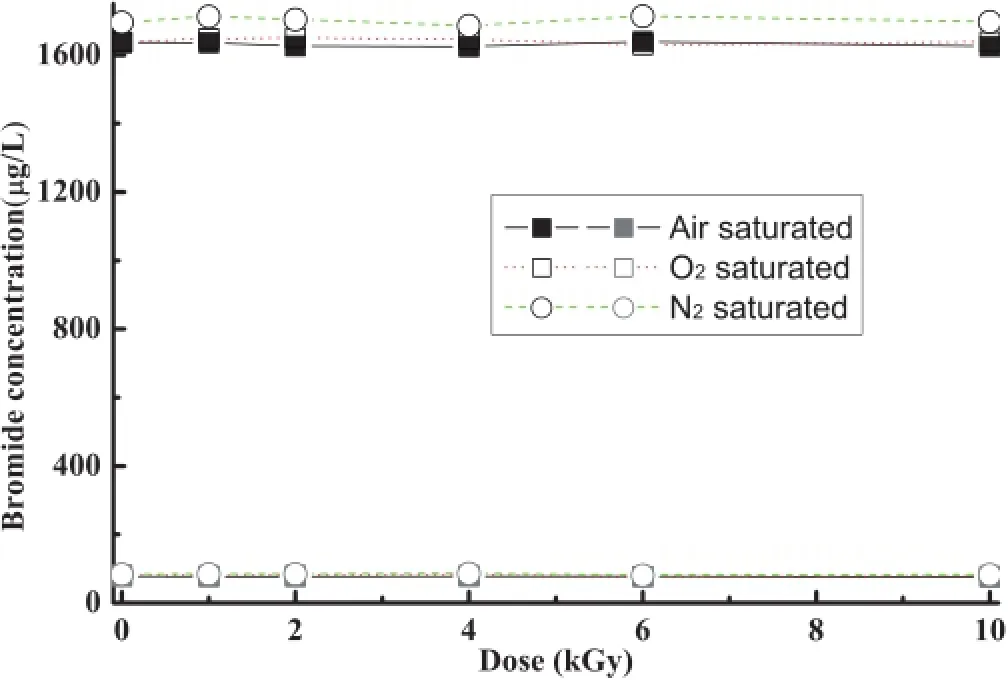
Fig.1.(Color online)Br–concentration as a function of absorbed dose and atmosphere.The datum points in square(□)and circle (◦)are of initial Br–concentration of approximately 80µgl−1and 1650µgl−1,respectively.
The results from samples saturated with air,N2,or O2and withoutotheradditives,areillustrated inFig.1.Forbothinitial Br–concentration of approximately 80µgl−1and 1650µgl−1, the concentration of Br–almost remained unchanged for all samples,and the BrO3–concentrations were all below the detection limit.It could be conclude from Fig.1 that Br–could not be oxidized into BrO3–under such conditions.In Ar saturated solutions with initial Br–concentrations from 800µgl−1to 80µgl−1.LaVerneet al.[7]found that the Br–concentrations kept constant up to 100kGy.
It is well known that·OH radicals could oxidize Br–to BrOH–·,which then transforms to Br·,Br2–·and other bromine species[8–10].From Fig.1,the oxidized bromine species could be reduced effectively bye−aq,H·and/or O2–·/HO2·andBr–reformed.Possible reactions are listed below[7,9]:
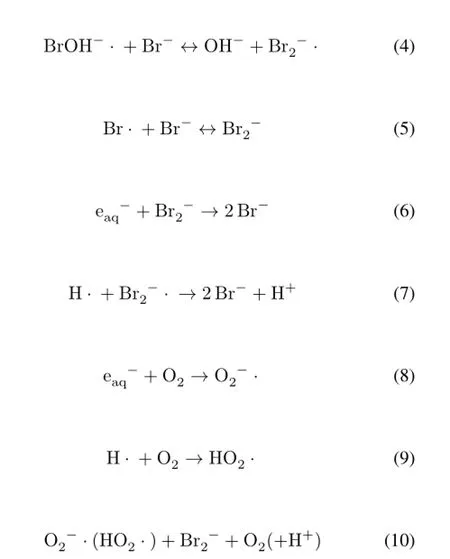
The numbers in Eq.(1)are called G-values,def i ned as the number of formed or decomposed molecules per 100eV absorbed energy.The reactions in Eqs.(8)–(10)take place in aqueous solutions saturated with air or O2.
On the other hand,as shown in Fig.2,the majority of bromide was oxidized to bromate in N2O saturated solutions even at 0.5kGy.The formation of bromate increased with increasing doses and initial bromide concentrations.At 2.0kGy,for initial bromide concentrations of 180.2µgl−1,416.9µgl−1, 663.1µgl−1and 823.9µgl−1approximately 79.5%,84.0%, 87.3%and 88.3%of bromide ions were transformed to bromate,respectively.And the analysis of bromide indicated that over 95%of the oxidized bromide was transformed to bromate forallthesamplesinFig.2.Thismeansthattheconcentrations of intermediates were low.
In the irradiating N2O-saturated solutions,the primary reactive radicals were·OH radicals due to reactions(11)and(12).

Whentert-butanol(acted as an·OH scavenger)was added to the solution of 0.1–25mM concentration,no bromate was detected after 4.0kGy irradiation for N2O saturated solutions with 823.9µgl−1Br–.From the results,one knows that·OH radicals can oxidize bromide to bromate.Von Gunten and Oliveras reported that bromate formed with·OH radicals being the only oxidants and HOBr/OBr–are requisite intermediates[11].

Fig.2.(Color online)Effect of initial Br–concentration and absorbed dose on bromate formation in N2O saturated bromide solutions.
B.Effect of pH
Figure 3 shows the effect of pH value on the formation of bromate in N2O saturated bromide-containing waters.The formation of bromate was nearly the same at pH 3.5–7.0,and decreased by about 5%at pH 2.5.At the pH 10.8,however, the bromate concentration decreased to 46.0µgl−1from about 550µgl−1in neutral conditions.
This shows the pH value dependence of G-values of the species formed in water radiolysis[12].While the G-values of·OH,e−aqand·H are almost constant in near neutral conditions, theH+in higher concentration can react with eaq−(H++ eaq−→·H),hence the decreased concentration of·OH radicals according to Eq.(11)[12],which resulted in a small decrease of bromate concentration compared to neutral conditions.In alkaline conditions,the G-value of·OH radicals decreases as they are transformed to less reactive·O–species, and more reductive species,like eaq–and less reactive HO2–·, were formed[12].The oxidized bromide ions may be reduced by these reductive species.Therefore,the bromate formed at pH 10.8 was much less than that at lower pH values.
C.Effect of common anions
Natural waters are complex matrixes,containing anions that may interfere with the oxidation of bromide by competing with the free radical species.To investigate the inf l uence of common anions in drinking waters,0.1–25mM of Cl–,CO32–/HCO3–,NO3–or SO42–were added to bromidecontaining solutions.The results from solutions saturated with air,N2orO2showedthatnobromatewasfoundat0.5–6.0kGy for solutions in initial bromide concentrations of 81.7µgl−1and 1636.6µgl−1.
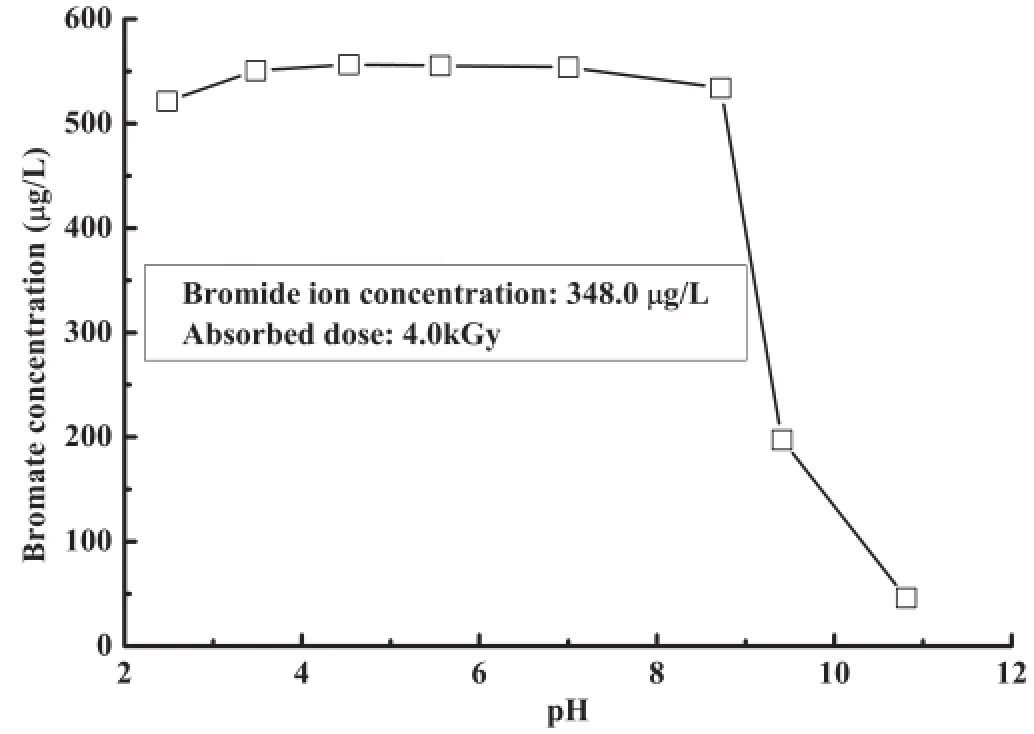
Fig.3.Effect of pH on bromate formation for N2O saturated bromidecontaining waters.

Fig.4.(Color online)Effect of common anions on bromate formation for N2O saturated bromide-containing waters.
Figure 4 depicts the inf l uence of the selected common anions on the formation of bromate in N2O saturated waters. The amounts of bromate ion formed were unaffected by SO42–ion,and increased slightly with the Cl–ions.However,in the presence of CO32–/HCO3–(predominantly HCO3–when the solution was neutral),the bromate concentration decreased notably to 44.2µgl−1from 554.0µgl−1added with 1mM CO32–/HCO3–after 4.0kGy irradiation.The bromate concentration decreased signif i cantly only at high NO3–concentrations,as depicted in Fig.4.This shows that the bromate concentration was not substantially affected by 1mM NO3–addition,whereas added with 10mM NO3–,it decreased from 554.0µgl−1to 190.1µgl−1.
Cl–ions could react with·OH radical quickly to form ClOH·(k=4.3×109mol−1s−1),but ClOH–·may reform·OH radical by the fast reverse reaction(k=6.1×109s−1) in neutral solutions[13].Then,the slightly increased bromate formation in the presence of Cl–ion might be due to less radical recombination of·OH radicals(such as·OH+·H→H2O,·OH+·OH→H2O2,etc.)
Both CO32–and HCO3–react with·OH radicals and form·CO3–radicals[13].The results in Fig.4 indicated that the·CO3–radical only cannot oxidize Br–and other bromine species to bromate.In the ozonation process,however,·CO3–radicals,which are also produced from·OH,can oxidize BrO–toBrO·(k=4.3×107molL−1s−1)andpotentiallyleadtoan increase in bromate due to the presence of O3[14].
NO3–ion is not as eff i cient an·OH scavenger as CO32–/HCO3–,asrevealedinFig.4,butforhigher NO3–ion concentrations,NO3–ion can compete with N2O for·eaq−(NO3−+·eaq−→NO32−,k= 9.7×109molL−1s−1),resulting in the notably decrease of·OH concentration and then the decrease of bromate formed.
IV.CONCLUSION
γ-rays irradiation of bromide-containing aqueous solution in different conditions was investigated.It is found that bromate can be formed only in N2O saturated solutions,in which the primary reactive radicals were·OH radicals.Adding CO32–/HCO3–or NO3–ions to N2O saturated bromide solutions can decrease the bromate formation.Bromide concentrations are found to remain constant in irradiated N2,O2or air saturated bromide solutions irradiated to 0.5–6kGy.When NO3–,Cl–,CO32–/HCO3–,SO42–or tert-butanol was added, no bromate was found in the irradiated bromide solutions saturated by N2,O2or air.This study indicated that,instead of ozonation,γ-rays irradiation can be used as a disinfection process especially in high-bromide waters,because of a continuous pressure from regulators to further lower bromate drinking water standards,and the fact that few practical methods can be used to reduce bromate formation in ozonation or remove bromate after its formation to levels well below 10µgL−1or even below the current detection limits.
[1]World Health Organization,Guidelines for drinking–water quality.Geneva,Switzerland,2011.
[2]ChinaMinistryofHealth,GB5749–2006,2006,ChinaStandard Press:Beijing.
[3]Von Gunten U.Water Res,2003,37:1469–1487.
[4]Antoniou M G,Andersen H R.Environ Technol,2012,33: 1747–1753.
[5]Ozekin K,Westerhoff P,Amy G L,et al.J Environ Eng–Asce, 1998,124:456–462.
[6]Mizuno T,Yamada H,Tsuno H.Ozone–Sci Eng,2004,26:573–584.
[7]LaVerne J A,Ryan M R,Mu T.Radiat Phys Chem,2009,78: 1148–1152.
[8]Matheson M S,Mulac W A,Weeks J L,et al.J Phys Chem, 1966,70:2092–2099.
[9]Mamou A,Rabani J,Behar D.J Phys Chem,1977,81:1447–1448.
[10]Zehavi D,Rabani J.J Phys Chem,1972,76:312–319.
[11]Von Gunten U,Oliveras Y.Environ Sci Technol,1998,32:63–70.
[12]Getoff N.Radiat Phys Chem,1996,47:581–593.
[13]Buxton G,Greenstock C,Helman W,et al.J Phys Chem Ref Data,1988,17:513–886.
[14]Hofmann R,Andrews R C.Water Res,2006,40:3343–3348.
10.13538/j.1001-8042/nst.25.010301
(Received May 29,2013;accepted in revised form December 15,2013;published online February 20,2014)
∗Supported by National Natural Science Foundation of China(No. 10979020)
†Corresponding author,caochangqing@sinap.ac.cn
 Nuclear Science and Techniques2014年1期
Nuclear Science and Techniques2014年1期
- Nuclear Science and Techniques的其它文章
- Feasibility study on optical vortex generation at Shanghai deep ultraviolet free-electron laser∗
- Lattice design and optimization of the SSRF storage ring with super-bends
- Temperature and carrier-density dependent excitonic absorption spectra of semiconductor quantum wires∗
- Adsorption behavior of uranyl ions onto amino-type adsorbents prepared by radiation-inducedgraft copolymerization∗
- A 16-Channel high-resolution time and charge measurement module for the external target experiment in the CSR of HIRFL∗
- Radiation tolerance studies on the VA32 ASIC for DAMPE BGO calorimeter∗
