硝酸对N,N-二甲基羟胺γ-辐解及液态辐解产物的影响
王锦花 曹晓军 李 春 吴明红 包伯荣郑卫芳 何 辉 张生栋
(1上海大学环境与化学工程学院,射线应用研究所,上海200444;2中国原子能科学研究院放射化学研究所,北京102413)
硝酸对N,N-二甲基羟胺γ-辐解及液态辐解产物的影响
王锦花1,*曹晓军1李 春1吴明红1包伯荣1郑卫芳2何 辉2张生栋2
(1上海大学环境与化学工程学院,射线应用研究所,上海200444;2中国原子能科学研究院放射化学研究所,北京102413)
N,N-二甲基羟胺(DMHA)是用于动力堆乏燃料后处理U与Pu和Np分离的新型无盐还原剂,本文研究了硝酸对DMHA γ-辐解及液态辐解产物的影响.研究结果表明:在U、Pu分离循环和Pu纯化循环的辐照剂量下,在0.3-1.0 mol·L-1的硝酸溶液中,0.1和0.5 mol·L-1DMHA具有较好的辐照稳定性.当吸收剂量为5-25 kGy时,DMHA硝酸溶液的液态辐解产物主要有单甲基羟胺、甲醛、甲酸和亚硝酸.有机物的浓度远远高于亚硝酸浓度,且随着剂量和硝酸浓度的增加而增大.对于相同的硝酸浓度和剂量,0.1 mol·L-1DMHA辐解产生的一甲基羟胺的浓度高于0.5 mol·L-1DMHA,但前者辐解产生的甲醛浓度低于后者;当硝酸浓度较高时,0.1 mol· L-1DMHA辐解产生的甲酸浓度高于0.5 mol·L-1DMHA.亚硝酸浓度与硝酸浓度及剂量的关系取决于起始DMHA和硝酸浓度.
N,N-二甲基羟胺;硝酸;γ-辐解;液相辐解产物;乏燃料后处理
©Editorial office ofActa Physico-Chimica Sinica
Key Words:N,N-dimethyl hydroxylamine;Nitric acid;γ-Radiolysis;Radiolytic liquid product; Reprocessing of spent fuel
1 Introduction
With increasing in population and higher living standards the demand for energy has increased quickly.However,fossil fuels are being exhausted and the long-term use of fossil fuels has resulted in serious environmental pollution.Therefore,clean energy will gradually replace fossil fuels.Nuclear,hydro,wind,and solar energy are clean sources of energy.The Chinese government has decided to develop significant nuclear energy capability.The quick development of nuclear energy will result in a great amount of spent nuclear fuel.To sustainably develop nuclear energy, China has adopted the closed nuclear fuel cycle.This includes the reprocessing of spent fuel to isolate and recycle unused U and Pu generated in the burned fuel.The PUREX(Plutonium Uranium Recovery by Extraction)process is the main process used for the industrial scale reprocessing of spent fuel.1In this process,U(VI) and Pu(IV)are co-extracted into an organic phase and Pu(IV)is then reduced to organic-insoluble Pu(III).The U(VI)in the organic phase is not affected.Sulfamate-stabilized Fe(II)and hydrazine-stabilized U(IV)are two common reductants that are used in U/Pu separation.Sulfamate and hydrazine are holding reductants that are used to destroy traces of HNO2and to stabilize Pu(III)in HNO3.2,3However,neither sulfamate nor hydrazine is appreciably extracted into the tributyl phosphate(TBP)phase and, therefore,they cannot destroy HNO2in the organic phase resulting in Pu(III)oxidation.This is the reason for a large of excess reductant being required for acceptable Pu separation.4-6The excess consumption of ferrous sulfamate increases the volume of radioactive waste,which obviously increases the cost of the final disposal of radioactive waste.Additionally,U(IV)-NH2NH2may potentially produce explosive HN3.The Np content is high in spent fuel7and neither ferrous nor U(IV)can control the valence of Np.This causes Np to deport to different phases.8Because237Np is a highly toxic nuclide,the allowable Np content in the U product is severely limited.9The irradiated product of237Np is238Pu,and Np is supposed to deport with Pu.1Research results10-20have shown that N,N-dimethyl hydroxylamine(DMHA)can quickly reduce Pu(IV)and Np(VI)to Pu(III)and Np(V),respectively and they are not extractable in TBP.Pu(III)and Np(V) are stable for specific periods of time in acid solutions and, therefore,DMHA is a good prospect in this regard.Our research group21-23has studied the radiolysis and radiolytic products of aqueous DMHA solutions and found that DMHA has good radiation stability.The main radiolytic liquid products are N-methyl hydroxylamine,HCHO,and HCOOH,and the concentrations of N-methyl hydroxylamine and HCHO are much higher than that of HCOOH.The main radiolytic gaseous products are hydrogen and methane,and the volume fraction of hydrogen is higher than that of methane.In the PUREX process,the separation of U from Pu and Np is carried out in HNO3solution.HNO3may affect the radiation stability of DMHAas well as the radiolytic product.We thus report on the effect of HNO3on the radiolysis of DMHAand its radiolytic liquid products.Our results are an important reference for the application of DMHA to the reprocessing of spent fuel.
2 Experimental
2.1 Reagent
DMHA was supplied by the China Institute of Atomic Energy and its purity was 98%.N-Methylhydroxylamine hydrochloride was supplied by theAcros Organics Company,USA,and its purity was higher than 98%.p-Aminobenzenesulfonamide and N-(1-naphthyl)ethylenediamine dihydrochloride were supplied by the China Chemical Reagent Co.,Ltd.,and they were of analytical purity.
2.2 Main equipment and accessories
The60Co γ-ray radiation facility supplied 1.295×1015Bq radiation and is located at the Shanghai Institute of Applied Physics, the Chinese Academy of Sciences.For gas chromatography we used a GC7890A gas chromatograph from Agilent Technologies Co.,Ltd.,USA.Spectrophotometry was conducted using a TU-1901 UV-Vis spectrophotometer that was purchased from the Beijing General Instruments Co.,Ltd.Ion chromatography was conducted using a Metrohm ion chromatograph(research-style) that was purchased from the Metrohm Co.,Ltd.,Switzerland.
2.3 Sample preparation and irradiation
The preparation of 0.1 mol·L-1DMHAcontaining 0.3 mol·L-1HNO3was as follows.A specific amount of DMHA was put into a beaker and the beaker was placed in an ice-water bath.20 mLof 0.5 mol·L-1HNO3was added dropwise into the beaker with stirring for the protonation of DMHA.A specific amount of slightly diluted HNO3was then added into the beaker to give a final HNO3concentration of 0.3 mol·L-1.The solution was added to a 100-mL measuring flask and made up to a constant volume to give the desired solution.Other solutions were prepared in the same manner as 0.1 mol·L-1DMHAcontaining 0.3 mol·L-1HNO3. 4 mL of each solution was placed in 7 mL penicillin bottles and the bottles were covered and sealed with a sealing machine.The samples were irradiated at the60Co radiation facility.The absorbed doses were 5,10,15,20,and 25 kGy and monitoring was done using dichromate dosimeters.
2.4 Calculation of the oxidative degradation and radiolytic rate of DMHA
The oxidative degradation rate(OR)is defined as follows:

where c0is the original DMHAconcentration and Ctis the DMHA concentration in the controlled samples.The radiolytic rate(RR) of DMHAis defined as follows:

where cris the DMHAconcentration in the irradiated samples.
3 Results and discussion
3.1 Effect of HNO3on DMHA radiolysis
The concentration of the reductant used in the U/Pu separation cycle is lower in the PUREX process than that in the Pu purification cycle.Theeffect of HNO3on the radiolysis and radiolytic products of DMHA was investigated using different DMHA concentrations.Fig.1 shows the DMHAconcentration in irradiated DMHA-HNO3as a function of absorbed dose.
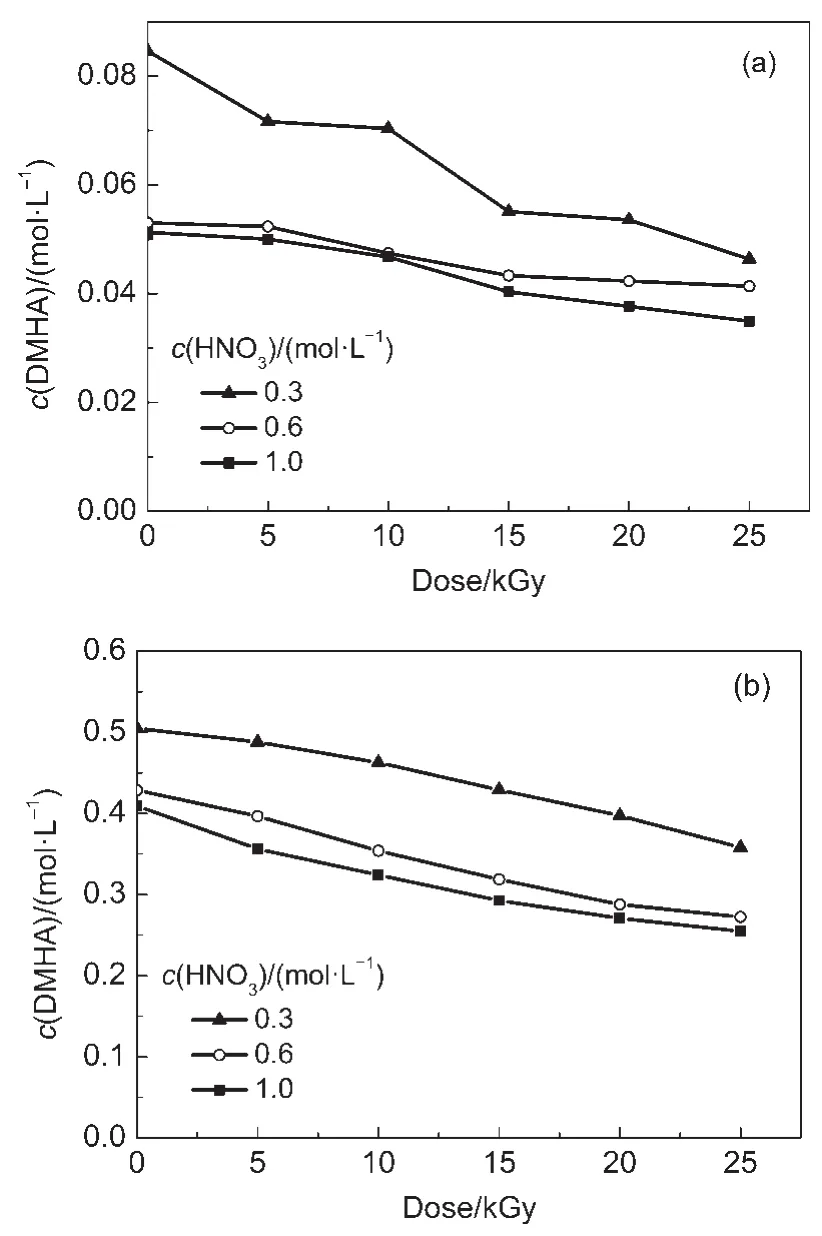
Fig.1 DMHAconcentration in irradiated DMHA-HNO3as a function of absorbed dose
Fig.1 shows that the DMHAconcentration decreases obviously with an increase in HNO3concentration from 0.3 to 0.6 mol·L-1, however,when the HNO3concentration is higher than 0.6 mol·L-1, the DMHA concentration does not change much.The DMHA concentration decreases with an increase in dose.For the 0.1 mol· L-1DMHA solutions,the DMHA concentration decreases obviously with an increase in dose at a HNO3concentration of 0.3 mol· L-1;however,it decreases slowly when the HNO3concentration is≥0.6 mol·L-1.For 0.5 mol·L-1DMHA-HNO3,the DMHA concentration decreases gradually with an increase in dose.Li et al.24and Karraker25reported that the reagent-absorbed dose during the U/Pu separation cycle and the Pu purification cycle was lower than 10 kGy.The effect of HNO3on the oxidative degradation and radiolytic rate of DMHAat 10 kGy is listed in Table 1.

Table1 OR and RR at 10 kGy of DMHAin HNO3
Table 1 shows that for the same HNO3concentration the OR of DMHAin 0.1 mol·L-1DMHA-HNO3is much higher than that of 0.5 mol·L-1DMHA-HNO3.For the 0.1 mol·L-1DMHA-HNO3that was irradiated at 10 kGy and at a HNO3concentration of 0.3 mol·L-1,the OR of DMHA was found to be lower than its RR. However,at a HNO3concentration≥0.6 mol·L-1the OR of DMHA is much higher than its RR.For 0.5 mol·L-1DMHAHNO3both the OR and RR increase with HNO3concentration but the OR is always lower than the RR.HNO3is an oxidant and DMHA is a reductant,and they may interact with each other as follows:26,27
3(CH3)2NOH+2HNO3→3HCHO+3CH3NHOH+2NO+H2O(1)
At a higher HNO3concentration more DMHA is consumed upon reacting with HNO3.This is the reason for the OR of DMHA increasing with HNO3concentration.Additionally,the DMHA concentration in 0.1 mol·L-1DMHA-HNO3is five times lower than that in 0.5 mol·L-1DMHA-HNO3.This means that the relative HNO3concentration in 0.1 mol·L-1DMHA-HNO3is much higher than that in 0.5 mol·L-1DMHA-HNO3.This is the reason for the OR of DMHAfor 0.1 mol·L-1DMHA-HNO3being much higher than that of 0.5 mol·L-1DMHA-HNO3.In addition,when water is exposed to γ-rays:H·,OH,eaq,and other active species are produced.28,29


The N,N-dimethyl nitroxide radical may self-react to form N,N-dimethyl nitrone,which can hydrolyze to produce N-methyl hydroxylamine and HCHO:

At a higher dose the·OH concentration increases(Eq.(2))and the amount of DMHAconsumed by the reaction between DMHA and·OH(Eq.(5))also increases.Therefore,the DMHAconcentration decreases with an increase in dose.
3.2 Effect of HNO3on the radiolytic liquid products of DMHA
Our research group has studied the radiolysis of aqueous DMHA solutions and their radiolytic liquid organics.23For the 0.1-0.5 mol·L-1DMHAsolutions that were irradiated from 5 to 25 kGy,the main radiolytic liquid organics are N-methyl hydrox-ylamine,HCHO,and HCOOH.The concentration of N-methyl hydroxylamine and HCHO is much higher than that of HCOOH. The radiolytic liquid organics of the DMHA-HNO3solutions were studied using the same methods and results show that the main radiolytic organics are N-methyl hydroxylamine,HCHO,and HCOOH.When HNO3is exposed to radiation,NO3-may react with ea-qand H·to produce NO32-·and HNO3-·(Eqs.(3,4)).Both these reactive radicals may continue reacting to produce HNO2:30-32

As HNO2can oxidize Pu(III)to Pu(IV),it may affect the reprocessing of spent fuel and HNO2must thus be further studied. A qualitative analysis of HNO2was carried out using anion chromatography33and N-(1-naphthyl)-1,2-diaminoethane spectrophotometry.34Results show that HNO2is present in the irradiated DMHA-HNO3solutions.
3.2.1 Effect of HNO3on the N-methyl hydroxylamine produced by the radiolysis of DMHA
The concentration of N-methyl hydroxylamine in irradiated DMHA-HNO3as a function of dose is shown in Fig.2.This figure shows that the concentration of N-methyl hydroxylamine increases with increasing the dose and HNO3concentration. However,the effect of HNO3on the concentration of N-methyl hydroxylamine in 0.5 mol·L-1DMHA-HNO3is higher than that in 0.1 mol·L-1DMHA-HNO3.At doses from 5 to 25 kGy and HNO3concentrations from 0.3,0.6 to 1.0 mol·L-1,the concentrations of N-methyl hydroxylamine in irradiated 0.1 mol·L-1DMHA-HNO3are(29.9-38.2)×10-3,(27.5-49.4)×10-3,and(33.0-54.3)×10-3mol·L-1,respectively.The concentrations in irradiated 0.5 mol·L-1DMHA-HNO3are(14.3-34.7)×10-3,(23.7-40.8)×10-3, and(30.0-49.0)×10-3mol·L-1,respectively.A comparison of these data reveals that the concentration of N-methyl hydroxylamine in irradiated 0.1 mol·L-1DMHA-HNO3is higher than that in 0.5 mol·L-1DMHA-HNO3.
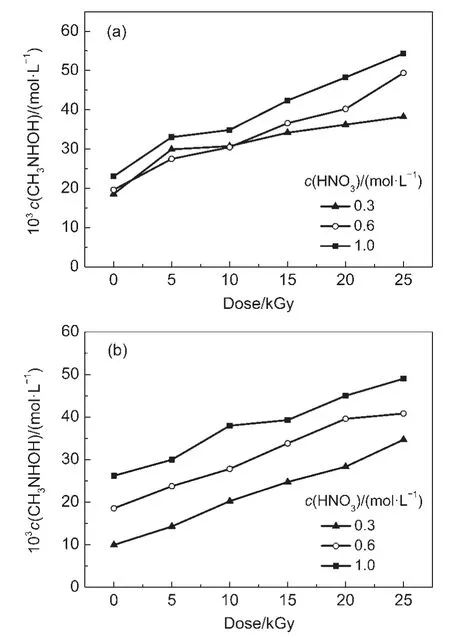
Fig.2 Concentration of N-methyl hydroxylamine in irradiated DMHA-HNO3as a function of dose
3.2.2 Effect of HNO3on the HCHO produced by the radiolysis of DMHA
Fig.3 shows that the HCHO concentration increases with increasing the dose and HNO3concentration.At doses from 5 to 25 kGy and HNO3concentrations from 0.3,0.6 to 1.0 mol·L-1,the HCHO concentrations in irradiated 0.1 mol·L-1DMHA-HNO3are (23.7-34.2)×10-3,(28.7-34.9)×10-3,and(29.0-36.6)×10-3mol· L-1,respectively;these concentrations in irradiated 0.5 mol·L-1DMHA-HNO3are(37.9-50.2)×10-3,(41.7-52.9)×10-3,and(47.6-55.2)×10-3mol·L-1,respectively.The HCHO concentration in irradiated 0.1 mol·L-1DMHA-HNO3is lower than that in irradiated 0.5 mol·L-1DMHA-HNO3.
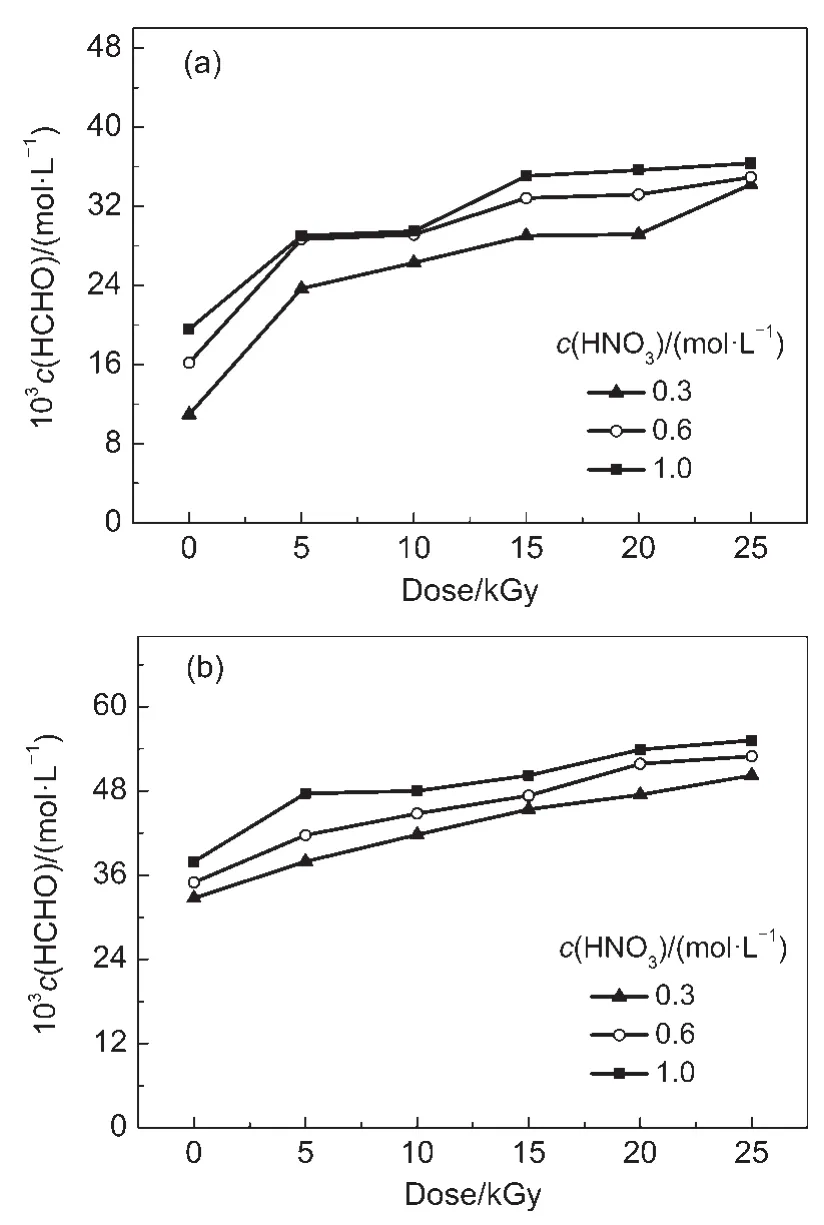
Fig.3 HCHO concentration in irradiated DMHA-HNO3as a function of dose
From Eq.(1),a higher HNO3concentration leads to higher N-methyl hydroxylamine and HCHO concentrations.This is the reason for the concentrations of N-methyl hydroxylamine and HCHO,produced by the oxidation of DMHA,increasing with HNO3concentration.In addition,a higher dose results in a higher ·OH concentration(Eq.(2)),and the concentration of the N,N-dimethyl nitroxide radical(Eq.(5))is also higher.The N,N-dimethyl nitroxide radical can hydrolyze into N-methyl hydroxylamine and HCHO(Eqs.(6,7)).Therefore,the concentrations of N-methyl hydroxylamine and HCHO increase with dose.
In Eqs.(1,7),the molar ratio of N-methyl hydroxylamine and HCHO is 1:1.The molar concentrations of N-methyl hydroxylamine and HCHO should thus be equal.However,different concentrations were obtained experimentally.At the same dose and HNO3concentration,the concentration of N-methyl hydroxylamine in irradiated 0.1 mol·L-1DMHA-HNO3is higher than that in irradiated 0.5 mol·L-1DMHA-HNO3,but the HCHO concentration in 0.1 mol·L-1DMHA-HNO3is lower than that in 0.5 mol·L-1DMHA-HNO3.
3.2.3 Effect of HNO3on the HCOOH produced by the radiolysis of DMHA
Fig.4 shows that the HCOOH concentration in irradiated DMHA-HNO3increases with increasing the HNO3concentration and dose but the effect of HNO3on the HCOOH concentration in 0.1 mol·L-1DMHA-HNO3is larger than that in 0.5 mol·L-1DMHA-HNO3.At doses from 5 to 25 kGy and HNO3concentrations from 0.3,0.6 to 1.0 mol·L-1,the HCOOH concentrations in irradiated 0.1 mol·L-1DMHA-HNO3are(1.8-6.0)×10-3,(4.6-18.5)×10-3,and(6.1-25.5)×10-3mol·L-1,respectively;the concentrations in irradiated 0.5 mol·L-1DMHA-HNO3are(2.0-8.1)× 10-3,(3.5-8.7)×10-3,and(4.0-9.6)×10-3mol·L-1,respectively.At a HNO3concentration of 0.3 mol·L-1the HCOOH concentration in 0.1 mol·L-1DMHA-HNO3is a little lower than that in 0.5 mol· L-1DMHA-HNO3.For a HNO3concentration≥0.6 mol·L-1the HCOOH concentration of 0.1 mol·L-1DMHA-HNO3is obviously higher than that of 0.5 mol·L-1DMHA-HNO3.DMHA can be oxidized to HCHO by HNO3and it can also be radiolyzed to HCHO.HCHO may be oxidized to HCOOH:
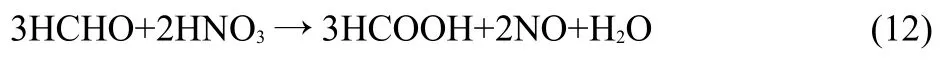
The HCOOH concentration increases with HNO3concentration. Additionally,HCHO may also react with·OH to produce HCOOH:
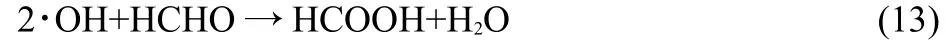
At a higher dose the·OH concentration increases and,therefore,the HCOOH concentration increases with dose.Fig.3 shows that the HCHO concentration in irradiated 0.5 mol·L-1DMHAHNO3is obviously higher than that in irradiated 0.1 mol·L-1DMHA-HNO3.Fig.4 shows that the HCOOH concentration in 0.5 mol·L-1DMHA-HNO3is only slightly higher than that in 0.1 mol· L-1DMHA-HNO3at a HNO3concentration of 0.3 mol·L-1.The relative HNO3concentration in 0.1 mol·L-1DMHA-HNO3is much higher than that in 0.5 mol·L-1DMHA-HNO3,which means that the oxidizability of 0.1 mol·L-1DMHA-HNO3is higher than that of 0.5 mol·L-1DMHA-HNO3.Therefore,more HCHO can transform into HCOOH in 0.1 mol·L-1DMHA-HNO3than that in 0.5 mol·L-1DMHA-HNO3.In addition,as the DMHA concentration in 0.1 mol·L-1DMHA-HNO3is much lower than that in 0.5 mol·L-1DMHA-HNO3,the H2O concentration in 0.1 mol·L-1DMHA-HNO3is higher than that in 0.5 mol·L-1DMHA-HNO3. The·OH concentration in 0.1 mol·L-1DMHA-HNO3is higher than that in 0.5 mol·L-1DMHA-HNO3resulting in more HCHO being transformed into HCOOH.At a HNO3concentration of 0.3 mol·L-1,although the HCHO concentration in irradiated 0.1 mol· L-1DMHA-HNO3is obviously lower than that in irradiated 0.5 mol·L-1DMHA-HNO3,because more HCHO transforms into HCOOH,the HCOOH concentration in 0.1 mol·L-1DMHAHNO3is only slightly lower than that in 0.5 mol·L-1DMHAHNO3.At a HNO3concentration≥0.6 mol·L-1far more HCHO transforms into HCOOH in the irradiated 0.1 mol·L-1DMHAHNO3compared with the irradiated 0.5 mol·L-1DMHA-HNO3. This results in a higher HCOOH concentration in 0.1 mol·L-1DMHA-HNO3compared with that in 0.5 mol·L-1DMHA-HNO3. This is also the reason for the HCHO concentration in the irradiated 0.1 mol·L-1DMHA-HNO3being lower than that in 0.5 mol· L-1DMHA-HNO3.

Fig.4 HCOOH concentration in irradiated DMHA-HNO3as a function of dose
3.2.4 Effect of HNO3on the HNO2produced in irradiated DMHA-HNO3
Fig.5 shows that in irradiated 0.1 mol·L-1DMHA containing 0.3-0.6 mol·L-1HNO3and in irradiated 0.5 mol·L-1DMHA containing 0.3-1.0 mol·L-1HNO3,the HNO2concentration increases with the increases in HNO3concentration and dose. However,for 0.1 mol·L-1DMHAcontaining 1.0 mol·L-1HNO3, the HNO2concentration is dependent on the dose.The HNO2concentration increases with an increase in dose at doses lower than 10 kGy.However,it decreases upon increasing the dose to higher than 10 kGy.A quantitative analysis of HNO2was carried out using anion chromatography and N-(1-naphthyl)-1,2-diami-noethane spectrophotometry.The results were found to be consistent.At doses from 5 to 25 kGy and HNO3concentrations from 0.3,0.6 to 1.0 mol·L-1,the HNO2concentrations in irradiated 0.1 mol·L-1DMHA-HNO3are(1.2-2.7)×10-4,(1.5-4.9)×10-4,and (1.0-2.8)×10-4mol·L-1,respectively.The concentrations in irradiated 0.5 mol·L-1DMHA-HNO3are(1.7-3.8)×10-4,(1.9-5.1)× 10-4,and(2.3-5.4)×10-4mol·L-1,respectively.The HNO2concentration in 0.1 mol·L-1DMHA-HNO3is slightly lower than that in 0.5 mol·L-1DMHA-HNO3.

Fig.5 HNO2concentration in irradiated DMHA-HNO3as a function of dose
From Figs.2-5,the concentrations of N-methyl hydroxylamine and HCHO are higher than that of HCOOH;the concentrations of these organics are much higher than that of HNO2.Some HNO2remains in the irradiated DMHA-HNO3solutions and this means that DMHA alone does not completely destroy HNO2.This may initiate the oxidation of Pu(III)and Np(V)causing Pu and Np to deport to different phases.Therefore,a holding reductant should be added when DMHAis used to separate U from Pu and Np.
4 Conclusions
DMHA is a novel salt-free reductant used in the separation of U from Pu and Np in the reprocessing of spent fuel.For 0.1 and 0.5 mol·L-1DMHAcontaining 0.3-1.0 mol·L-1HNO3,we found that DMHA has good radiation stability upon receiving a reasonable radiation dose during the U/Pu separation cycle and the Pu purification cycle.At doses of 5-25 kGy the main radiolytic liquid products are N-methyl hydroxylamine,HCHO,HCOOH, and HNO2.The concentration of organics is much higher than that of HNO2and it increases with an increase in HNO3concentration and dose.At the same HNO3concentration and dose,the concentration of N-methyl hydroxylamine in irradiated 0.1 mol·L-1DMHA-HNO3is higher than that in irradiated 0.5 mol·L-1DMHAHNO3.However,the HCHO concentration of 0.1 mol·L-1DMHAHNO3is lower than that of 0.5 mol·L-1DMHA-HNO3.The HCOOH concentration in the irradiated 0.1 mol·L-1DMHAHNO3is higher than that in irradiated 0.5 mol·L-1DMHA-HNO3at a higher HNO3concentration.As some HNO2is still present in irradiated DMHA-HNO3solutions,a holding reductant should be used when DMHA is applied to the separation of U from Pu and Np.
(1)Choppin,G.R.;Morgenstern,A.J.Radioanal.Nucl.Chem.2000,243(1),45.doi:10.1023/A:1006754927614
(2)Taylor,R.J.;Denniss,I.S.;Wallwork,A.L.Nucl.Energy1997,36(1),39.
(3)Schlea,C.S.;Caverly,M.R.;Henry,H.E.;Jenkins,W.J. Uranium(VI)Nitrate as a Reducing Agent for Plutonium(IV)in the Purex Process(DP-808);E.I.Du Pont De Nemours& Company:Alken,South Carolina,1963;pp 1-20.
(4)Sze,Y.K.;Clegg,L.J.;Gerwing,A.F.;Grant,G.R.Nucl. Technol.1982,56,527.
(5)Sze,Y.K.;Gosselin,J.A.Nucl.Technol.1983,63,431.
(6)Mckibben,J.M.;Bercaw,J.E.Hydroxylamine Nitrate as a Plutonium Reductant in the Purex Solvent Extraction Process (DP-1248);E.I.Du Pont De Nemours&Company:Alken, South Carolina,1971;pp 1-22.
(7)Ochsenfeld,W.;Petrich,G.Sep.Sci.Technol.1983,18, 1685.doi:10.1080/01496398308056121
(8)Biddle,P.;Miles,J.H.J.Inorg.Nucl.Chem.1968,30, 1291.doi:10.1016/0022-1902(68)80558-5
(9)Zheng,W.F.;Zhang,Z.P.;Lin,Z.J.;Chang,Z.Y.;Zhu,J.M.; Zhu,Z.W.Chin.J.Nucl.Sci.Eng.2001,21(4),369.[郑卫芳,章泽浦,林漳基,常志远,朱建民,朱兆武.核科学工程,2001,21(4),369.]
(10)Koltunov,V.S.;Baranov,S.M.;Zharova,T.P.Radiokhimiya1993,35(4),49.
(11)Zhang,A.Y.;Li,K.;He,H.;Hu,J.X.;Zhang,X.Y.;Wang,F. D.Chin.J.Appl.Chem.2001,18(3),180.[张安运,厉 凯,何 辉,胡景炘,张先业,王方定.应用化学,2001,18(3),180.]
(12)Koltunov,V.S.Stabilization of Pu and Np Valances in Purex Process:Problems and Outlook,RECOD′98,the 5th International Nuclear Conference on Recycling,Condition and Disposal,Nice,France,1998,The French Nuclear Society and the European Nuclear Society,France,1998;pp 425-431.
(13)He,H.;Hu,J.X.;Zhang,X.Y.;Xiao,S.T.;Zhu,W.B.;Wang, F.D.J.Nucl.Radiochem.2001,23(2),65.[何 辉,胡景炘,张先业,肖松涛,朱文彬,王方定.核化学和放射化学,2001,23(2),65.]
(14)Zhang,A.Y.;Hu,J.X.;Zhang,X.Y.;Wang,F.D.At.Energy Sci.Technol.2001,35(1),83. [张安运,胡景炘,张先业,王方定.原子能科学技术,2001,35(1),83.]
(15)He,H.;Hu,J.X.;Zhang,X.Y.;Wang,F.D.At.Energy Sci. Technol.2002,36(2),101.[何 辉,胡景炘,张先业,王方定.原子能科学技术,2002,36(2),101.]
(16)Li,X.G.;Ye,G.A.;He,H.;Tang,H.B.;Li,B.;Li,H.R.At. Energy Sci.Technol.2010,44(2),129. [李小该,叶国安,何辉,唐洪彬,李 斌,李会容.原子能科学技术,2010,44(2), 129.]
(17)Chen,Y.X.;Tang,H.B.;Liu,J.P.J.Radioanal.Nucl.Chem.2011,289(1),41.doi:10.1007/s10967-011-1030-1
(18)Liu,J.P.;He,H.;Tang,H.B.;Chen,Y.X.J.Radioanal.Nucl. Chem.2011,288(2),351.doi:10.1007/s10967-010-0917-6
(19)Li,G.L.;He,H.J.Radioanal.Nucl.Chem.2011,287(3), 673.doi:10.1007/s10967-010-0912-y
(20)Zuo,C.;Yan,T.H.;Zheng,W.F.;Li,C.B.;Wang,X.R. J.Radioanal.Nucl.Chem.2010,283(2),417.doi:10.1007/ s10967-009-0371-5
(21)Wang,J.H.;Li,Q.;Wu,M.H.;Xu,G.;Li,C.;Bao,B.R.; Zheng,W.F.;He,H.;Zhang,S.D.J.Radioanal.Nucl.Chem.2012,292(1),249.doi:10.1007/s10967-011-1468-1
(22)Wang,J.H.;Li,C.;Wu,M.H.;Xu,G.;Bao,B.R.,Zheng,W. F.;He,H.;Zhang,S.D.Nucl.Sci.Techniq.2010,21(4),233.
(23)Wang,J.H.;Wu,M.H.;Bao,B.R.;Li,Z.;Wang,Q.Y.;Zhang, X.Y.;Ye,G.A.J.Radioanal.Nucl.Chem.2007,273(2), 371.doi:10.1007/s10967-007-6842-7
(24)Li,H.B.;Su,Z.;Cong,H.F.;Song,F.L.;Wang,X.R.;He,H.; Liu,Z.Y.;Lin,C.S.J.Nucl.Radiochem.2012,34(5),281. [李辉波,苏 哲,丛海峰,宋凤丽,王孝荣,何 辉,刘占元,林灿生.核化学与放射化学,2012,34(5),281.]
(25)Karraker,D.G.Radiation Chemistry of Acetohydroxamic Acid in the Urex Process(WSRC-TR-2002-00283);U.S.Department of Commerce,National Technical Information Service: Springfielf,2002;pp 1-8.
(26)Adamic,K.;Bowman,D.F.;Gillan,T.;Ingold,K.U.J.Am. Chem.Soc.1971,93(4),902.doi:10.1021/ja00733a018
(27)Deng,J.X.J.Chengdu Coll.Edu.2001,15(11),70.[邓加旭.成都教育学院学报,2001,15(11),70.]
(28)Wu,J.L.;Qi,S.C.Radiation Chemistry;Atomic Energy Publisher:Beijing,1993;pp 156-198.[吴季兰,戚生初.辐射化学.北京:原子能出版社,1993:156-198.]
(29)Spinks,J.W.T.;Woods,R.J.An Introduction to Radiation Chemistry,2nd ed.;AWiley-Interscience Publication,John Wiley&Sons:New York,1976;pp 247-295.
(30)Alfassi,Z.B.N-Centered Radicals;John Wiley&Sons Ltd.: Hoboken,New Jersey,1998;pp 393-412.
(31)KozŁowska-Milner,E.;Broszkiewicz,R.K.Radiat.Phys. Chem.1977,11(5),253.
(32)Katsumura,Y.;Jiang,P.Y.;Nagaishi,R.;Oishi,T.;Ishigure,K. J.Phys.Chem.1991,95,4435.doi:10.1021/j100164a050
(33)Lou,Y.W.;Ji,Q.Z.Chin.J.Health Lab.Tech.2010,20(12), 3227.[楼颖伟,季巧珍.中国卫生检验杂志,2010,20(12), 3227.]
(34)Editorial Committee of China Environmental Protection Bureau for the book“Analysis Methods for Water and Waste Water”. Analysis Methods for Water and Waste Water,4th ed.;China Environmental Publisher:Beijing,2002;Vol.12,271-274. [国家环境保护总局《水和废水检测分析方法》编委会.水和废水监测分法方法(第四版).北京:中国环境出版社,2002:12卷,271-274.]
Effect of HNO3on the γ Radiolysis and Radiolytic Liquid Products of N,N-Dimethylhydroxylamine
WANG Jin-Hua1,*CAO Xiao-Jun1LI Chun1WU Ming-Hong1BAO Bo-Rong1ZHENG Wei-Fang2HE Hui2ZHANG Sheng-Dong2
(1Shanghai Applied Radiation Institute,School of Environmental and Chemical Engineering,Shanghai University,Shanghai 200444,P.R.China;2Radiochemistry Department,China Institute of Atomic Energy,Beijing 102413,P.R.China)
N,N-dimethylhydroxylamine(DMHA)is a novel salt-free reductant used for the separation of U from Pu and Np in the reprocessing of spent fuel.We investigated the effect of HNO3on the radiolysis of DMHA and its radiolytic liquid product.Results show that 0.1 and 0.5 mol·L-1DMHA containing 0.3-1.0 mol· L-1HNO3have good radiation stability at a reasonable dose level during the U/Pu separation cycle and the Pu purification cycle.For 5-25 kGy the main radiolytic liquid products are N-methyl hydroxylamine,HCHO, HCOOH,and HNO2.The concentration of these organic compounds is much higher than that of HNO2and increases with both HNO3concentration and absorbed dose.At the same HNO3concentration and dose, the concentration of N-methyl hydroxylamine in irradiated 0.1 mol·L-1DMHA-HNO3is higher than that in irradiated 0.5 mol·L-1DMHA-HNO3.However,the HCHO concentration in 0.1 mol·L-1DMHA-HNO3is lower than that in 0.5 mol·L-1DMHA-HNO3.The HCOOH concentration in 0.1 mol·L-1DMHA-HNO3is higher than that in 0.5 mol·L-1DMHA-HNO3at higher HNO3concentrations.The relationship of HNO2concentration with HNO3concentration and dose depends on the original DMHAand HNO3concentrations.
O644.2
10.3866/PKU.WHXB201501092www.whxb.pku.edu.cn
Received:September 23,2014;Revised:January 9,2015;Published on Web:January 9,2015.
∗Corresponding author.Email:jinhuawang@staff.shu.edu.cn;Tel:+86-21-66137506.
The project was supported by the National Natural Science Foundation of China(20771074,11025526)and Program for Innovative Research Team in University,China(IRT13078).
国家自然科学基金(20771074,11025526)和创新团队发展计划(IRT13078)资助项目

