Evaluation of the use of surrogateLaminaria digitatain eco-hydraulic laboratory experiments*
PAUL Maike
Forschungszentrum Küste, Leibniz Universität Hannover, Hannover, Germany, E-mail: paul@fzk-nth.de
HENRY Pierre-Yves T.
Department of Marine Technology, Norwegian University of Science and Technology, Trondheim, Norway
Evaluation of the use of surrogateLaminaria digitatain eco-hydraulic laboratory experiments*
PAUL Maike
Forschungszentrum Küste, Leibniz Universität Hannover, Hannover, Germany, E-mail: paul@fzk-nth.de
HENRY Pierre-Yves T.
Department of Marine Technology, Norwegian University of Science and Technology, Trondheim, Norway
(Received March 27, 2014, Revised May 11, 2014)
Inert surrogates can avoid husbandry and adaptation problems of live vegetation in laboratories. Surrogates are generally used for experiments on vegetation-hydrodynamics interactions, but it is unclear how well they replicate field conditions. Here, surrogates for the brown macroalgaeLaminaria digitatawere developed to reproduce its hydraulic roughness. Plant shape, stiffness and buoyancy ofL. digitatawere evaluated and compared to the properties of inert materials. Different surrogate materials and shapes were exposed to unidirectional flow. It is concluded that buoyancy is an important factor in low flow conditions and a basic shape might be sufficient to model complex shaped plants resulting in the same streamlined shape.
bending modulus, buoyancy, hydraulic forcing,Laminaria digitata, plant surrogate
Introduction
The interaction between hydrodynamics and vegetation is of ecological interest, and also concerns questions regarding coastal protection and river management. Coastal vegetation attenuates waves and mitigates storm flood effects[1]and can potentially be used for coastal protection measures[2,3]. Riparian vegetation can stabilize river banks[4]and submerged plants and plant parts can reduce flow rates[5,6], resulting in increased sedimentation rates[7]and potentially causing flow blockage and hence increase the risk of flooding. In order to predict these effects and their variability as well as assessing associated risks, it is important to understand the processes involved. One important tool for gaining insight into bio-physical interactions is the use of physical models, but such experiments face major challenges when using live vegetation in the laboratory. Requirements with respect to plant health, vitality and adaptation to different and changing environmental conditions limit the use of vegetation in hydraulic laboratories.
As an alternative, abiotic surrogates have been used to investigate how individual plant properties affect flow[8]and wave motion[9]or a combination of the two[10]. Bouma et al.[9]used materials with varying stiffness and identical shape to investigate how plants with different growth strategies performed under identical hydrodynamic forcing. Other studies used surrogates that resembled certain plant species in size, shape and stiffness[10,11]. However, bending behaviour was only compared visually between real plants and surrogates. It is not known whether such an optical similarity also reflects a hydraulic comparability and hence it is uncertain whether gained results can be transferred to real vegetation in the natural environment.
The aim of this study is therefore to evaluate how well abiotic surrogates can reproduce the hydraulic roughness of live vegetation and to investigate to what extend simplifications are possible while still yielding significant and transferrable results. The experiments are part of the eco-hydraulic work package (PISCES) of the HYDRALAB IV project which compares results from physical experiments to those obtained in the field. Vegetation, substrate and hydraulic conditions have been monitored at a field site and will be repro-duced in the laboratory as closely as possible. Consequently, hydrodynamic conditions in the close vicinity of plants in the field and laboratory will be measured using identical instruments (see Ref.[12]). The dominant species at the field site is the brown macroalgaeLaminaria digitatawhich was chosen as an example species for the present study.
1. Material and methods
1.1Plant species
The brown macroalgaeLaminaria digitatawill be modelled. This species was chosen as it dominates the region that is used for other field studies within the PISCES project.L. digitatacolonises the upper subtidal along rocky shores of the temperate zone. It consists of a holdfast, a single stipe and a large oval blade that can grow up to 2 m in length. The blade does not have a midrib and has a smooth glossy surface which is dark brown in colour and tapers slightly towards the tip where it can be ripped due to exposure to wave action[13].

Fig.1(a) Location of Hopavågen bay (63o35′37″N, 9o32′11″E)
Fig.1(b) Aerial view of the sampling area (www.norgeskart.no)
Specimens for this study were collected in the Hopavågen Bay, Sør-Trøndelag, Norway (Fig.1). The bay is connected to the Trondheim Fjord by a small inlet, which shelters it from waves and limits tidal variation. The tidal curve is strongly asymmetric and flood dominated, tidal elevation varies from 0.3 m during neap and up to 1 m during spring tides. The substrate is mainly comprized of sand, but is interspersed with gravel, cobbles and mussel shells which provide support forL. digitata. A more detailed description of the sampling location was given in Ref.[12].
1.2Measurement of plant and material properties
At the site, 20 specimens ofL. digitatawere collected and analysed for their morphological and physical properties in the laboratory within 48 h of collection. During this time period, the macroalgae were stored in salt water from the bay at 4oC and samples were kept under a damp cloth between tests.
Prior to analysis, the holdfasts were removed and were hence not part of this study. The thickness of stems and blades were measured with a calliper gauge to the nearest 5×10-5m. The surface area of the blades was obtained from digital photographs. Photographs were taken vertically from approx. 1.5 m height on a white background. Markers on the background were used to rectify the images in MATLAB®. From the RGB colour channels, the blue channel provided the strongest contrast between plant and background. Plant size was evaluated by defining pixels with a blue value <50 as part of the plant and computing the projected plant area from the number of pixels and the set pixel size of 10-6m2.
Each specimen was dried with a paper towel to remove excess water from the surface and was then weighed with a precision of ±0.0001kg . Mass density (mass/volume) and hence buoyancy was determined by measuring the plant’s displacement in unfiltered fresh water at 20oC.
In order to quantify the stiffness of plants, a three-point-bending test was performed for stems and parts of the blade cut from the base and the tip respectively. Stems were used in one piece while blade sections were cut to strips of 0.18 m length and 0.04 m width. Samples were clamped horizontally between metal plates, the distance between plates was 0.043 m for stems and 0.155 m for blade sections. Consequently, a metal bar of 0.015 m width was lowered manually onto the centre of the sample. The bar was attached to a force meter (FMI-250A5 from Alluris, resolution 0.001 N, precision ±0.05%). The required force to bend the sample was recorded in conjunction with the resulting deflection (resolution 10-5m). Tests were terminated when applied forces exceeded the maximum measurable load of the force meter (5 N). Manual operation of the test apparatus may have caused strain rate variations and hence may have introduced uncertainty into the measured material properties.Moreover, the tissue may creep under applied loads due to viscoelasticity[14]. Both sources of error were considered negligible in this case as algal tissues are considered reasonable insensitive to strain rate variations[15]and each loading lasted less than 5 s.
The force deflection curve was used to derive flexural rigidityJaccording to beam theory for a cantilever with one fixed end[16]

wheresis the distance between clamped ends of the sample,Pthe applied force andhthe resulting vertical deflection. For deflections ≤10% of the cantilever length, a best fit forP/hand consequentlyJwas computed after[16]and the bending/tangent modulusEwas derived. In this case,Eis defined as the ratio between flexural rigidity and the area second moment of inertiaIat the point of application of the force:

The latter is a geometric quantity that differs for samples with different cross-sectional shapes as it accounts for the size and shape of a sample.L. digitatastems have a relatively stiff wall and a soft pith in the centre. Strength and stiffness are solely attributed to the wall and it is assumed that the pith does not contribute to the stem's stability[14]. The cross-section forL. digitatastems is therefore approximated as a hollow circular shape (Is), while the blade sections are considered to be rectangular in cross-section (Ib):

withdis the diameter (outer and inner respectively),bis the sample width andtis the blade thickness.
The same method of analysis was applied to several inert materials in order to find materials with properties comparable toL. digitatastems and blades with the aim to produce adequate surrogates. Plastic tubing of 0.09 m length was investigated as possible material for stem models as they are hollow cylinders. For blade models, various materials were tested that were all cut to 0.18 m×0.04 m. For each material, three samples were tested.
1.3Surrogate development
Surrogates were developed from the chosen materials. The stems for all surrogates were produced from the same material with the length of 0.1 m, while three different materials (moosipren, artificial leather, geotextile) were used to mimic the blade ofL. digitata. To compare material performance, the surrogates all had the same elliptic shape with a surface area ofa= 0.13 m2. Additionally, different shaped surrogates with identical surface area (a=0.38 m2)were produced from one of the chosen materials (artificial leather) to assess the impact of shape complexity on hydrodynamics. These surrogates approximate the natural shape of aL. digitatablade to a closer (hand shape), intermediate (ellipse) or lesser (rectangle) degree.
1.4Laboratory experiments
The surrogates were exposed to unidirectional flow under controlled laboratory conditions to evaluate their effect on velocity and turbulence levels. Experiments were conducted in a 20 m long flume with a cross-sectional area of approx. 1 m×1 m at Franzius-Institute in Hannover, Germany. It is equipped with a pumping system that allows discharges of up to 200 l/s and a weir at the end to regulate water depth. Water depth was set to the maximum to provide full submergence of the surrogates and enable sufficient depth to collect velocity profiles that also cover the area above the surrogate. For the chosen flow velocities (0.1 m/s and 0.2 m/s) these were >0.4 m above the test section. The set velocities cover the typical velocity range encounteredL. digitatain the Hopavågen Bay.
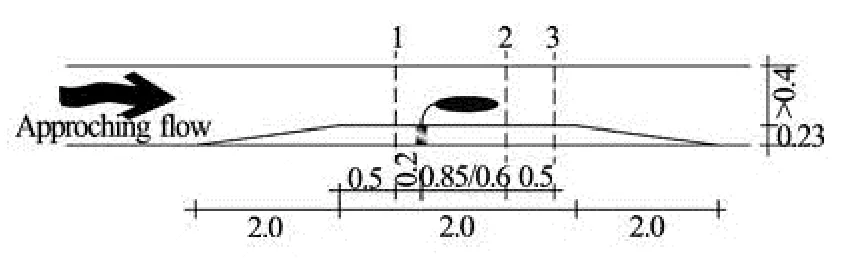
Fig.2 Experimental setup. dashed lines indicate ADV positions. dimensions (m)
The surrogates were deployed 10 m downstream of the inlet on an elevated test section of 2 m length (Fig.2). Profiles of the velocity components (u,vandwin the downstream, cross-stream and vertical direction, respectively) were recorded with an acoustic Doppler velocimiters (ADV, Sontek) placed 0.2 m in front of the surrogate, 0.15 m and 0.65 m behind the surrogate to monitor the size of a turbulent wake cau-sed by the surrogates. As the size of the surrogates varied, the two latter positions were not constant with respect to the flume setup but changed, depending on the experimental run. Velocity profiles consisted of 10 measurement points each, with a vertical spacing of 0.02 m, starting 0.04 m above the floor due to the limitations of the ADVs. Each measurement was taken at 100 Hz and lasted 4 min. Additionally, measurements were performed without surrogates, but with the test section in place for reference.

Table 1 Properties ofL. digitataparts and selected abiotic materials (± standard deviation). Mass density is given for dry/wet material
From the ADV records time averaged velocities (,and) and their fluctuations (u',v' andw') were computed at each sampling location and turbulent kinetic energy (TKE, Jm-3) was calculated withρbeing the density of water

Previous studies on the effect of vegetation on flow fields and turbulence have used the vegetation Reynolds numberRevto describe the vegetation-flow interaction non-dimensionally[17-19].Revrequires a length scale specific for the vegetation and for simple, strip like vegetation (e.g., seagrass) the width of the plant can be used. For complex shaped vegetation and surrogates, however, it is debatable which length scale is adequate to enable comparison between specimens. It was therefore refrained from usingRevin this study.
2. Results and discussion
2.1Property comparison
TheL. digitataspecimens tested during this study (Table 1) had a mean weight of 0.2037± 0.0907kgand amass density of 1001.5±102.7 kg/m3which makes them almost neutrally buoyant. The plants had a projected area of 0.21± 0.05 m2and were hence relatively small for this species. In comparison to other populations,L. digitatain Hopavågen produced short stems (0.093±0.019 m) which may have been caused by the habitat’s substrate conditions. Pebbles and shells, to which holdfasts can attach, are spaced widely apart within the Hopavågen Bay andL. digitataspecimens cannot grow close to one another. Consequently, specimens do not have to compete for light availability and it is sufficient to produce a short stipe.

Fig.3 Force deflection curves forL. digitataand curves for selected materials
The bending moduli for stems (28.67± 13.22 MPa) are approx. one third of bending moduli derived for anL. digitatapopulation (83.8± 84.1MPa) on the island of Helgoland, Germany[20]. The high standard deviation in Harder’s[20]data indicates a high natural variability and hence a careful interpretation of the differences to the present study isrequired. The two populations are likely to be exposed to different biotic and abiotic conditions (e.g., salinity, water temperature, nutrient availability) and the Helgoland population will also experience higher wave exposure than the specimens in the Hopavågen Bay. It is assumed that such differences in environmental conditions can lead to differences in mechanical plant properties. Moreover, the testing methods applied in the two studies differed and in conjunction with possibly different environmental conditions during analysis may have led to the different values obtained for plants of the same species[16,21].

Table 2 Time and space averaged velocities and TKE (±standard deviation) for all profiles and velocity settings
Classic 3-point bending tests usually require a ratio equal or lower than 16 between sample length and thickness[21]. This condition could not be met for the tests onL. digitatablades and therefore E has been named “bending modulus” for the stems and “tangent modulus” for the blades[16]. Comparison of bending/ tangent moduli for stems and blades (Table 1) showed a distinct difference between plant parts and the difference between blade base (3073.55±1627.08 MPa) and tip (13355.75±8385.6 MPa)was statistically significant (p<0.01), highlighting the inhomogeneity of blade tissue. The disparity between stems and blades suggests a difference in bending behaviour which also reflects in the force deflection curves (see Fig.3). Within the force range applied during this study(<5 N) stems showed a linear relationship between force and deflection while blade sections resulted in a J-shaped curve with different slopes in the upper and lower parts of the relationship. These variations may be generated by the use of the same method for two different types of sample (stiff stems and very flexible blades). Therefore, direct comparison between bending moduli for stems and tangent moduli for blades should be avoided, particularly when attempting to compare absolute values to results obtained with other methods. However, qualitative comparison between stiff materials and stems or flexible materials and blades remains possible in order to determine a surrogate material with a similar flexibility.
A total of eight different tubes and 15 sheet like abiotic materials were tested to evaluate their comparability toL. digitatastems and blades, respectively. A comprehensive list can be found in the appendix and the materials considered most suitable for surrogate development are listed in Table 1.
2.2Results of laboratory experiments
In the control runs, velocity profiles were logarithmic and values averaged over the profiles indicated that the downstream flow dominated and both crossstream and vertical velocities were negligible (Table 2).
The three materials showed very different posture when exposed to the flow which can be attributed to their mass density. Moosipren is highly buoyant (wet mass density of 280.6 kg/m3) and remained upright in still water conditions, even penetrating the water surface. The slow flow scenario was not sufficient to overcome this buoyancy and the surrogate only bent and streamlined at the high flow velocity. The geotextile had a similar dry mass density (258.1 kg/m3) but had open pores. Consequently, it soaked with water resulting in a wet mass density(1 093.8 kg/m3) higher than water, it hovered close to the ground under both flow conditions. The artificial leather (wet mass density of 938.0 kg/m3) remained floating at the surface in still water conditions and the tips of the large hand shaped surrogate remained at the surface during low flow. But at a flow of 0.2 m/s all of the artificial leather surrogates streamlined at approx. 0.05 m-0.15 m above the ground.
Apart from the moosipren in low flow, the surrogates moved very little due to the lack of turbulence in the approaching water (Table 2). Only the upright posture of moosipren in a free stream flow velocity of 0.1 m/s caused an undulating motion that moved the surrogate beyond the vertical against the approaching flow for short periods of time.
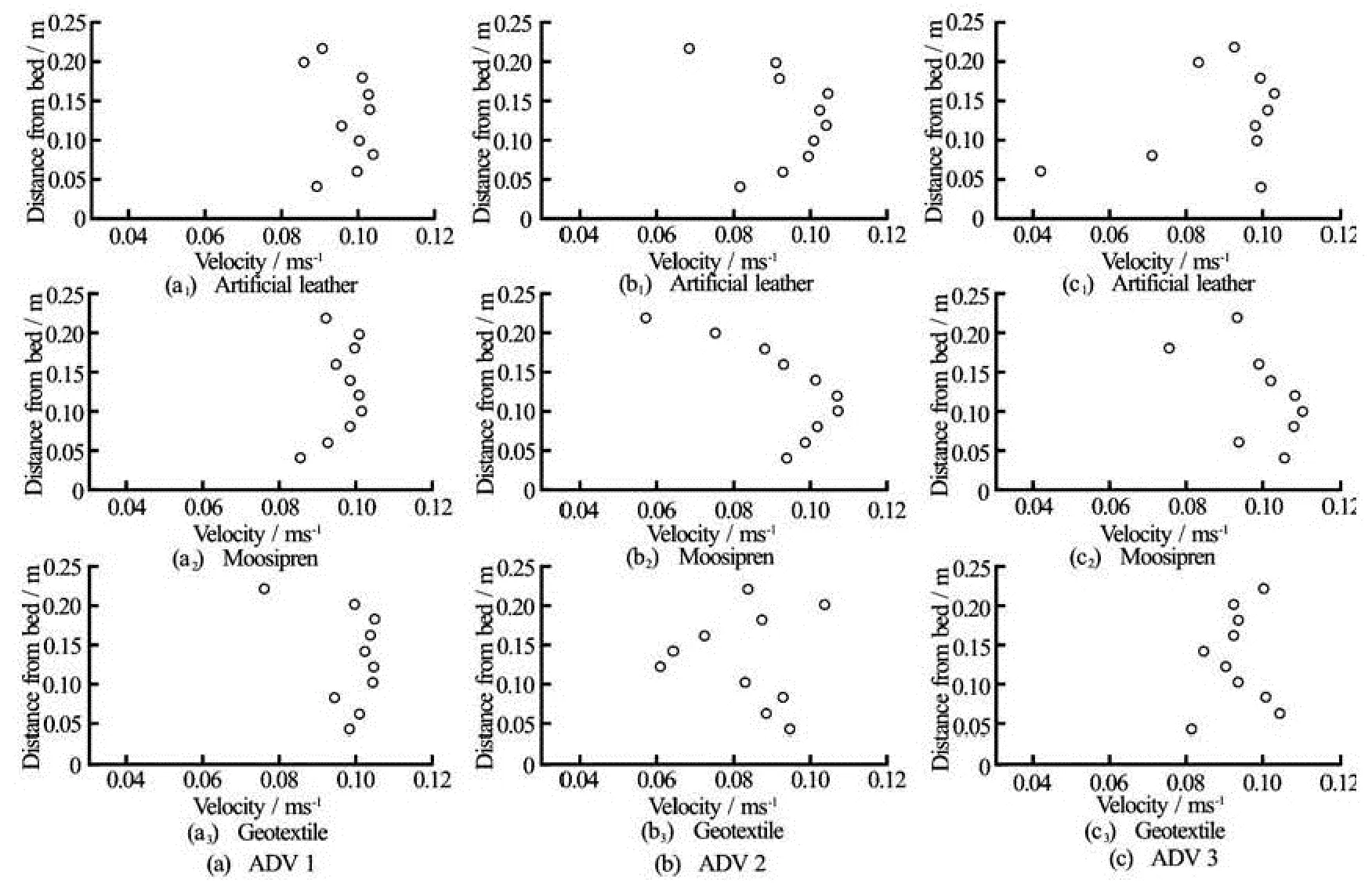
Fig.4 Velocity profiles () for elliptic surrogates (a=0.13m2)of different materials under identical hydrodynamic forcing
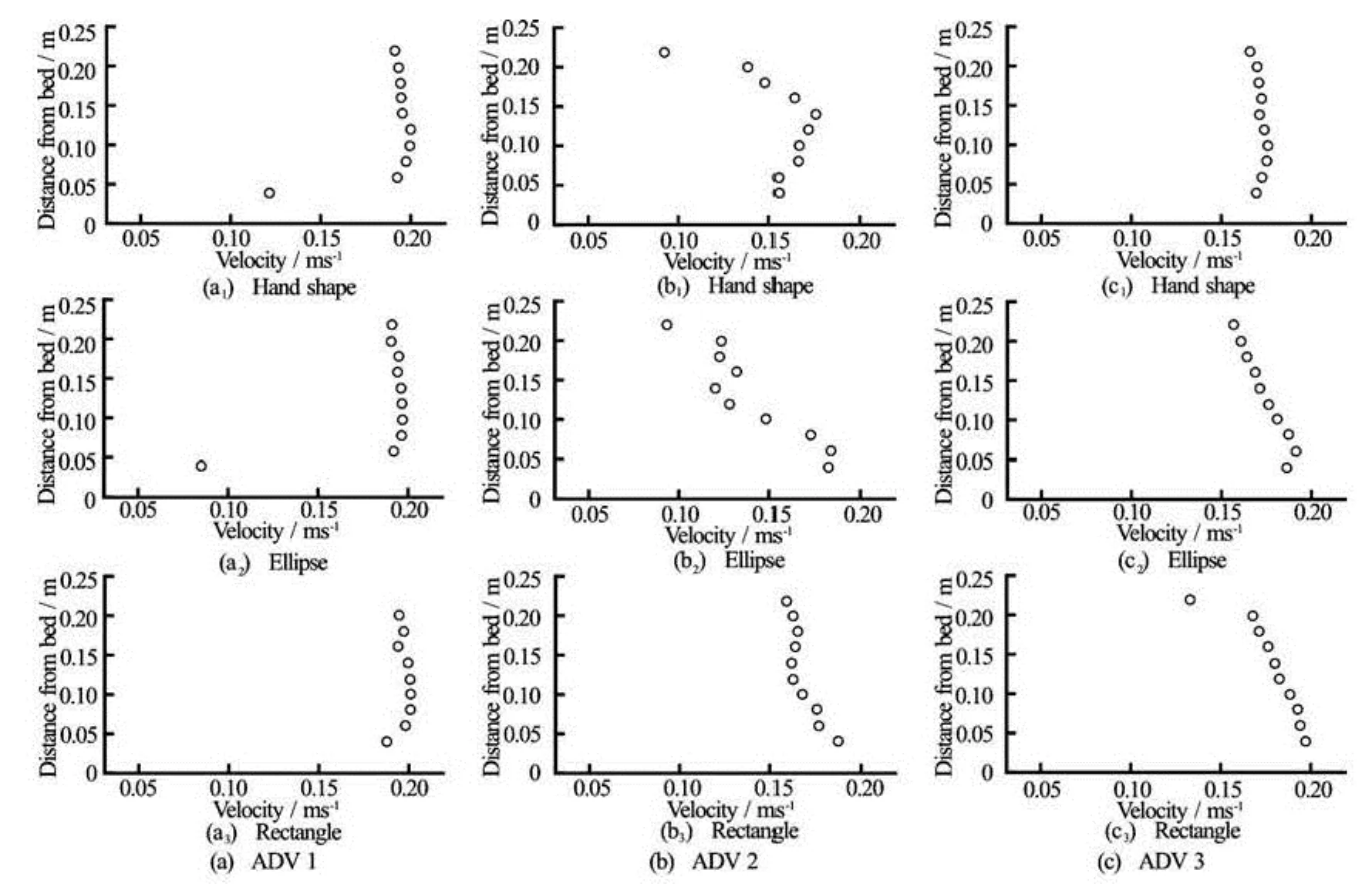
Fig.5 TKE for elliptic surrogates (a=0.13m2)of different materials under identical hydrodynamic forcing, corresponding to velocity profiles in Fig.4
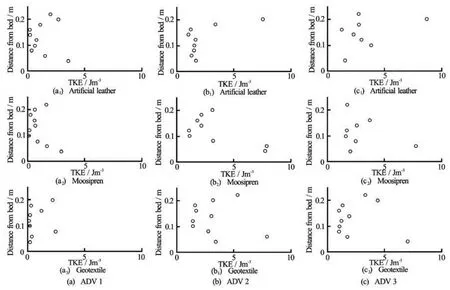
Fig.6 Velocity profiles () for artificial leather surrogates (a=0.38 m2)of different shapes under identical hydrodynamic forcing
Velocity profiles were affected by the presence of surrogates, irrespective of their material and posture. This included the profiles 0.2 m in front of them (ADV 1). At this position, the velocity profile was still roughly logarithmic (see Fig.4) but the values ofwere more scattered than in the profiles of the control runs. This upstream effect of flexible structures agrees with observations made during measurements in a vegetated stream, where the effect of aRanunculus penicillatuspatch could be observed in the velocity profiles 0.5 m upstream[22]although the flow diversion during the present study was not so pronounced. This could be explained with the different values of the ratio between stream/flume width and size of the vegetation. In the field study,Ranunculus penicillatusoccupied a large part of the stream’s cross-section, leading to a blockage high enough to cause water head loss[22], while in the present study no water head loss could be observed due to the small ratio of flume cross-section to frontal area of a single surrogate.
Directly behind the surrogates (ADV 2), the artificial leather and moosipren surrogates yielded similar velocity profiles despite the difference in posture. They showed a peak in the centre of the profile and reduced velocities just above the bed and above the surrogate (see Fig.4). The geotextile, on the contrary, produced a scatteredprofile without a clear trend. At the last position 0.5 m further downstream (ADV 3), the effect of surrogate presence reduced again to a scattered profile similar to ADV 1 and no difference between materials or posture was observed. Especially the profile similarity for moosipren and artificial leather was unexpected, given their difference in posture at low flow. However, the observed differences reflect in the caused turbulence at ADV 2. Moosipren obstructed the flow mainly in the upper part (z>0.1m) of the profile where the blade was positioned, while the artificial leather surrogate bent and hence obstructed the flow in the lower part of the profile (z<0.15 m). These areas of obstruction correspond to lower TKE while turbulence is increased in unobstructed profile regions for both surrogates (Fig.5). This trend still persists 0.5 m further downstream (ADV 3), but is not as pronounced anymore.
Under high flow, the moosipren surrogate also deflected and blade posture changed from buoyancy to stiffness dominated[23]. The obtained posture was similar to the one of the artificial leather, while the geotextile remained closer to the bed. This behaviour reflects the observations made during bending tests, where the force-deflection curves for moosipren and artificial leather were not significantly different while the geotextile yielded a different curve (Fig.3(b)).

Fig.7 TKEfor artificial leather surrogates (a=0.38 m2)of different shapes under identical hydrodynamic forcing, corresponding to velocity profiles in Fig.6
Tests with the different shaped surrogates yielded a decrease inin the upper part of the profile at ADV 1, although the intensity of decrease varied with shape (see Fig.6). The effect continued further downstream (ADV 3) for the rectangle and ellipse, resulting in the same almost linear profile, while the profile for the hand shaped surrogate returned back to the profile shape in front of the surrogate. All three shapes led to increased TKE in the upper part of the profile, but the profile for the hand shaped surrogate was more scattered than the other two (Fig.7). This increased scatter may be due to the higher overall length of the hand shaped surrogate. Hence the tip of the surrogate was closer to the profile than for the other two surrogates and turbulence levels may fluctuate more closer to the surrogate. At ADV 3, however, no difference in turbulence could be observed between the three shapes. In addition to the shape and biomechanic properties, other parameters such as the blade surface roughness can affect vegetation-flow interactions and may need to be considered in designing surrogates. However, Albayrak et al.[24]found that leaf surface roughness does not affect the flow field at spatial scales considered in this study and this aspect was therefore not addressed here. Nevertheless, it should be part of future work to improve plant modelling, particularly for numerical applications.
All surrogates curled to the shape of a funnel under hydrodynamic forcing. The diameter of the funnel increased with increasing buoyancy for the three surrogate materials, but was independent of shape. This curling may be an artefact of the flume, where wall effects may have caused lateral forces that lead to a curling response of the surrogates, although no significant lateral velocities existed in the obtained velocity profiles that may have indicated such an effect. No such curling has been observed for naturalL. digitataspecimen in the field (Paul, pers. obs.) but have been reported for fresh water macrophytes in laboratory settings (E. Penning, pers. com.). Hence, further tests with natural plants and surrogates under identical conditions are required to evaluate this behaviour.
3. Conclusions
This preliminary study confirms the observations that flexible structures streamline under hydrodynamic forcing depending on their buoyancy and stiffness[23]and in return affect velocities and turbulence levels downstream as well as upstream of the structure.
Identically shaped surrogates of different materials lead to differences in velocity and turbulence profiles directly behind the surrogates. Velocity profiles are more scattered with increased flexural rigidity while turbulence profiles depend on the posture of the surrogates in the water column with higher turbulence levels in the regions that are not directly obstructed by the surrogates. However, further 0.5 m downstream, no significant differences in profiles between the different materials could be observed.
For the different shaped surrogates, the differences in velocity and turbulence are reduced with in-creasing flow velocity, which may be due to the overall similar posture of the surrogates after streamlining. The present study therefore suggests that a simple shaped surrogate may be an adequate substitute for real complex shaped plants, given it assumes the same posture when streamlining. However, detailed research is necessary to evaluate whether the observed curling represents natural behaviour under the given hydrodynamic forcing.
Acknowledgements
The authors thank the members of the PISCES project for their assistance during field work. The work described in this publication was supported by the European Community’s 7th Framework Programme through the grant to the budget of the Integrated Infrastructure Initiative HYDRALAB-IV, Contract No. 261520.
[1] KOCH E. W., BARBIER E. B. and SILLIMAN B. R. et al. Non-linearity in ecosystem services: Temporal and spatial variability in coastal protection[J].Frontiers in Ecology and the Environment,2009, 7(1): 29-37.
[2] FEAGIN R. A., MUKHERJEE N. and SHANKER K. et al. Shelter from the storm? Use and misuse of coastal vegetation bioshields for managing natural disasters[J].Conservation Letters,2010, 3(1): 1-11.
[3] FEAGIN R. A., IRISH J. L. and MÖLLER I. et al. Short communication: Engineering properties of wetland plants with application to wave attenuation[J].Coastal Engineering,2011, 58(3): 251-255.
[4] THOMAS R. E., POLLEN-BANKHEAD N. Modeling root-reinforcement with a fiber-bundle model and Monte Carlo simulation[J].Ecological Engineering,2001, 36(1): 47-61.
[5] BAL K. D., BOUMA T. J. and BUIS K. et al. Trade-off between drag reduction and light interception of macrophytes: Comparing five aquatic plants with contrasting morphology[J].Functional Ecology,2011, 25(6):1197-1205.
[6] MILER O., ALBAYRAK I. and NIKORA V. I. et al. Biomechanical properties of aquatic plants and their effects on plant-flow interactions in streams and rivers[J].Aquatic Science,2012, 74(1): 31-44.
[7] BOS A. R., BOUMA T. J. and De KORT G. L. J., et al. Ecosystem engineering by annual intertidal seagrass beds: Sediment accretion and modification[J].Estuarine, Coastal and Shelf Science,2007, 74(1-2): 344-348.
[8] GHISALBERTI M., NEPF H. M. Mixing layers and coherent structures in vegetated aquatic flows[J].Journal of Geophysical Research: Oceans,2002, 107(C2): 3-1-3-11.
[9] BOUMA T. J., De VRIES M. B. and LOW E. et al. Trade-offs related to ecosystem engineering: A case study on stiffness of emerging macrophytes[J].Ecology,2005, 86(8): 2187-2199.
[10] PAUL M., BOUMA T. J. and AMOS C. L. Wave attenuation by submerged vegetation: Combining the effect of organism traits and tidal current[J].Marine Ecology Progress Series,2012, 444: 31-41.
[11] STRATIGAKI V., MANCA E. and PRINOS P. et al. Large-scale experiments on wave propagation over Posidonia oceanica[J].Journal of Hydraulic Research,2011, 49(Supp1.): 31-43.
[12] THOMAS R. E., Mclelland S. J. and FROSTICK L. E. The impact of macroalgae on mean and turbulent flow fields[C].Proceedings of the 35th IAHR World Congress: The Wise Find Pleasure in Water.Chengdu, China, 2013.
[13] LOBBAN C., HARRISON P.Seaweed ecology and physiology[M]. Cambridge, UK: Cambridge University Press, 1997.
[14] DUNN H., DABNEY S. M. Modulus of elasticity and moment of inertia of grass hedge stems[J].Transactions of the ASAE,1996, 39(3): 947-952.
[15] GAYLORD B., HALE B. B. and DENNY M. W. Consequences of transient fluid forces for compliant benthic organisms[J].Journal of Experimental Biology,2001, 204: 1347-1360.
[16] PAUL M., HENRY P. T. and THOMAS R. E. Variation of mechanical properties for four northern European brown macroalgae[J].Coastal Engineering,2014, 84: 73-80.
[17] PAUL M., AMOS C. L. Spatial and seasonal variation in wave attenuation over Zostera noltii[J].Journal of Geophysical Research: Oceans,2011, 116(C8): C08019.
[18] BRADLEY K., HOUSER C. Relative velocity of seagrass blades: Implications for wave attenuation in lowenergy environments[J].Journal of Geophysical Research: Earth Surface,2009, 114(F1): 1-13.
[19] MÉNDEZ F. J., LOSADA I. J. and LOSADA M. A. Hydrodynamics induced by wind waves in a vegetation field[J].Journal of Geophysical Research: Oceans, 1999, 104(C8): 18383-18396.
[20] HARDER D., HURD C. and SPECK T. Comparison of mechanical properties of four large, wave-exposed seaweeds[J].American Journal of Botany,2006, 93(10): 1426-1432.
[21] BOWER A. F.Applied mechanics of solids[M]. Boca Raton, USA: CRC Press, 2010.
[22] PAUL M., THOMAS R. E. and KEEVIL G. M. Concurrent field measurements of turbulent velocities, plant reconfiguration and drag forces on Ranunculus penicillatus[C].10th International Conference on Fluvial Sedimentology.Leeds, UK, 2013.
[23] LUHAR M., NEPF H. M. Flow-induced reconfiguration of buoyant and flexible aquatic vegetation[J].Limnology and Oceanography,2011, 56(6): 2003-2017.
[24] ALBAYRAK I., NIKORA V. I. and MILER O. et al. Flow-plant interactions at a leaf scale: Effects of leaf shape, serration, roughness and flexural rigidity[J].Aquatic Science,2012, 74(2): 267-286.
Appendix

Table A1 Properties of tested abiotic sheet materials as possible blade surrogates. All samples were 0.04 m wide and 0.18 m long, mass and density were only evaluated for one sample each
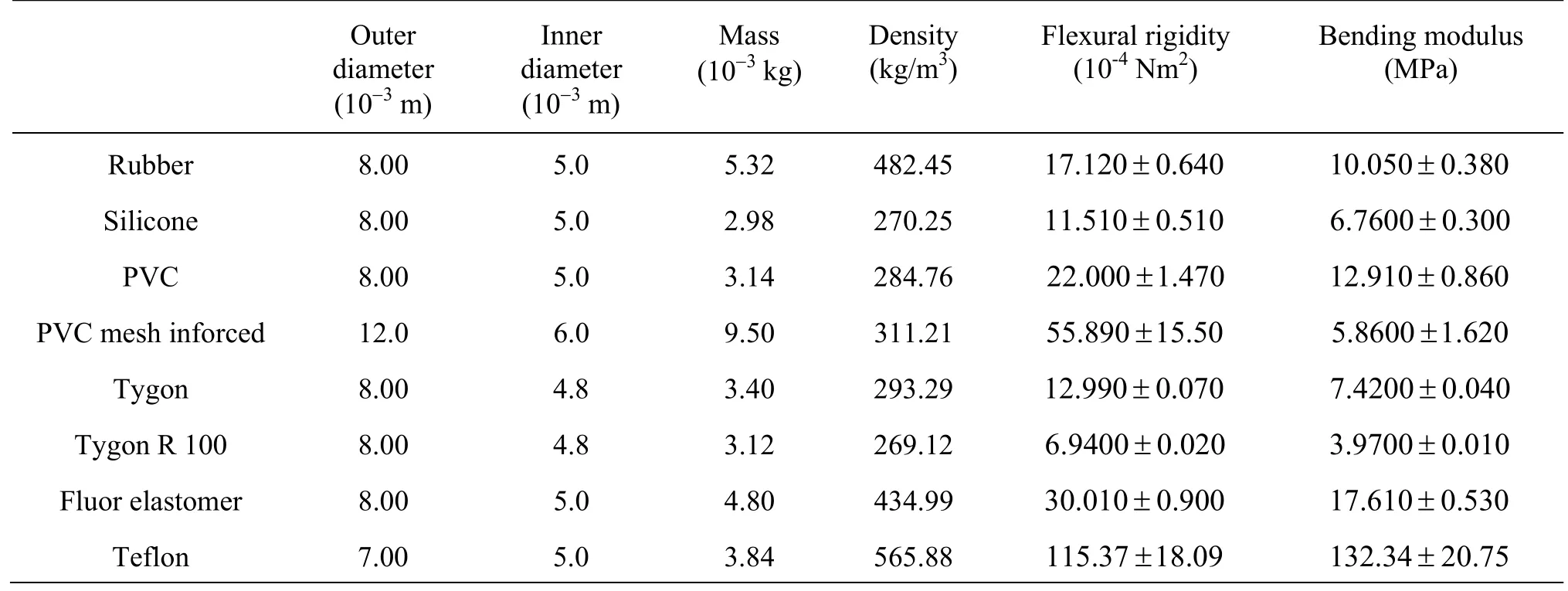
Table A2 Properties of tested abiotic tube materials as possible stem surrogates. All samples were 0.09 m long
10.1016/S1001-6058(14)60042-1
* Biography: Maike Paul (1978-), Female, Ph. D., Researcher
- 水动力学研究与进展 B辑的其它文章
- Numerical simulation of landslide-generated impulse wave*
- A random walk simulation of scalar mixing in flows through submerged vegetations*
- Scaling of maximum probability density function of velocity increments in turbulent Rayleigh-Bénard convection*
- Dispersion in oscillatory electro-osmotic flow through a parallel-plate channel with kinetic sorptive exchange at walls*
- A numerical study on dispersion of particles from the surface of a circular cylinder placed in a gas flow using discrete vortex method*
- Numerical simulation of the aerodynamic characteristics of heavy-duty trucks through viaduct in crosswind*

