Regulation of Moxibustion on the Expression of NF-κBp65 and PPARγ mRNA in Colon of Rats with Ulcerative Colitis
1 Shanghai Research Institute of Acupuncture and Meridian, Shanghai 200030, China
2 Changhai Hospital of Shanghai,the Second Military Medical University, Shanghai 200433, China
BASIC STUDY
Regulation of Moxibustion on the Expression of NF-κBp65 and PPARγ mRNA in Colon of Rats with Ulcerative Colitis
Feng Xiao-ming1, Cheng Tong-bin1, Wu Huan-gan1, Liu Hui-rong1, Zhou Shuang2
1 Shanghai Research Institute of Acupuncture and Meridian, Shanghai 200030, China
2 Changhai Hospital of Shanghai,the Second Military Medical University, Shanghai 200433, China
Author: Feng Xiao-Ming, M.D., research assistant
Objective: To explore the mechanism of herb-partitioned moxibustion for ulcerative colitis (UC) through observing the colonic mucosal histopathological changes and the expression of nuclear factor kappaB (NF-kB) and peroxisome proliferators-activated receptor γ (PPARγ) mRNA of UC rats.
Methods: Male SD rats were randomly divided into a normal group and a model group. UC model was established by general immunological plus local irritation method. After model identification, rats in the model group were randomly divided into a model group, a herb-partitioned moxibustion (HPM) group and a Western medicine (Salicylazosulfapyridine, SASP) group. Rats in the HPM group received treatment at bilateral Tianshu (ST 25) and Dachangshu (BL 25), two cones for each point, once a day for 7 d. SASP group rats were gavaged with SASP. The pathological scores were evaluated according to hematoxylin-eosin (HE) staining of colonic tissues. We used light microscopy to observe degree of colonic mucosal damage and the quantitative polymerase chain reaction (QPCR) to detect the expression of NF-κBp65 and PPARγ in colorectal mucosa.
Results: Compared with the normal group, histopathological scores were significantly higher in the model group (P<0.05); compared with the model group, the scores were decreased significantly in the HPM group and the SASP group. Compared with the normal group, NF-κBp65 mRNA expression was increased with statistical significance in the model group (P<0.05); compared with the model group, NF-κBp65 mRNA expressions were decreased significantly in the HPM group and the SASP group. Compared with the normal group, PPARγ mRNA expression was increased significantly in the model group (P<0.05); compared with the model group, PPARγ mRNA expressions were decreased significantly in the HPM group and the SASP group.
Conclusion: HPM could improve the mucosa damage of UC rats, which is possibly through down-regulating NF-κBp65 to achieve anti-inflammatory effect. Whether decreasing the PPARγ mRNA is possibly involved in preventing precancerosis will need further study.
Colitis, Ulcerative; Moxibustion Therapy; Indirect Moxibustion; NF-kappa B; PPAR gamma; Rats
Ulcerative colitis (UC) is a non-specific inflammatory bowel disease that is chronic and recurrent, mainly involving colon mucosa. The main symptoms are abdominal pain, diarrhea mixed with blood and mucus. The duration is long, with varying severity and alternative mitigation and episodes. UC canoccur at any age, but is more common in young adults with no sex difference. UC has become a recognized precancerous state and a refractory disease. Its pathology is mainly the interaction between susceptibility genes, environmental factors and immune factors that cause activation of mucosal immunocytes to produce inflammatory cytokines and mediators, which caused intestinal mucosa inflammation. Clinical and experimental studies have shown that acupuncture has a good effect on UC to relieve abdominal pain, diarrhea and other symptoms, and therefore it is worthy of further study[1-2].
Immune dysfunction is one of the major causes of UC. Nuclear factor κappa B (NF-κB) was highly expressed in the colon of patients with UC. NF-κB regulates transcription and release of interleukin-1 β (IL-1β), tumor necrosis factor-α (TNF-α) and IL-10 etc.[3], and thus participates in the process of inflammation and immune response in the intestine of patients with UC, which suggests NF-κB activation may be the trigger of UC occurrence and development. Peroxisome proliferators-activated receptor γ (PPARγ) is a member of ligand-activated nuclear receptor superfamily, and plays an important role in inflammation and tumor formation. This study investigated the effects of moxibustion on NF-κBp65 and PPARγ expression in UC rat colon to provide scientific experimental evidence for the mechanism of moxibustion treatment.
1 Material and Methods
1.1 Experimental animals
Forty healthy and clean male Sprague-Dawley (SD) rats weighing 180-200 g were purchased from Shanghai Super B&K Laboratory Animal Company (certificate number: 2008001619613), fed by the Experimental Animal Center of Fudan University. The feeding conditions were clean grade, temperature at 18-22 ℃, and relative humidity of 50%-70%, free access to water and food.
1.2 Main instruments and reagents
Tissue dehydration, embedding machine and slicing machine (Leica, Germany), hand-held homogenizer (Polytron, USA), LC96 PCR amplification (ROCHE, Switzerland), Elx800 microplate reader (Bio-TEK, USA), NanoDrop 2000 minim spectrophotometer (Thermo, USA), ethanol and isopropanol (Sinopharm Chemical Reagent Co., Ltd., China), chloroform (Shanghai Leimeng Technology Co., Ltd., China), Trizol (Invitrogen, USA), iScript™ cDNA Synthesis Kit and iQ™ SYBR® Green Supermix (BIO-RAD, USA), primers synthesized by Shanghai Biological Engineering Technology Services Co. Ltd.: NF-κBp65 (sense: 5’-ATC TGT TTC CCC TCA TCT TTC C-3’; anti-sense: 5’-TGC GTC TTA GTG GTA TCT GTG C-3’), PPARγ (sense: 5’-CCT CCC TGA TGA ATA AAG ATG G-3’, anti-sense: 5’-GCA AAC TCA AAC TTA GGC TCC A-3’), β-actin (sense: 5’-TCA GGT CAT CAC TAT CGG CAA T-3’, anti-sense: 5’-AAA GAA AGG GTG TAA AAC GCA-3’).
1.3 Model establishment
Rats in all groups were given immunologic plus local irritation for modeling except those in the normal group.
In reference to thePharmacological Experiments Methodology, we used general immunological plus local irritation method to establish the model of UC[4]: the fresh colonic mucosa of patients with UC was homogenized with saline and centrifuged for 30 min (3 000 r/min), then the purified protein in the supernatant was measured and then mixed with complete Freund's adjuvant (CFA, Sigma, USA) at 1: 1 volume ratio. The 0.1 mL CFA emulsion was injected (containing 3 mg antigen) to the paw of each rat for the first time. On day 10, 17, 24 and 31, 0.2 mL CFA emulsion (containing 6 mg antigen) was respectively injected to paw, groin, abdomen and lower back. On day 38, after anesthetized with 2% Sodium Pentobarbital [30 mg/(kg·bw)], rats received 2% formalin 2 mL enema for 15 min, followed by antigen solution (4 g/L, without CFA) 2 mL enema for 1 h, then a saline wash.
1.4 Grouping and treatment
1.4.1 Herb-partitioned moxibustion (HPM) group
Acupoints: Bilateral Tianshu (ST 25) and Dachangshu (BL 25).
Operation: The locations of acupoints were according to theExperimental Acupuncture Science[5]. Herb cakes of 0.5 cm in diameter and 0.3 cm thick were made from powder ofFu Zi(Radix Aconiti Lateralis Preparata),Rou Gui(Cortex Cinnamomi) andMu Xiang(Radix Aucklandiae) mixed with rice wine. The herb cake was placed on the acupoint, and a refined moxa cone about 90 mg was placed on the cake and lit. Two moxa cones on each cake, once a day for 10 d.
1.4.2 Western medicine (Salicylazosulfapyridine, SASP) group
1.4.3 Model group
After modeling, rats in the model group were fixed as those in the treatment group without any treatment.
1.4.4 Normal group
Rats in the normal group only received the same fixation.
1.5 Index detection
1.5.1 Histopathological observation of the colon
Colonic pathological changes were observed under an optical microscope after hematoxylin-eosin (HE) staining, and evaluated according to pathological score standard[6].
1.5.2 Colon ultrastructure
Colon ultrastructure was observed and photographed using CM120 transmission electron microscope (Philips Company, Netherlands) in Department of Pathophysiology, Shanghai Medical College, Fudan University.
1.5.3 Colonic NFκBp65 and PPARγ expression detection
Primers that passed amplification efficiency testing were used to detect target genes, including the following steps.
Total RNA extraction: Total RNA extraction with Trizol reagent followed the protocol provided by Invitrogen Life Technology. Then concentration and purity of total RNA was detected by Nanadrop spectrophotometer using 1 μL of total RNA solution.
One-step reverse transcription into cDNA: According to iScript™ cDNA Synthesis Kit protocol, an 80 μL system was built and followed the procedure of 25 ℃for 5 min, then 42 ℃ for 30 min, and finally 85 ℃ for 5 min for reverse transcription.
Quantitative polymerase chain reaction (QPCR)was according to iQ™ SYBR® Green Supermix protocol. A 20 μL system was established for QPCR. The results were statistical analyzed using the 2ˉ△△Ctformula.
1.6 Data analysis
All experimental data were analyzed using the SPSS 18.0 version software. Methods were selected according to the data distribution features. For data that complied with normal distribution and homogeneity of variance, One-way ANOVA was used, and the least significant difference (LSD) test was employed for multiple pairwise comparisons among groups. For data of non-normal distribution or heterogeneity of variance, One-way ANOVA or nonparametric test was applied after variable transformation.P<0.05 was considered statistically significant.
2 Results
2.1 Model identification
2.1.1 Histopathological observation of the colon (HE staining)
The mucosa epithelium coating was integrated in normal rat colon without ulcers; the structure of layers were clear; the glands were arranged neatly; there were only a small amount of inflammatory cells infiltrated in mucous layer; and no interstitial edema. In model rats, the colonic epithelial cells shed; the mucosal glands decreased; there were a large number of lymphocytes, plasma cells, neutrophils and tissue cells infiltrated in stroma and submucosa, with small vascular proliferation (Figure 1).
2.1.2 Colonic ultrastructure observation of rats in the normal group and the model group
The ultrastructure of normal rat colon displayed consecutive and integrated mucosa; the cells arranged in neat rows with clear and integrated organelles; the intercellular gap junctions and tight junctions were clear. The ultrastructure of the model group showed incomplete epithelial coating and cell disorder; the membrane ruptured and organelles scattered extracellularly; a large amount of inflammatory cells infiltrated perivascularly (Figure 2).
These results suggested that the experimental rat model of UC was successfully established.

Figure 1. Colonic tissue HE staining of the normal group and the model group (×200)

Figure 2. Colonic ultrastructure of rats in the normal group and the model group
2.2 Histopathological observation and colonic damage score
The colon damage score of the model group was higher than that of the normal group, with a statistically significant difference (P<0.05); the score of HPM group was lower than the model group with a statistically significant difference (P<0.05); the score of SASP group was lower than that of the model group with a significant difference (P<0.05); there was no significant difference between the HPM group and the SASP group (Figure 3).
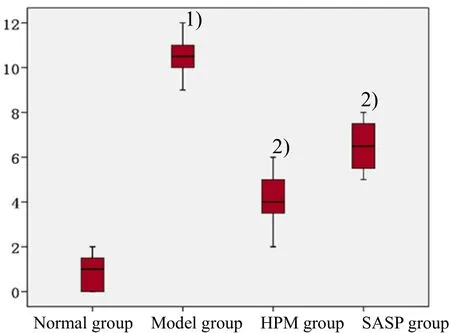
Figure 3. Colon damage score of rats in each group [Note: Compared with the normal group, 1) P<0.05; compared with the model group, 2) P<0.05]
The light microscope showed that normal colonic mucosa epithelium was intact without ulcer; the structure of layers were distinct and glands were arranged neatly; only a small amount of inflammatory cells infiltrated in mucosa layer; no interstitial edema. The colonic epithelial cells shedded in model rats; the mucosal glands decreased; there were a large number of lymphocytes, plasma cells, neutrophils and tissue cells infiltrated in stroma and submucosa, with small vascular proliferation; some folliculi lymphaticus has formed. Compared with the model group, the inflammatory cells decreased significantly in the HPM group, and the structure was close to normal and the surface coating epithelial was integrated. Compared with the model group, inflammatory cells decreased in the SASP group, the tissue structure was close to that of the normal group, the residential glandular atrophied, and the surface coating epithelial was integrated (Figure 4).
2.3 Colon tissue ultrastructure observation
The ultrastructure of normal rat colon was consecutive with intact mucosa; the cells were arranged neatly; the organelles were clear and integrated. The ultrastructure of the model group showed incomplete epithelial coating; cell necrosis and edema appeared; the membrane was ruptured and organelles scattered extracellularly. The mucosal epithelium was relatively complete in the HPM group, endoplasmic reticulum expanded bubbly, cells were like low column; epithelium was integrated in the SASP group, and gap junctions were damaged and the intercellular space became larger (Figure 5).

Figure 4. Histopathological observation of colonic tissues in each group

Figure 5. Colonic ultrastructure observation of rats in each group
2.4 NF-κBp65 mRNA expression in colon tissue
QPCR results showed that NF-κBp65 mRNA level of colon in the model group was higher than that of the normal group, with a significant difference (P<0.05); the level in the HPM group was lower than that of the model group with a statistically significant difference (P<0.05); and the level in the SASP group was lower than that of the model group, with a statistically significant difference (P<0.05). But there was no significant difference between the HPM group and the SASP group (P>0.05). Changes in NF-κBp65 mRNA levels in colon tissues of rats are shown in Figure 6.

Figure 6. Comparison of colonic NF-κBp65 mRNA expression [Note: Compared with the normal group, 1) P<0.05; compared with the model group, 2) P<0.05]
2.5 PPARγ mRNA expression in colon tissue
西达里亚油田矿区面积6.3平方公里,为三叠系碎屑岩油藏。1991年至1996年,该油田原油年产量占西北油田总产量的95%以上,最高达31.8万吨。2004年至2009年,西达里亚油田连续5年综合递减率控制在5%以下,原油年产量保持在10万吨以上。
QPCR results showed that PPARγ mRNA level of colon in the model group was higher than that in the normal group, with a significant difference (P<0.05); those in the HPM group and the SASP group were lower than that in the model group, with significant differences (bothP<0.05). But there was no significant difference between the HPM group and the SASP group (P>0.05). Expression changes of PPARγ mRNA levels are shown in Figure 7.

Figure 7. Comparison of colonic PPARγ mRNA expression [Note: Compared with the normal group, 1) P<0.05; compared with the model group, 2) P<0.05]
3 Discussion
According to Chinese medicine, UC is mainly caused by exogenous pathogenic factor invading the large intestine and subsequent conduction dysfunction, stagnation of qi and blood and injury to intestine collaterals. Pathological factors include dampness, heat and blood stasis, while pathological nature includes cold or hot. Related Zang-fu organs involve the large intestine, liver, spleen, lung and kidney. By contrasting symptom improvement in patients with UC, Wu HG, et al[7]reported that herb-partitioned moxibustion is better than bran-partitioned moxibustion in improving diarrhea, flatus, lassitude, tenesmus and low back soreness. Tan LY, et al[8]reported that moxibustion can significantly inhibit collagen Ⅰ and Ⅳ synthesis of colonic fibroblast (CFB) in UC rats, suggesting that moxibustion may be effective in preventing intestinal fibrosis. Zhou EH, et al[9]reported that moxibustion can promote heat shock protein 70 (HSP70) and its mRNA expression of spinal cord and colon, which may suppress inflammation and protect colon mucosa cells. In this study, we used general immunological plus local irritation method to establish UC model. Results showed that model rats displayed colonic epithelium shedding, reduced mucous glands, and a large number of inflammatory cells infiltration in interstitial and submucosa area. HPM and SASP both reduced injury score, suggesting both therapies can relieve colonic damage and inflammatory response of UC rats.
NF-κB is an important factor in the regulation of gene transcription, specifically binding with a variety of immunoglobulin light chain gene promoter κB sequence (5'-GGGACTTTCC-3') and to promote transcription. The most common NF-κB dimer is a heterodimer formed of p65 and p50. It combines with inhibitor of κBs (IκBs) to form a trimer in the resting state, and exists in the cytoplasm in an inactive mode. When cells are stimulated by external signals (e.g. lipopolysaccharide, tumor necrosis factor α, etc.), IκBs is phosphorylated and degradated, then NF-κB is activated and shifted into the nucleus, recognizing specific DNA sequences. Its subunit combines with specific binding site of target gene and gene transcription is initiated. Several studies have shown that NF-κBp65 plays an important role in intestinal inflammation[10-11]. NF-κBp65 antisense inhibits NF-κB activation, decreases TNF-α and IL-1γ expression, increases IL-10 expression, and significantly reduces inflammation of colitis, thus playing a key role in UC, and can be a target of a lot of anti-inflammatory drugs[12-13]. The expression of cytoplasm NF-κBp65 in UC tissues reflects inflammatory states. There is no or low NF-κBp65 expression in normal tissues, butoverexpression in active UC tissues[14], indicating that NF-κBp65 is closely associated with inflammatory bowel disease. NF-κBp65 can regulate gene transcription of intestinal inflammatory cytokines, which may be one major mechanism of its action.
PPAR is a member of transcription factor superfamily that regulates intranuclear receptor expression[15-16]. PPARγ is mainly expressed in adipose tissue and immune system, and is closely related to fat cell differentiation, immune and insulin resistance. The findings on PPARγ are quite different and even reach to opposite conclusions. For instance, PPARγ is widely expressed in colonic epithelial cells, macrophages and lymphocytes, and may be involved in colonic inflammation reaction[17-19]. However, some studies have shown that excess PPARγ has toxic side effects. For example, PPARγ agonists caused overweight, edema and tumor formation in rats[20]. Other studies indicate PPARγ may intermediately link a high-fat diet and colon polyps[21]. Although PPARγ is upregulated in colorectal cancer, as to its role in cancer, different researches have drawn different even opposite conclusions. PPARγ agonists and antagonists both decrease cell proliferation[22-24]. A PPARγ inhibitor, 2, 3-cyclic phosphatidic acid (cPA), can suppress its activation and antagonize its function, to prevent cell proliferation and invasion, thus to be a potential therapeutic agent for cancer[22]. Clinical study has shown that NF-κBp65 increased in patients with UC than in the normal people, while PPARγ expression was lower than in the normal people. However, the expressions of PPARγ and NF-κBp65 didn’t show obvious correlation, suggesting that the up-regulation of NF-κBp65 was not caused by PPARγ[25].
This study selected Dachangshu (BL 25) and Tianshu (ST 25) for moxibustion therapy. Dachangshu (BL 25) is the Back-Shu point of the large intestine, and Tianshu (ST 25) is the Front-Mu point of large intestine. Combination of Back-Shu and Front-Mu points can regulate qi movement of large intestine and is an effective treatment for bowel disease. This study showed that colonic NF-κBp65 and PPARγ mRNA were both up-regulated in the model group compared with those in the normal group (P<0.05). After treatment, moxibustion can down-regulate colonic NF-κBp65 and PPARγ mRNA expression with a statistically significant difference compared with the model group (P<0.05). Expression of NF-κBp65 and PPARγ in the model group didn’t show opposite trend, indicating that the up-regulation of NF-κBp65 was not caused by PPARγ pathway. Whether or not the regulation of PPARγ by herb-partitioned moxibustion has the effect of preventing precancerous lesion needs further study.
Conflict of Interest
The authors declared that there was no conflict of interest.
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 81173331); Scientific Research Budget Project of Shanghai Municipal Education Commission (No. 2011JW46); Science and Education Department Project for Youth, Shanghai Municipality Health Bureau (No. 20124Y004).
Statement of Human and Animal Rights
In this experiment, the treatment of animals conformed to the ethical criteria.
[1] Wu HG, Chen HP, Hua XG, Shi Z, Zhang LS, Chen JL. Clinical therapeutic effect of drug-separated moxibustion on chronic diarrhea and its immunologic mechanisms. Zhongyi Zazhi, 1997, 17(4): 253-258.
[2] Wu HG, Chen HP, Liao BS, Shi Z, Zhang LS. Effect of partition-herb moxibustion on immune function and β-endorphin of ulcerative colitis rats. Zhongguo Zhenjiu, 1997, 17(3): 163-165.
[3] Moynagh PN. The NF-kappaB pathway. J Cell Sci, 2005, 118 (Pt20): 4589-4592.
[4] Xu SY, Bian RL, Chen X. Pharmacological Experiments Methodology. Beijing: People’s Hygiene Publishing House, 1991: 1554.
[5] Li ZR. Experimental Acupuncture Science. Beijing: China Press of Traditional Chinese Medicine, 2007: 71.
[6] Araki Y, Andoh A, Fujiyama Y, Bamba T. Development of dextran sulphate sodium-induced experimental colitis is suppressed in genetically mast cell-deficient Ws/Ws rats. Clin Exp Immunol, 2000, 119(2): 264-269.
[7] Wu HG, Shi Z, Zhu Y, Ma XP, Yao Y, Cui YH, Zhao TP, Liu HR. Clinical study of herb-partitioned moxibustion on ulcerative colitis. Shanghai Zhenjiu Zazhi, 2007, 26(4): 3-4.
[8] Tan LY, Liu HR, Wang J, Qin XD, Huang WY, Zhao TP, Wu HG. Effect of moxibustion on CFB collagen synthesis in ulcerative colitis fibrosis rats. Shanghai Zhenjiu Zazhi, 2009, 28(2): 63-66.
[9] Zhou EH, Mu JP, Wu HG, Cui YH, Tan LY, Zhao TP, Shi Z. Study the mechanisms of herb-partition moxibustion therapy on ulcerative colitis rats in view of the hot shock protein 70 expression. 2009 Academic Annual Conference Proceedings of China Association for Acupuncture and Moxibustion, 2009: 419-425.
[10] Yang CH, Chen XY, Yang F, Ran ZH, Liu WZ, Xiao SD. Correlation of C-reactive protein with activity of Crohn’s disease. Zhonghua Yixue Zazhi, 2006, 86(18): 1253-1255.
[11] Li BN, Lü NH, Xie Y, Chen H, Zhu X, Yi J. Effects of NF-κBp65 antisense oligonucletide on the expression of cytokines and NF-κB in colonic intestinal mucosa of mice with TNBS-induced colitis. Shijie Huaren Xiaohua Zazhi, 2008, 16(17): 1926-1931.
[12] Dubuquoy L, Jansson EA, Deeb S, Rakotobe S, Karoui M, Colombel JF, Auwerx J, Pettersson S, Desreumaux P. Impaired expression of peroxisome proliferator-activated receptor gamma in ulcerative colitis. Gastroenterology, 2003, 124(5): 1265-1276.
[13] Andresen L, Jørgensen VL, Perner A, Hansen A, Eugen-Olsen J, Rask-Madsen J. Activation of nuclear factor kappa B in colonic mucosa from patients with collagenous and ulcerative colitis. Gut, 2005, 54(4): 503-509.
[14] Miao XP, Ouyang Q, Wei H. Significance of COX-2, PPARγ and NF-κBp65 expression in ulcerative colitis. Shijie Huaren Xiaohua Zazhi, 2010, 18(25): 2660-2665.
[15] Seegers H, Grillon E, Trioullier Y, Väth A, Verna JM, Blum D. Nuclear factor-kappa B activation in permanent intraluminal focal cerebral ischemia in the rat. Neurosci Lett, 2000, 288(3): 241-245.
[16] Marx N, Mach F, Sauty A, Leung JH, Sarafi MN, Ransohoff RM, Libby P, Plutzky J, Luster AD. Peroxisome proliferatoractivated receptor-gamma activators inhibit IFN-gammainduced expression of the T cell-active CXC chemokines IP-10, Mig, and I-TAC in human endothelial cells. J Immunol, 2000, 164(12): 6503-6508.
[17] Dubuquoy L, Dharancy S, Nutten S, Pettersson S, Auwerx J, Desreumaux P. Role of peroxisome proliferator-activated receptor γ and retinoid X receptor heterodimer in hepatogastroenterological diseases. Lancet, 2002, 360 (9343): 1410-1418.
[18] Spiegelman BM. PPARgamma in monocytes: less pain, any gain? Cell, 1998, 93(2): 153-155.
[19] Sánchez-Hidalgo M, Martín AR, Villegas I, Alarcon De La Lastra C. Rosiglitazone, an agonist of peroxisome proliferator-activated receptor gamma, reduces chronic colonic inflammation in rats. Biochem Pharmacol, 2005, 69(12): 1733-1744.
[20] Lebovitz HE. Differentiating members of the Thiazolidinedione class: a focus on safety. Diabetes Metab Res Rev, 2002, 18 (Suppl 2): S23-S29.
[21] Saez E, Tontonoz P, Nelson MC, Alvarez JG, Ming UT, Baird SM, Thomazy VA, Evans RM. Activators of the nuclear receptor PPARgamma enhance colon polyp formation. Nat Med, 1998, 4(9): 1058-1061.
[22] Tsukahara T, Hanazawa S, Kobayashi T, Iwamoto Y, Murakami-Murofushi K. Cyclic phosphatidic acid decreases proliferation and survival of colon cancer cells by inhibiting peroxisome proliferator-activated receptor. Prostaglandins Other Lipid Mediat, 2010, 93(3-4): 126-133.
[23] Tsukahara T, Haniu H. Peroxisome proliferator- activated receptor gamma overexpression suppresses proliferation of human colon cancer cells. Biochem Biophys Res Commun, 2012, 424(3): 524-529.
[24] Lee G, Elwood F, McNally J, Weiszmann J, Lindstrom M, Amaral K, Nakamura M, Miao S, Cao P, Learned RM, Chen JL, Li Y. T0070907, a selective ligand for peroxisome proliferator-activated receptor gamma, functions as an antagonist of biochemical and cellular activities. J Biol Chem, 2002, 277(22): 19649-19657.
[25] Jansson, Emmelie A. Pivotal role of the nuclear receptor PPARgamma in colon epithelial cells. Doctoral Thesis of Karolinska Institute, 2004.
Translator:Feng Xiao-ming
Received Date:June 10, 2014
Zhou Shuang, post doctorate, associate professor, associate chief physician.
E-mail: zhoushuanghome@163.com
R2-03
: A
he SASP group
intragastric SASP administration. According to thePharmacological Experiments Methodology[4], the daily dosage of the drug was calculated at the ratio of 1:0.018 as adult (70 kg body weight) versus rat (200 g body weight). The drug was administered twice a day, continuously for 10 d.
 Journal of Acupuncture and Tuina Science2014年5期
Journal of Acupuncture and Tuina Science2014年5期
- Journal of Acupuncture and Tuina Science的其它文章
- Warm Needling Combined with Iontophoresis of Chinese Medicine for Temporomandibular Joint Disorder
- Effect of Electroacupuncture Combined with Tuina on Lumbar Muscle Tone in Patients with Acute Lumbar Sprain
- Comparative Study on the Analgesic Effects of Different Moxibustion Methods with Tai-yi Moxa Stick in Treating Primary Dysmenorrhea
- Clinical Analysis of Acupuncture Combined with Tuina in Treating Cervical Vertigo
- Chinese Herbal Foot Bath plus Acupoint Massage Beneficial to the Improvement of Grade 0 Diabetic Foot
- Therapeutic Observation on Tuina plus Electroacupuncture for Lateral Humeral Epicondylitis
