Synthesis and Properties of Two Energetic Salts from 3,3′-Dinitroamino-4,4′-azoxyfurazan
-, , , -, -,
(Xi′an Modern Chemistry Research Institute, Xi′an 710065, China)
1 Introduction
Furazan is a kind of energetic structure unit with high density, high nitrogen content and oxygen balance[1]. Therefore, the combination of furazan with other energetic functional groups was favorable for maintaining a high level of energetic material in recent years[2-4]. 3,3′-Dinitroamino-4,4′-azoxyfurazan (NOF) was first obtained in 2014 through introducing nitramine (—NH—NO2) and azoxy [—NN(O)—] groups onto furazan backbones by Shreeve et al[5]. The crystal density, calculated detonation velocity, calculated detonation pressure and calculated specific impulse of NOF is 1.96 g·cm-3, 9746 m·s-1, 44.1 GPa and 283 s respectively, whose comprehensive energy performance is at the same proficiency level to CL-20. However the decomposition temperature of NOF is only about 90 ℃, which limits application in secondary explosive and propellant. It is well-known that the strong electron-withdrawing effect of nitro group in nitramine can deprotonate amine (—NH—) to form energetic salts when paired with bases[6-7]. And this is an effective method to improve the thermal stability of NOF.
In this study, NOF was prepared by through nitration 3,3′-diamino-4,4′-azoxyfurazan (AOF). And two new nitrogen-rich energetic salts, carbohydrazidium 3,3′-dinitroamino-4,4′-azoxyfurazan (NOF-CBH) and aminoguanidium 3,3′-dinitroamino-4,4′-azoxyfurazan (NOF-AG), were synthesized from NOF via metathesis reaction with carbohydrazide hydrochloride and aminoguanidine hydrochloride. These compounds were characterized using infrared and elemental analysis as well as multinuclear NMR spectroscopy. The thermal behaviors of compounds were studied by thermogravimetry derivative thermogravimetry (TG-DTG) method. The comparative study of molecular geometry and stability between NOF and its anion
(NOF2-) were conducted by quantum chemistry calculation. In addition, the energetic properties of NOF-CBH and NOF-AG were also estimated.
2 Experiment section
2.1 Synthetic route2.2 Materials and Instrument
All chemical reagents and solvents were obtained from Sigma-Aldrich Inc. or Chengdu Kelong Reagent Company (analytical grade) and were used without further purification. 3,3′-Diamino-4,4′-azoxyfurazan (AOF) was prepared according to the literature [8] procedure. Infrared spectra were obtained using a Nexus 87 Fourier transform infrared spectrometer (Nicolet USA). Organic elemental composition was analyzed on a Vario EL III elemental analyzer (Elementar Germany). Purity analysis with HPLC was carried out on a LC-20A system equipped with a C18 column (250 mm×4.6 mm, 5 mm, Agela) and a UV detector set at 254 nm (Shimadzu Japan).1H NMR (500.13 MHz) and13C NMR (125.76 MHz) spectra were recorded on a V500 spectrometer (Bruker, Germany). Chemical shifts were reported as aδvalue in parts per million and tetramethylsilane was used as the internal standard.1H NMR and13C NMR spectra were recorded in DMSO-d6.
2.3 Synthesis
2.3.1 3,3′-Dinitroamino-4,4′-azoxyfurazan (NOF)
AOF (2 g,9.5 mmol) was added slowly to 100% nitric acid (10 mL) and stirred to dissolving completely for 20 minutes at -5 ℃. The resulting mixture was held for an additional 4 h at 0 ℃ and then poured into ice water. The colorless precipitate was filtered, yielding 2.55 g. Its purity was 97.5%.1H NMR (DMSO-d6)δ: 9.85 (s, 2H).13C NMR (DMSO-d6)δ: 155.4, 153.3, 151.1, 148.4. IR (KBr,ν/cm-1): 342 (w), 3393(w), 3279(m), 1620(s), 1562(s), 1499(vs), 1389 (m), 1315 (m), 1230 (m), 1007 (m), 950 (w).Anal. calcd for C4H2N10O7: C, 15.90; H, 0.67; N 46.36. found C,15.46; H, 0.70; N, 45.87.
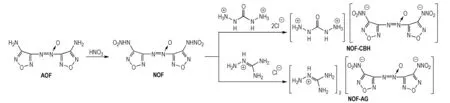
Scheme1Synthetic route of NOF-CBH and NOF-AG
2.3.2 Carbohydrazide 3,3′-dinitroamino-4,4′-azoxyfurazan (NOF-CBH)
A solution of NOF (0.6 g,2 mmol) in ethanol (10 mL) was stirred at room temperature while a slight excess of carbohydrazide (0.81 g,5 mmol) was added. After 2 h the yellow precipitate was filtered and air-dried, yielding 0.71 g. Its purity was 98.9%.1H NMR (DMSO-d6)δ: 9.73(s, 2H),δ: 3.60 (s, br, 6H).13C NMR (DMSO-d6,)δ: 156.9, 155.1, 154.5, 153.9, 151.2. IR (KBr,ν/cm-1): 3525(w), 3493(w),3315(m), 2966(vw), 2713 (w), 1708 (s), 1663 (w), 1639 (vw), 1614(vw), 1580(w), 1561(w), 1531(w), 1517(w), 1489(m), 1473(m), 1421(s), 1399(s), 1324(vs), 1300(s), 1203(m), 1053(m), 1015(m), 966(w), 937(m), 896(w), 863(vs), 836(m), 799(vw), 772(vw), 740(vw), 723(m), 668 (w), 641(vw), 591(vw), 567(v), 468(vw), 405(vw). Anal. calcd for C5H8N14O8·5H2O: C, 12.45; H, 3.76; N 40.66. found C,12.56; H, 3.78; N, 40.42.
2.3.3 Aminoguanidine 3,3′-dinitroamino-4,4′-azoxyfurazan (NOF-AG)
NOF-AG was prepared according to the method of NOF-CBH. The yellow precipitate was filtered, yielding 0.84 g. Its purity was 99.0%.1H NMR (DMSO-d6)δ: 8.58(s, 1H), 7.11(s, 2H), 4.99(s, 4H).13C NMR (DMSO-d6)δ: 158.7, 155.1, 153.8, 153.1, 151.1. IR(KBr,ν/cm-1): 3381(m), 3245(w), 3172(m), 1687(vs), 1665(vs), 1560(m), 1524(m), 1468(s), 1441(vw), 1410(m), 1387(s), 1294(vs), 1177(w), 1073(vw), 1044(m), 015(vw), 982(vw), 950(m), 908(vw), 883(vw), 872(vw), 827(m), 773(w), 745(vw), 716(w), 675(vw), 585(m), 565(m), 511(vw), 468(w), 404(vw). Anal (%) calcd for C6H14N18O7·2H2O: C, 14.82; H, 3.73; N 51.84. found C, 14.95; H, 3.63; N, 50.86.
2.4 Thermal decomposition conditions
TG-DTG was conducted on a SDT-Q600 apparatus (TA, USA) under a nitrogen atmosphere at a flow rate of 100 mL·min-1. The sample mass used in test was 0.5 mg and the heating rate was 5 ℃·min-1. The temperature range was from room temperature to 500 ℃.
3 Results and discussions
3.1 Thermal Decomposition of NOF-CBH and NOF-AG
The thermal behavior of NOF-CBH and NOF-AG was studied by TG-DTG. As shown in Fig.1, the TG-DTG curves indicate that the thermal behaviors of NOF-CBH and NOF-AG are similar to each other and all can be divided into one endothermic dehydrating crystal water stage and one obvious exothermic decomposition stage at 50-200 ℃. The dehydration process of NOF-CBH occurs at 50-100 ℃ corresponding to a mass loss of about 20%, and the sharp maximum dehydration rate is at 71.4 ℃. The dehydration mass loss is consistent with theoretical value of elemental analysis corresponding to the loss of 5 mol crystal water molecule. The exothermic decomposition of NOF-CBH starts at 144.9 ℃ with a mass loss of about 66%, and the tiptop temperature of decomposition is 173.1 ℃. The dehydration process range of NOF-AG is from 79 ℃ to 92 ℃, and the whole mass loss of dehydration process is only about 8%. The sharp maximum dehydration rate is at 86.3 ℃. The result agrees well with theoretical value of elemental analysis corresponding to the loss of 2 mol crystal water molecule. The exothermic decomposition of NOF-AG starts at 151.6 ℃ with a mass loss of about 62%, and the tiptop temperature of pyrolysis is 191.3 ℃. The decomposition temperature of NOF-CBH and NOF-AG are above 140 ℃, which can prove that their thermal stabilities are far better than 90 ℃ of NOF.

a. NOF-CBH
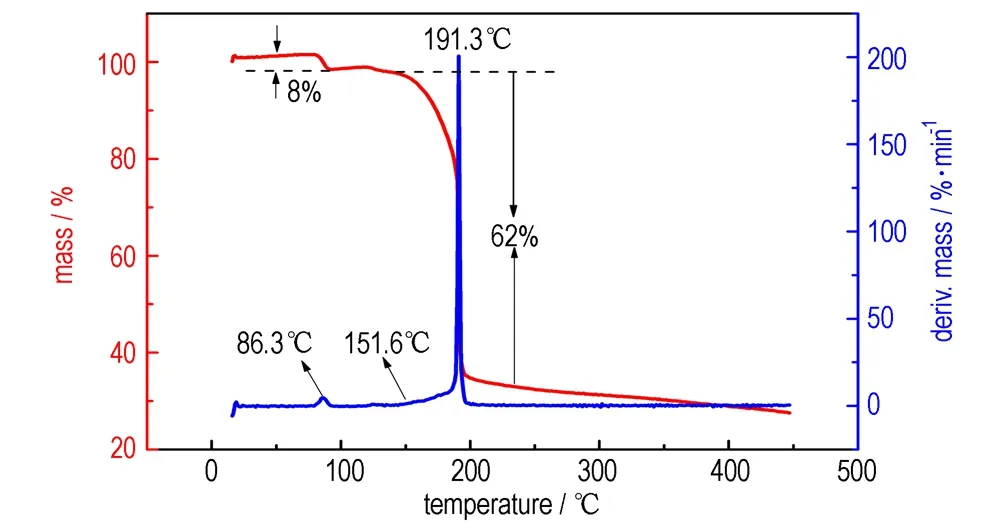
b. NOF-AG
Fig.1TG-DTG curves of NOF-CBH and NOF-AG
3.2 Optimized molecular geometry of NOF and NOF2-
To gain a better understanding of structures for NOF and its anion (NOF2-), the molecular geometry has been optimized using various ab initio methods including PM3 and B3LYP[9-10], and the most stable molecule conformations are shown in Fig.2. The optimized structures show that molecule conformations of NOF and NOF2-arevery semblable. The two furazan rings connecting with azoxy are not at the same plane, and the torsion angles (C(3)—C(2)—C(8)—C(12)) are 87.57° and 87.82° ,respectively. In molecule conformation of NOF, the torsion angles between furazan rings and nitramine are 113.12° and 135.71°, and the bond lengths of N—NO2are 1.43 Å and 1.41 Å. In contrast to molecule conformation of NOF, the furazan ring and nitramine of NOF2-are nearly at the same plane, and the bond lengths of N—NO2are only 1.35 Å and 1.34 Å, much smaller than that of NOF. The results indicate preliminarily that the conjugate action between furazan ring and nitramine will be strengthened when the deprotonation of NOF is occurred to form NOF2-. So the structure stability of NOF2-is better than that of NOF.
It is significant and objective to further make comparison of the weakest bond order between NOF and NOF2-for evaluating theoretically the stability. Through the bond order calculation, N—NO2was determined as the weakest bond[11], and the N—NO2length and order of NOF and NOF2-were listed in Table 1. The two N—NO2order values of NOF2-(N(13)—N(16) and N(14)—N(19)) are 1.1589 and 1.0910 respectively, which are all greater than those of NOF (0.9833 and 0.9887). The data show that the N—NO2of NOF2-is stronger than that of NOF. Therefore the structure stability of NOF2-is better than NOF.

a. NOF
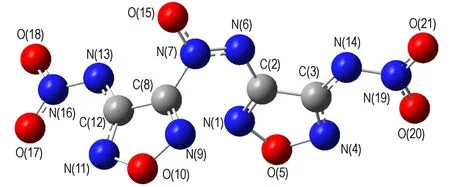
b. NOF2-
Fig.2The molecular geometry of NOF and its anion (NOF2-)
Table1The bond length and order of NOF and NOF2-
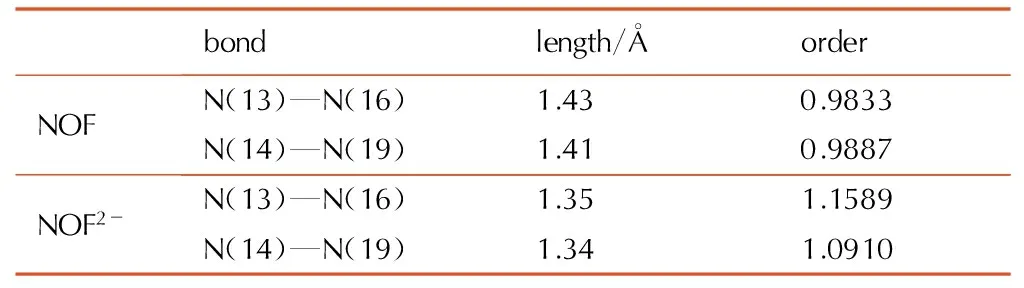
bondlength/ÅorderNOFN(13)—N(16)1.430.9833N(14)—N(19)1.410.9887NOF2-N(13)—N(16)1.351.1589N(14)—N(19)1.341.0910
3.3 The detonation performances of NOF-CBH and NOF-AG
The detonation performances of NOF-CBH and NOF-AG were calculated by Monte-Carlo method[12], Atomization scheme[13]and Kamlet-Jacobs formula[14]based on the optimized molecular geometry, and listed in Table 2. Standard molar enthalpies of formation of NOF-CBH and NOF-AG are 515.86 kJ·mol-1and 815.96 kJ·mol-1respectively, revealing highly positive heat of formation. The calculated densities of NOF-CBH and NOF-AG are 1.82 g·cm-3and 1.75 g·cm-3, respectively. The detonation velocities of NOF-CBH and NOF-AG are all over 8500 m·s-1, revealing that NOF-CBH and NOF-AG have the detonation performance level approaching that of RDX.
Table2Calculated performances of NOF-CBH and NOF-AG in comparison to RDX
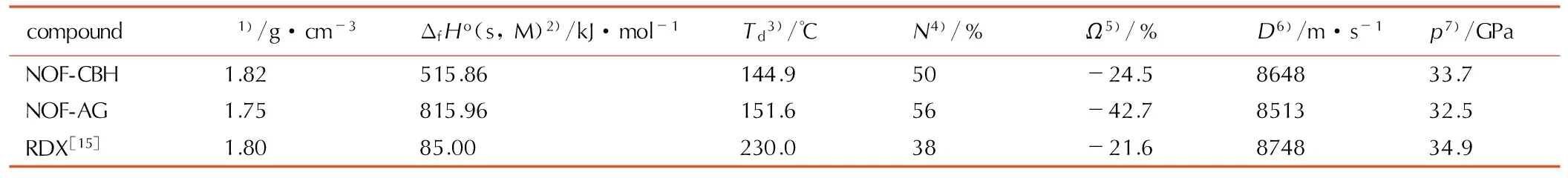
compoundρ1)/g·cm-3ΔfHo(s,M)2)/kJ·mol-1Td3)/℃N4)/%Ω5)/%D6)/m·s-1p7)/GPaNOF-CBH1.82515.86144.950-24.5864833.7NOF-AG1.75815.96151.656-42.7851332.5RDX[15]1.8085.00230.038-21.6874834.9
Note: 1) density; 2) standard molar enthalpy of formation; 3) decomposition temperature; 4) nitrogen content; 5) oxygen balance; 6) detonation velocity; 7) detonation pressure.
4 Conclusions
Two new nitrogen-rich energetic salts, NOF-CBH and NOF-AG are synthesized. Their initial decomposition temperatures are 144.9 ℃ and 151.6 ℃ respectively, which are far higher than 90 ℃ of NOF. The structure stability of NOF2-is better than NOF. The standard molar enthalpy of formation, density and detonation velocity are 515.86 kJ·mol-1, 1.82 g·cm-3and 8648 m·s-1for NOF-CBH and 815.96 kJ·mol-1, 1.75 g·cm-3and 8513 m·s-1for NOF-AG, revealing that they have highly positive heat of formation and the detonation performance level approaching that of RDX.
[1] LI Hong-zhen, HUANG Ming, HUANG Yi-gang. Progress in diaminoazofurazan and diaminoazoxyfurazan[J].ChineseJournalofEnergeticMaterials(HannengCailiao), 2005, 13(3): 192-195.
[2] Thottempudi V, YIN P, Zhang J, et al. 1,2,3-Triazolo[4,5,-e]furazano[3,4, -b]pyrazine 6-oxide-a fused heterocycle with a roving hydrogen forms a new class of insensitive energetic materials[J].Chemistry-AEuropeanJournal, 2014, 20(2): 542-548.
[3] Stepanov R S, Kruglyakova L A, Astakhov A M. Effect of structure on the rate and mechanism of thermolysis of some 4-substituted 3-methylfuroxans[J].RussianJournalofGeneralChemistry, 2009, 79(5): 1047-1048.
[4] Makhova N N, Ovchinnikov I V, Kulikov A S, et al. Monocyclic and cascade rearrangements of furoxans[J].PureandAppliedChemistry, 2004, 76(9): 1691-1703.
[5] Zhang J, Shreeve J. 3, 3′-Dinitroamino-4, 4′-azoxyfurazan and its derivatives: an assembly of diverse NO building blocks for high-performance energetic materials[J].JournaloftheAmericanChemicalSociety, 2014, 136(11): 4437-4445.
[6] Gao H, Shreeve J. Azole-based energetic salts [J].ChemicalReviews, 2011, 111(11): 7377-7436.
[7] LI Hui, Yu Qian-qian, WANG Bo-zhou, et al. Synthesis and thermal properties of 3,3′-bis(tetrazol-5-yl)-4,4′-azofurazan and its energetic Salts[J].ChineseJournalofEnergeticMaterials(HannengCailiao), 2013 ,21(6): 821-824.
[8] LIN Zhi-hui, GAO Li, LI Min-xia, et al. Synthesis, Thermal behavior and prediction of theoretical detonation performance for some energetic compounds derived from Furazan[J].ChineseJournalofExplosives&Propellants, 2014, 37(3): 6-10.
[9] Yu T, Chang H B, Lai W P, et al. Computational study of esterification between succinic acid and ethylene glycol in the absence of foreign catalyst and solvent[J].PolymerChemistry, 2011, 2(4): 892-896.
[10] Klapötke T M, Krumm B, Scherr M, et al. Experimental and theoretical studies on some energetic functionalized trimethylamine derivatives[J].Chemistry-AEuropeanJournal, 2009, 15(42): 11341-11345.
[11] LIAN Peng, LAI Wei-peng, WANG Bo-zhou, et al. Design of synthetic route and prediction of properties for a novel high energetic density compound 3,6-bis(3,5-dinitro-1,2,4-triazol-1-yl)-1,2,4,5-tetrazine-1,4-dioxide[J].ActaChimicaSinica, 2009, 67(20): 2343-2348.
[12] BI Fu-qiang, FAN Xue-zhong, XU Cheng, et al. Synthesis and theoretical study of 1,1′-dihydroxy-5,5′-bitetrazole[J].ChineseJournalofExplosives&Propellants, 2013, 36(4): 22-25.
[13] Curtiss L A, Raghavchari K, Redfern P C, et al. Gaussian-3 (G3) theory for molecules containing first and second-row atoms[J].TheJournalofChemicalPhysics, 1998, 109(18): 7764-7776.
[14] Kamlet M J, Jacobs S. Chemistry of detonations. I. A simple method for calculating detonation properties of C-H-N-O explosives[J].TheJournalofChemicalPhysics, 1968, 48(1): 23-35.
[15] Meyer R, Homburg A. Explosives[M]. Wiley. 2007: 156-157.

