油泥经热化学处理转化成多孔炭材料的可行性
Shohreh Mohammadi, Nourollah Mirghaffari
油泥经热化学处理转化成多孔炭材料的可行性
Shohreh Mohammadi, Nourollah Mirghaffari
(Department of Natural Resources,Isfahan University of Technology,Isfahan,Iran)
研究燃油储罐中产生的油泥转化为多孔炭材料的表征和可行性。油泥含有80%碳,主要以脂肪族化合物形式存在。经600℃热处理和KOH存在下的热化学裂解得到2种碳质材料。热化学处理可显著提高所制多孔炭的织构特性,即微孔和介孔结构。该多孔炭表面积、总孔容与微孔比表面积分别为327.95 m2·g-1、0.21 cm3·g-1和89.10 m2·g-1,其在水溶液中对Cd的吸附性能优于商业活性炭。油泥经热化学转化的多孔炭吸附剂能应用于污水处理,是一种转化废弃物的有效途径。
油泥;多孔炭;热化学处理;吸附
1 Introduction
Porous carbonaceous materials are known as powerful adsorbents,owing to their high surface area and adsorptive capacity[1,2].Activated carbons,as one class of them,have been widely utilized in different applications such as the adsorbent for the removal of pollutants from the gaseous or liquid phases,catalysts and catalystsupports[1,3].They can be produced from various carbonaceous precursors including agricultural waste[3],petroleum coke[1]and sewage sludge[1,4]by physical and/or chemical processes[1,3].In recent years,particular emphasis has been focused on the production of activated carbons from solid wastes,which are expected to have lower cost than the commercial ones[1].
Oily sludges contain heavy metals,polycyclic aromatic hydrocarbons,and oily compounds.Their production is one of the most important environmental issues in the oil and petrochemical industries[5].Annually,large quantities of oily sludges(more than 60 million tons)are generated in oil-waterseparators, dissolved air flotation units,heat exchanger cleanings,and tank bottom cleanings in oil refineriesworldwide[5,7-9].Oily sludges have a complex and variety structure depending on the quality of crude oil as well as the processes used for water-oil separation.In general,oily sludges may be defined as heavy oily residues with the composition of oily matters,water, and particulates[5,8,9].
The prevalent methods for the oily sludges treatment and disposal are landfilling and incineration. However,landfill has been limited due to the large space required for sludge disposal[4,6],leakage and leaching of toxic components to the underground[6,10],high costs[4,10]as well as greenhouse gas emission[10].Recentlly,the use of incineration technology to dispose the oily sludges has received more attentions[4].In addition to the high cost,this method can contribute largely to the air pollution by the formation and emission of hazardous gases[4-6,10].
It seems preferable to process and recover the oily sludges because of environmentalimpacts and the huge amount of production.Some researchers have tended to develop the innovative and commodious solutions for resolving the problems of oily sludges such as solidification[5,7,11],bioremediation[5,10], road applications,and fuel source[5,11].Based on the composition of these organic wastes,they could be converted into porous carbonaceous materials through various processes.Previous studies focused on the preparation of porous carbon from oily sludges by carbonization withoutchemicalor physicalactivation[12]. Seredych et al.[12]used a composite of sewage sludge and industrial oily sludge,which was pyrolyzed at 650 and 950℃without activation,for the removal of copper.However,the production and application of porous carbon prepared from oily wastes have not been extensively investigated yet.It should be stated that the conversion of oily sludges into porous carbon could be a promising approach from environmental and economic points of view.Accordingly,the conversion of this waste into carbonaceous adsorbents could be attractive for the removal of pollutants from wastewater.In addition,the volume and the costs of sludge disposal may be decreased.
The main objective of this research is to characterize oily sludge generated from a fuel oil storage tank to investigate possibility to convert it into a porous carbonaceous material via thermal and thermochemical treatments.
2 Experimental
2.1Oily sludge collection and preparation
The oily sludge in the form of semi-solid was collected randomly from a fuel oil storage tank in the oil refinery of Isfahan,Iran.The sludge sample was dried in an oven for 72 h at 80 to 90℃until the weight became constant.
2.2Characterization of oily sludge
Ash content of the oily sludge sample was determined using ASTM method D 482-87[13].The ASTM method D 4980-89[14]was used to determine the pH of oily sludge sample using a pH meter(Research pH Meter 3330).The elemental composition including carbon,nitrogen,sulfur and hydrogen in the dry sludge sample was measured by an elementalanalyzer (Elementar Vario EL III).The oily sludge was digested by diluted nitric acid(v/v 1∶1)with a ratio of solid/acid(m/v 1∶5)according to the ASTM method D 5198-92[15].The mixture was heated at90 to 95℃for 2 h,cooled,diluted with distillated water and filtered using a filter paper(Whatman 40).The heavy metals concentration in the extract was measured by flame atomic absorption spectrometry (FAAS,Perkin-Elmer,A Analyst 700).
The thermal behavior of oily sludge sample was examined by thermo-gravimetric analysis(TGA). The sample was placed into the instrument with 10℃·min-1heating rate from 25℃to 1 200℃under N2flow.The weight loss was recorded as a function of temperature.The functional groups of oily sludge were identified in the wave number range of 600-4 000 cm-1using Fourier transform infrared spectroscopy(FT-IR)(Tensor 27,Bruker)with an attenuated total reflection(ATR)mode.
The organic volatile compounds of oily sludge were detected using a gas chromatograph(GC,Hewlett-Packard HP 6890)equipped with a TRB-5MS column with 30 m length,250μm internal diameter, 0.25μm layer thickness of the stationary phase and a mass detector(HP 5973).The temperature program of GC oven was set to increase at a rate of 5℃·min-1from 50(the initial time 6 min)to 150℃in the first step,and then to 290℃(the final time 5 min)at 10℃·min-1.Helium was used as the carrier gas at a constant flow of 1 mL·min-1.In this method,the sample was poured into a container and heated to 90℃for 45 min.The sampling was performed from the upper space and the gas phase was injected to the apparatus(headspace method)[16].Finally,the compounds were identified according to their mass spectra,GC retention times and comparison with the library mass spectra.In order to estimate the distribution of compounds present in the oily sludge,a semi-quantitative method was employed using the means of the percentage of area under the curve for each peak of the GC analysis.
2.3Preparation of porous carbonaceous materials
Two porous carbonaceous materials were produced through carbonization without activation(thermal treatment)and with activation(thermochemical treatment).For producing porous carbon without activation,the pyrolysis of sample was carried out in a vertical furnace under the nitrogen atmosphere with a heating rate of 10℃·min-1.The final pyrolysis temperature was 600℃with a residence time of 60 min. Then,the sample was cooled down under nitrogen flow before it was removed from the furnace.The non-activated carbonized product(NA)was ground and sieved into a uniform size of less than 1.0 mm and stored in a well-closed container for characterization and adsorption experiments.
For thermochemical treatment,the oily sludge was directly mixed(physical mixing)at room temperature with KOH(99%purity)as a chemical activating agent with a mass ratio of KOH to oil sludge 2∶1.The mixture was pyrolyzed and prepared in the same conditions as those of the carbonization.The activated carbonized product(AC)was washed with distilled water until the pH of the filtrate became around 7.The washed sample value was dried in an oven for 24 h from 70 to 80℃.
2.4Characterization of porous carbonaceous materials
The surface area and the porosity of porous carbons were detemined via nitrogen adsorption-desorption at 77 K by a surface area analyzer(Bel Co., Belsorp.mini).The surface area(SBET)and the total pore volume(Vt)of the porous products were calculated,respectively,using Brunauer-Emmett-Teller (BET)method and amount of nitrogen adsorbed at the p/p0of 0.98.The mean pore diameter(Dp)was estimated from BET surface area and the total pore volume as follows[17]:

The t-plot method was used to measure the external surface area(Sexternal),the micropore surface area (Smic),the micropore volume(Vmic),and the mean micropore diameter(Dmic)of the porous carbons.In addition,the volume(Vmeso)and distributions of mesopores were calculated using the Barrett-Joyner-Halenda(BJH)method[17].
A scanning electron microscope(SEM,Tescan, Vega II)was applied to observe the surface morphology of the porous carbons.The surface functional groups of the porous carbons were studied by the FT-IR spectroscopy using the ATR system in the wavenumber range of 600-4000 cm-1.
Porous carbons were digested according to ASTM method D 5198-92[15]for heavy metal analysis.The release of heavy metals from the porous carbons was tested by two methods:the distillated water leaching test[18]and the toxicity characteristic leaching procedure(TCLP)according to 1311 standard method,which was proposed by United States Environmental Protection Agency(US EPA)[19].In the leaching test with distillated water,0.5 g of carbonaceous sample was mixed with 100 mL of distilled water, shaken for 24 h at160 r/min and filtered using a filter paper[18].Then,heavy metals analysis was performed using the FAAS.In the TCLP test,the sample was treated by an extraction fluid(5.7 mL of concentrated glacial acetic acid and 64.3 mL of 1 N NaOH diluted into 1 L distilled water)in a ratio of 20∶1 liquid to solid atthe fixed pH of4.93±0.05 and extraction time of 18 h.The liquid extract was filtered using a filter paper,and the concentration of heavy metals in the filtrates was analyzed using the FAAS.
2.5Adsorption experiments
The adsorption characteristics of the porous carbons were evaluated by the preliminary adsorption experiments of Cd from synthetic solution under batch. Furthermore,the efficiency of the porous carbon adsorbents was compared with three commercial activated carbons(South African:Jacobi,Holland:Norit and Iranian).The stock solution of Cd (1 000 mg·L-1)was prepared by dissolving CdCl2·H2O in distilled water.The pH of solutions was adjusted with 0.1 and 1 mol·L-1HCl or NaOH (2%and 5%)using a pH meter(Jenway,Research pH meter 3330).50 mL of Cd solution with a concentration of10 mg·L-1and pH of6 was added to 1 g of adsorbents in a 100 mL flat bottom polyethylene flask,and shaken at 150 r/min for 60 min at room temperature with two replications.
The solutions were filtrated using a filter paper, and Cd concentration was analyzed using the FAAS. The adsorption percentage of Cd was calculated by the equation 2.

Where Ciand Ceare the initial and final cadmium concentrations(mg·L-1),respectively.
3 Results and discussion
3.1Characterization of oily sludge
The pH value of oily sludge was 8.01,within the permitted limit of 6.5-8.5[20].The ash content (13%)was low.In addition to the carbon content,the ash contentis an importantfactor in the production of porous carbons[1,21].
The C,H,N,and S contents of oily sludge were 80.48%,14.58%,4.80%and 0.44%,respectively.The amount of C was very high,indicating that oily sludge could be used as a precursor for the pyrolysis and preparation of carbonaceous materials.The concentrations of some heavy metals in the oily sludge are shown in Table 1.Only the concentration of Cr is significantly higher than the allowed maximum level proposed by European Union(EU) for hazardous waste landfilling[8].In addition,the Fe content in the sample is very high,which is probably caused by contamination of storage tank[22].

Table 1 Metals concentration(mg·kg-1)in the oily sludge.
According to the TGA curve in Fig.1,the thermal behavior of the oily sludge shows three main steps.A weight loss of about 18%-20%is observed from 100 to 200℃in the first step,which can be ascribed to the evaporation of moisture from the sample during heating.The second weight loss is about20%-65%,occurring between 200 to 600℃and is constant up to 900℃.This mass loss is accompanied by pyrolysis that is related to the volatilization and decomposition of the organic compounds in the oily sludge.Thus,the temperature of 600℃was selected as the appropriate temperature for the preparation of the porous carbon.The third weight loss after 900℃is associated with the decomposition of inorganic compounds[23].

Fig.1 Thermogravimetric analysis curve of the oily sludge.
Fig.2 shows the FT-IR spectra of oily sludge. The bands around 2 857 and 2 925 cm-1can be assigned to the symmetric and asymmetric C—H stretching vibrations in the aliphatic compounds.The two peaks at1 376 and 1 459 cm-1may be associated to the bending mode of methyl(CH3)and methylene (CH2)[2].The band at 1 640 cm-1is attributed to the aromatic C= C vibrations[2,17,24].The peaks around 871,813 and 725 cm-1are ascribed to the ben ding mode of out-of-plane C—H[25].The band around 700 cm-1is attributed to benzene derivatives in the structure of oily sludge[17].The similar results of FT-IR for heavy fuel compounds were observed by Varlla et al.[25].

Fig.2 FT-IR spectrum of the oily sludge.
The results of GC-MS show that the oily sludge has a complex composition with numerous chemical compounds including aliphatic,mono-aromatics,polycyclic aromatics,carboxylic acids,et al.Some of the main identified compounds from the GC-MS chromatogram(Fig.3),and their percentages in the area under curve are summarized in Table 2.Generally, the oily sludge is composed of aliphatic compounds (alkanes and alkenes),in which the abundance of alkanes is higher than that of alkenes.The presence of aliphatic compounds is very importantfor the recovery of sludge as a fuel source[26].The most abundant polycyclic aromatic compounds are naphthalene,1-ethyl naphthalene,2,6-dimethylnaphthalene,2,7-dimethyl naphthalene,1,3-dimethylnaphthalene,1,4-dimethyl naphthalene,1,6,7-trimethyl naphthalene and 2,3,6-trimethyl naphthalene.In addition,the mono-aromatic compounds include benzene and toluene.

Fig.3 GC-MS chromatogram of the oily sludge.
3.2Characterization of the porous carbons
The surface area,pore volume,and pore diameter of the porous carbons are summarized in Table 3. The results indicate that the pore characteristics of AC are notably better than those of NA.The BET surface areas of AC and NA are 327.97 and 3.63 m2·g-1, respectively.The external surface area(the surface area of mesopores and macropores),the micropore surface area(subtracting the total surface area from the external surface area),the micropores volume, and the average micropore diameter are estimated to be 38.87 m2·g-1,289.10 m2·g-1,0.12 cm3·g-1, and 0.83 nm for AC,respectively.Overall,the thermochemical treatment of oily sludge could produce AC with a developed pore structure while the micropore and mesopore structures are not apparently formed by a simple pyrolysis in the NA.
The pores size was classified on the basis of the international union of pure and applied chemistry(IUPAC)classification into the micropores,mesopores, and macropores with pore diameter up to 2,2 to 50, and>50 nm,respectively[27].The average pore diameter(Dp)of AC was calculated to be 2.81 nm. The N2adsorption and desorption isotherms for AC are shown in Fig.4.According to IUPAC classification[27],the feature of this plot is a combination of I and IV types,indicating micropores and mesopores exist in the AC.According to this isotherm,the hysteresis loop of H4 type is observed in the adsorption and desorption for AC.This could be due to the capillary condensation of nitrogen gas in the mesopores. The H4 loop is often associated with the aggregates of plate-like particles,giving rise to narrow slit-shape mesopores[24].

Table 2 Main organic compounds identified in the oily sludge using GC-MS analysis.

Table 3 Surface area and the porosity of the porous carbons.
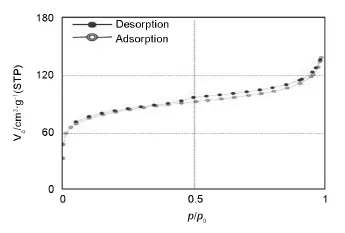
Fig.4 Nitrogen adsorption and desorption isotherm for the activated carbon obtained from the oily sludge.
The t-plotof AC shows two differentslopes(Fig.5 (a))including a sharp slope passing the original point and a more gradual slope,thus illustrating that the size ofthe micropores is homogenous.In the early adsorption stage,the adsorption amount increases strongly owing to the adsorption into the micropores,but the thickness of adsorption layerdoes notincrease so much.Consequently,the slope of t-plot became sharp.When the adsorption into the micropores is completed,the adsorption occurrs only on the surface,resulting in a gradual slope[28].On the other hand,the BJH plot(Fig.5(b)) shows that the AC has a wide mesopore distribution.
Table 4 summarizes the characteristics of porous carbons obtained from different wastes.The porous carbon from the oily sludge in this work has a higher SBETthan some carbonaceous materials produced from the precursor materials such as metalsludge and spent car oil[29]and the oily sludge[12,24].These differences can be related to the used raw materials,the treatment method and carbonization temperature.
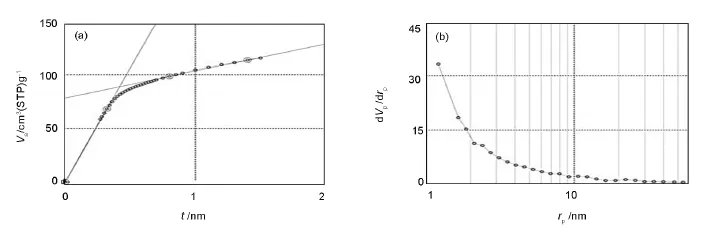
Fig.5 (a)t-plot and(b)BJH-plot for the activated carbon obtained from the oily sludge.
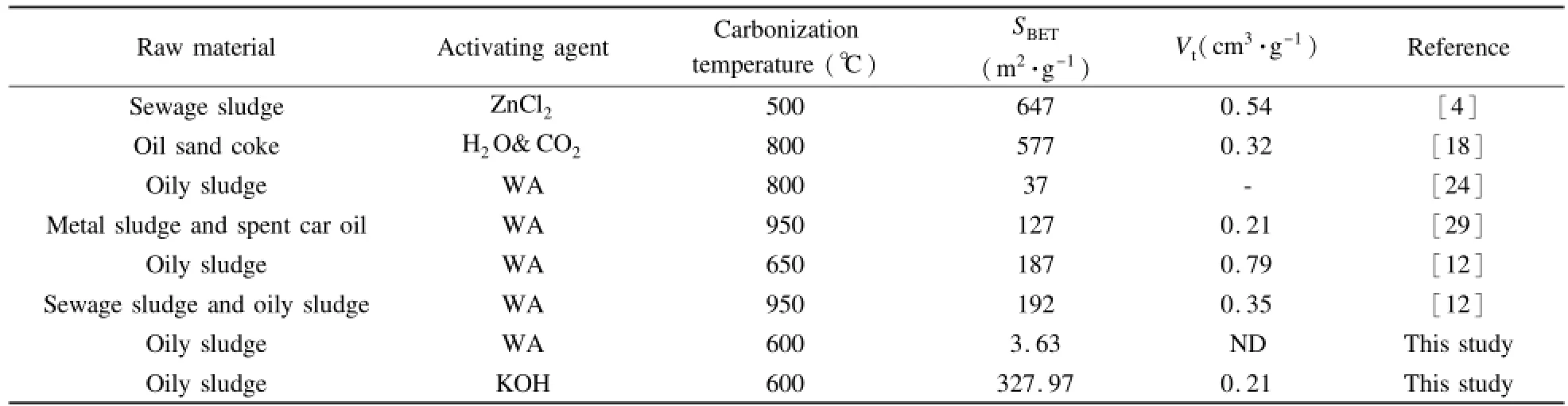
Table 4 Characteristics comparison of some porous carbon materials.
The SEM images of the porous carbons showed a pitted and heterogeneous surface(Fig.6).Based on the size and shape,differentpores are observed in the SEM photographs of the AC.The presence of salt grains in the AC surface could be related to the remaining KOH(Fig.6(b)).In addition,the sheetshaped forms are observed(Fig.6(b))on the surface of activated porous carbons,which might be attributed to the mineral structures or graphene sheets[24,30].
The concentrations of Cd,Cu,Zn,Mn,and Fe are enriched(Table 5)in the porous carbons compared with that of oily sludge(Table 1),as a result of the evaporation of volatile compounds during pyrolysis[6].However,the contents of Cr and Pb in both NA and AC as well as Ni in NA decrease,probably due to the formation of volatile metal compounds.For example,the reduction of Pb could be related to the volatilization of PbCl2[6].In addition, the contents of Cd,Cr,Mn and Fe in the NA arehigher than those in the AC.Chang etal.[6]indicated that the concentrations of Fe,Zn,Cu,Ni,and Cd in the oily sludge were enriched during pyrolysis while those of Pb and Cr were reduced.

Fig.6 SEM images of(a)NA and(b)AC.

Table 5 Concentration of heavy metals(mg·kg-1) in the porous carbons.
One of the main problems concerning the use of oily sludges as the raw materials for the production of porous carbons is the possibility of heavy metals leaching during their utilization.Therefore,the leaching tests of heavy metals were performed by distillated water and TCLP methods.Based on the obtained results(Table 6),the concentrations of the released heavy metals from the porous carbons by distilled water method,are in acceptable levels proposed by World Health Organization(WHO)for drinking water[31]except for Cr that is close to the acceptable level.Consequently,the porous carbons can not be used in drinking water treatment.In the TCLP test, the leaching of heavy metals increases from both carbons samples(Table 6)due to the lower pH of the extraction solution(≈4.9)compared to that of distillated water.However,the concentrations of heavy metals are lower than the acceptable limits proposed by EPA[11].Although the contents of heavy metals are enriched in the prepared porous carbons,they are released very little during both leaching procedures. This could be caused by either the bounding of heavy metals to the carbon structure or the affinity of carbon for metals adsorption[4].Moreover,the oily sludge contains a high conent of Fe(Tabel 1)that might alleviate the release of heavy metals.Karamalidis et al.[7]reported thatthe high levelof Fe could actas an immobilizing agent of heavy metals during the stabilization and solidification of refinery oily sludge owing to their sorption onto iron oxides.
The chemical structure of the raw oily sludge and those after pyrolysis were studied by the FT-IR analysis(Fig.7).The decrease of aliphaticity appears in the wavelength of2 857 and 2 925 cm-1in the NA and AC compared with the oily sludge,due to the decomposition of aliphatic compounds[32].In addition,the increase in the peak intensity at1 416 and 1 565 cm-1in the NA and AC could be assigned to the increase of aromatic compounds(aromaticity)after the treatment[29,32].The broad bands around 3 385 and 3 737 cm-1could be attributed to the O—H stretching in the hydroxyl functional groups in the AC[32,33]. The O—H functional group in the AC is observedwith much more intensity and width than thatof NA. The peaks at 2 313 and 2 355 cm-1with more intensity in the NA can be associated with the stretching vibrations of alkynes groups.The region around 1 128 cm-1may be related to the C—O stretching vibrations[33],whose intensity in the NA is more than that of AC.The peaks between 600 until 900 cm-1might be ascribed to the H-bending under different situations in the aromatic rings whose intensity in the NA is higher than that of AC[25].
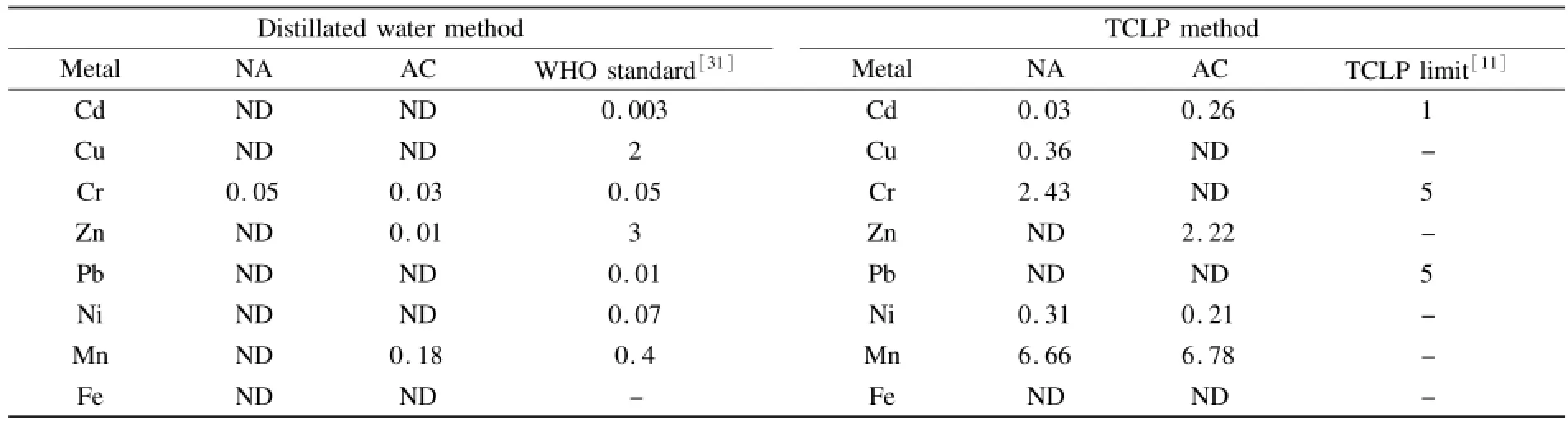
Table 6 Leaching tests of heavy metals(mg·L-1)using distilled water and TCLP methods.

Fig.7 FT-IR spectra of (a)the oily sludge,(b)NA and(c)AC.
3.3Cd adsorption
The results of preliminary adsorption(Table 7) indicate that the efficiency of Cd removal from aqueous solutions under given experimental conditions by AC(97.36%)is significantly higher than that of NA (77.74%).The higher Cd adsorption by AC could be related to its higher surface area and the better porosity structure than NA.In addition,the AC shows comparable adsorption efficiency with three commercial activated carbons while NA does not.

Table 7 Comparaison of Cd adsorption percent on the produced porous carbons and commercial activated carbons (10 mg·L-1Cd,20 g·L-1adsorbent,pH 6,and 60 min).
4 Conclusions
This study reveals thatthe porous carbons can be produced from the oily sludge.The thermochemical treatment of the oily sludge with KOH considerably increases the surface area and the porosity of porous carbon compared with those of the thermal method. The micropores and mesopores are appropriately formed in the activated carbon obtained by the thermochemical treatment.Moreover,the pyrolysis contributes to the stabilization of heavy metals present in the oily sludge and consequently,their leaching is effectively limited.The adsorption of Cd by the porous carbon from thermochemical treatment is comparable with that of commercial activated carbons.Finally, the recovery of oily sludges through their thermochemical conversion into the porous carbonaceous adsorbents can be suggested as an alternative technology for the conventionaldisposalmethods.However,further research is necessary to optimize the production processes and consider the environmentalimpacts such as the emission of the off-gas during thermal treatment.
Acknowledgements
The authors are thankful to the oil refinery of Isfahan for their efforts and supports.
[1] Dias J M,Alvim-Ferraz M C M,Almeida M F,et al.Waste materials for activated carbon preparation and its use in aqueousphase treatment:A review[J].Journal of Environmental Management,2007,85(4):833-846.
[2] Saka C.BET,TG-DTG,FT-IR,SEM,iodine number analysis and preparation of activated carbon from acorn shell by chemical activation with ZnCl2[J].Journal of Analytical and Applied Py-rolysis,2012,95:21-24.
[3] Moreno-Castilla C,Carrasco-Marín F,López-Ramón MV,et al. Chemical and physical activation of olive-mill waste water to produce activated carbons[J].Carbon,2001,39(9):1415-1420.
[4] Chen X,Jeyaseelan S,Graham N.Physical and chemical properties study of the activated carbon made from sewage sludge [J].Waste Management,2002,22(7):755-760.
[5] Hu G,Li J,Zeng G.Recent development in the treatment of oily sludge from petroleum industry:A review[J].Journal of Hazardous Materials,2013,261:470-490.
[6] Chang C Y,Shie J L,Lin J P,et al.Major products obtained from the pyrolysis of oil sludge[J].Energy&Fuels,2000,14 (6):1176-1183.
[7] Karamalidis A K,Voudrias E A.Release of Zn,Ni,Cu,SO42-and CrO42-as a function of pH from cement-based stabilized/solidified refinery oily sludge and ash from incineration of oily sludge[J].Journal of Hazardous Materials,2007,141(1): 591-606.
[8] Kriipsalu M,Marques M,Maastik A.Characterization of oily Sludge from a wastewater treatmentplantflocculation-flotation unit in a petroleum refinery and its treatment implications[J]. Journal of Material Cycles and Waste Management,2008,10 (1):79-86.
[9] Mazlova E A,Meshcheryakov S V.Ecological characteristics of oil sludges[J].Chemistry and Technology of Fuels and Oils, 1999,35(1):49-53.
[10] Kumar Mandal A,Manab Sarma P,Singh B,etal.Bioremediation:a sustainable eco-friendly biotechnological solution for environmental pollution in oilindustries[J].Journalof Sustainable Development&Environmental Protection,2011,1(3):5-23.
[11] Al-Futaisi A,Jamrah A,Yaghi B,et al.Assessmentof alternative management techniques of tank bottom petroleum sludge in Oman[J].Journal of Hazardous Materials,2007,141(3): 557-564.
[12] Seredych M,Bandosz T J.Removal of copper on composite sewage sludge/industrial sludge-based adsorbents:The role of surface chemistry[J].Journal of Colloid and Interface Science, 2006,302(2):379-388.
[13] ASTM.Standard test method for ash from petroleum products [S].PA(USA):ASTM International D 482-487,2005.
[14] ASTM.Standard test method for screening of pH in waste[S]. PA(USA):ASTM International D 4980-489,2005.
[15] ASTM.Standard practice for nitric acid digestion of solid waste [S].PA(USA):ASTM International D 5198-5192,2005.
[16] Hubschmann H J.Handbook of GC/MS[M].Germany:WILEY-VCH,2009:30-38.
[17] Deng H,Li G,Yang H,et al.Preparation of activated carbons from cotton stalk by microwave assisted KOH and K2CO3activation[J].Chemical Engineering Journal,2010,163(3): 373-381.
[18] Small C C,Hashisho Z,Ulrich A C.Preparation and characterization of activated carbon from oil sands coke[J].Fuel, 2012,92(1):69-76.
[19] U S Environmental Protection Agency.Method 1311[S].Toxicity characteristic leaching procedure(TCLP),1999.
[20] Asia I O,Enweani I B,Eguavoen I O.Characterization and treatment of sludge from the petroleum industry[J].African Journal of Biotechnology,2006,5(5):461-466.
[21] Shie J L,Chang C Y.Thermal degradation kinetics of oil sludge in the presence of carbon dioxide[J].Journal of Chinese Institute Environmental Engineering,2001,11(4):307-316.
[22] Rocha O,Dantas R,Duarte M M.Oilsludge treatmentby photocatalysis applying black and white light[J].Chemical Engineering Journal,2010,157(1):80-85.
[23] Liu J,Jiang X,Zhou L,etal.Pyrolysis treatmentof oil sludge and model-free kinetics analysis[J].Journal of Hazardous Materials,2009,161(3):1208-1215.
[24] Andrade P F,Azevedo T F,Gimenez I,et al.Conductive carbon-clay nanocomposites from petroleum oily sludge[J].Journal of Hazardous Materials,2009,167(3):879-884.
[25] Varela R,Andrade J M,Muniategui S,et al.The comparison of two heavy fuel oils in composition and weathering pattern, based on IR,GC-FID and GC-MS analyses:application to the prestige wreackage[J].Water Research,2009,43(4):1015-1026.
[26] Domínguez A,Menéndez J A,Inguanzo M,et al.Gas chromatographic-mass spectrometric study of the oilfractions produced by microwave-assisted pyrolysis of different sewage sludges [J].Journal of Chromatography A,2003,1012(2):193-206.
[27] Marsh H,Rodriguez-Reinoso F.Activated Carbon.1th ed[M]. London:Elsevier,2006:26-27,155-156.
[28] Lippens B C,Boer J H.Studies on pore systems in catalysts: V.the t method[J].Journal of Catalysis,1965,4(3):319-323.
[29] Kante K,Qiu J,Zhao Z,et al.Developmentof surface porosity and catalytic activity in metal sludge/waste oil derived adsorbents:effect of heat treatment[J].Chemical Engineering Journal,2008,138(1):155-165.
[30] Ruparelia J P,Duttagupta S P,Chatterjee A K,etal.Potential of carbon nanomaterials for removal of heavy metals from water [J].Desalination,2008,232(2):145-155.
[31] WHO.Guidelines for drinking-water quality.3rd ed.Volume 1:Recommendations[S].World Health Organization,2008, ISBN 978 92 4 1547604.
[32] Wang Y,Sheng F,Cao Z,et al.Assessment of maturity of vineyard pruning compost by fourier transform infrared spectroscopy,biological and chemical analyses[J].Landbauforschung VÖlkenrode,2004,54(3):163-169.
[33] KE Yi-hu,YANG Er-tao,LIU Xin,et al.Preparation of porous carbons from non-metallic fractions of waste printed circuit boards by chemical and physical activation[J].New Carbon materials,2013,28(2):107-114.
(柯义虎,杨二桃,刘 欣,等.用废弃印刷线路板非金属组分分离物制备多孔炭[J].新型炭材料,2013,28(2):107-114.)
A preliminary study of the preparation of porous carbon from oil sludge for water treatment by simple pyrolysis or KOH activation
Shohreh Mohammadi, Nourollah Mirghaffari
(DepartmentofNaturalResources,IsfahanUniversityofTechnology,Isfahan,Iran)
The production and disposal of large amounts of oil sludge are considered a most critical environmental issue in the petroleum industry.The possible conversion of oilsludge produced in a fueloilstorage tank to porous carbons by simple pyrolysis or KOH activation was investigated and the feasibility of their use to treat drinking water and to adsorb were evaluated.The oil sludge contains 80%of carbon and consists of mainly aliphatic compounds.The porous carbon obtained by KOH activation has a BET surface area,total pore volume and micropore surface area of 328.0 m2·g-1,0.21 cm3·g-1and 289.10 m2·g-1,respectively,while that produced by pyrolysis has a much lower surface area of 3.6 m2·g-1.Although Cd,Cu,Zn,Mn,and Fe are enriched in the porous carbons compared with the oil sludge,their leaching in distilled water is low and below the allowed standard limits except for Cr which is close to the limit.The Cd adsorption removal rates for the KOH activated porous carbon and the pyrolysed one are 97.36 and 77.74%,respectively.The former is comparable to three commercial activated carbons under the same conditions.The recovery of the oil sludge through KOH activation to prepare porous adsorbents for waste water treatment can be suggested as an alternative to the conventional disposal methods.
Oily sludge;Porous carbon;Thermochemical treatment;Adsorption
Nourollah Mirghaffari.E-mail:mnorolah@cc.iut.ac.ir
TB332
A
10.1016/S1872-5805(15)60192-5
Nourollah Mirghaffari.E-mail:mnorolah@cc.iut.ac.ir
1007-8827(2015)04-0310-09
Received date:2015-02-20;Revised date:2015-08-01
English edition available online ScienceDirect(http://www.sciencedirect.com/science/journal/18725805).

