丛枝菌根(AM)真菌与共生植物物质交换研究进展
舒 波, 李伟才, 刘丽琴, 魏永赞, 石胜友
(中国热带农业科学院南亚热带作物研究所, 农业部热带果树生物学重点实验室, 广东湛江 524091)
丛枝菌根(AM)真菌与共生植物物质交换研究进展
舒 波, 李伟才, 刘丽琴, 魏永赞, 石胜友*
(中国热带农业科学院南亚热带作物研究所, 农业部热带果树生物学重点实验室, 广东湛江 524091)
丛枝菌根(Arbuscular Mycorrhizal,AM)真菌能与约 80% 的陆生植物形成共生关系,植、 菌间矿质养分、 碳水化合物的物质交换是自然界物质循环的重要内容。目前,AM 真菌促进共生植物矿质养分吸收的研究相对较多。研究表明, AM 真菌可通过根外菌丝更小的吸收直径,加强矿质养分的空间有效性; 通过释放有机酸、 土壤酶,活化土壤中被固定的矿质养分; 通过根外菌丝上较低Km值的矿质养分转运蛋白,保证养分从土壤至根外菌丝的转运效率; 通过矿质养分在菌丝内运输形式的改变,增强养分的运输速率; 通过诱导共生植物矿质养分转运蛋白表达,提高植、 菌间养分的转运效率。相较于 AM 真菌促进共生植物养分吸收,植物反馈真菌碳水化合物的研究相对较少。鉴于 AM 真菌与植物共生关系在生态系统中的重要作用,明晰植、 菌间矿质养分和碳水化合物交换的具体场所(丛枝、 根内菌丝、 根外菌丝)、 具体形式(离子、 聚合物、 氨基酸、 蔗糖、 单糖)、 具体过程(主动运输)具有重要科学意义。本文对 AM 真菌与共生植物物质交换的丛枝、 菌丝双膜结构,氮(N)、 磷(P)、 糖等物质交换的具体形式以及跨双膜、 耗能量、 互耦连的物质交换过程进行综述,并从物质交换的场所、 形式、 过程三个方面提出了植、 菌物质交换的研究方向。
矿质养分; 碳水化合物; 膜结构; 转运蛋白

AM 真菌侵染植物后,植物矿质养分的吸收转变为两种方式,一种依赖于植物根毛吸收养分—根系直接吸收方式; 另一种则依赖根外菌丝吸收养分—菌根吸收方式。相较于根系直接吸收方式,菌根吸收方式存在诸多优势。AM 真菌通过根外菌丝更小的吸收直径,增加养分吸收面积,加强矿质养分的空间有效性[7]; 通过自身或诱导共生植物释放有机酸、 土壤酶等物质,活化土壤中被固定的矿质养分,提高矿质养分有效浓度[8-9]; 通过根外菌丝上较低Km值和较高 Vmax 值的矿质养分转运蛋白,保证养分从土壤转运至根外菌丝的效率[10-11]; 通过矿质养分在 AM 真菌菌丝内运输形式的改变,加快养分的运输速度[12-13]; 通过诱导定位于丛枝前体质膜(peri-arbuscular membrane)上共生植物矿质养分转运蛋白表达,提高植、 菌间养分的转运效率[14]。以此为交换,共生植物反馈 AM 真菌碳水化合物,以帮助此类严格活体营养型真菌完成其生活史[5]。具体地,共生植物光合作用产生的碳水化合物运抵根系丛枝细胞后,在丛枝前体质膜与丛枝膜(arbuscular membrane)上相关转运蛋白作用下穿过双膜结构,完成植物碳水化合物向 AM 真菌的转运[15-16]。
AM 真菌与共生植物间的物质交换相互促进、 互相关联。目前,关于 AM 真菌利用自身优势促进共生植物矿质养分吸收的研究已较为广泛,但涉及植、 菌间碳水化合物与矿质养分的交换过程却少有归纳。鉴于植、 菌共生关系在自然与农业生态系统中的重要作用,明确两者物质交换的过程有重要科学意义。本文对 AM 真菌与共生植物间矿质养分和碳水化合物物质交换场所、 物质交换形式、 物质交换过程进行梳理,以探讨植、 菌物质交换的研究方向。
1 AM 真菌与共生植物物质交换的场所
1.1AM 真菌与共生植物物质交换的双膜结构
AM 真菌与共生植物间绝大部分的物质交换发生于丛枝细胞内[17]。丛枝双膜结构的形成,标志真菌与植物互利共生关系的真正建立。丛枝是根内菌丝在特定细胞内的密集分支,其本质是 AM 真菌与共生植物于根系细胞内形成的双膜结构。丛枝内部为真菌膜(丛枝膜),外部则由植物膜包被(丛枝前体质膜),双膜之间为储存交换物质的间隙[18]。形式上,双膜结构将植、 菌双方间隔开来,丛枝膜一侧为 AM 真菌,丛枝前体质膜一侧为共生植物; 功能上,双膜结构又将植、 菌双方紧密联系起来,双膜结构上附着的大量转运蛋白与离子通道是植、 菌双方物质交换的载体[15-18]。虽然菌丝也可作为植、 菌间矿质养分与单糖物质交换的场所,但普遍认为其作用甚微[15]。
1.2双膜结构上的转运蛋白
丛枝双膜上附着的水分、 离子通道、 矿质养分以及单糖转运蛋白是植、 菌间物质交换的基础。真菌源离子通道、 转运蛋白定位于丛枝膜,植物源离子通道、 转运蛋白则定位于丛枝前体质膜,且 AM 真菌的侵染能特异诱导定位于丛枝前体质膜上的植物源矿质养分转运蛋白表达,以转运根外菌丝吸收的矿质养分[19-20]。


2 AM 真菌与共生植物物质交换的形式
2.1矿质养分的交换形式

2.2碳水化合物的交换形式
作为共生植物附加的一个 “库”,AM 真菌碳水化合物需求促使蔗糖从共生植物流向 AM 真菌[44]。蔗糖在运抵丛枝细胞后于丛枝前体质膜上蔗糖转运蛋白的作用下转运至双膜间隙。而后在植物蔗糖裂解酶、 蔗糖转移酶催化下,裂解为葡萄糖和果糖[45-46]。再经丛枝膜上 AM 真菌单糖转运蛋白交换至根内菌丝,最后葡萄糖和果糖在菌丝内转化为海藻糖与糖原,为 AM 真菌所用[45]。但新近研究表明,葡萄糖并非 AM 真菌碳源利用的唯一形式, 丛枝亦不是 AM 真菌获取碳源的唯一场所,即便是根外菌丝也能吸收诸如木糖,甘露糖等单糖[15]。因此,关于 AM 真菌碳源的获取还需要广泛深入的探索。
3 AM真菌与共生植物物质交换过程
3.1转运蛋白的结构
AM 真菌与共生植物间矿质养分、 碳水化合物的交换由跨膜转运蛋白实现。真菌源、 植物源矿质养分转运蛋白共同负责矿质养分跨过真菌膜与丛枝前体质膜由 AM 真菌向共生植物运输,而植物源、 真菌源蔗糖、 单糖转运蛋白则担负碳水化合物跨过丛枝前体质膜与真菌膜由共生植物向 AM 真菌转运[47-48]。真菌源与植物源磷转运蛋白具有一致的框架结构,总共都包括十二个跨膜域,N 端、 C 端各六个。十二个跨膜域在中间围成一个亲水环,具有“6-环-6”的二级结构[49]。细节上,不同亲和力磷转运蛋白在跨膜结构上存在差异。一般而言,高亲和力磷转运蛋白的亲水环在第六和第七个跨膜域之间,低亲和力磷转运蛋白的亲水环则在第八和第九个跨膜域之间[50-51],高亲和力磷转运蛋白氨基酸序列的 N 端和 C 端均朝向细胞膜内,低亲和力磷转运蛋白则相反。
植物源蔗糖、 单糖转运蛋白和真菌源单糖转运蛋白都属于 MFS 家族(major facilitator superfamily),两者均为高疏水性蛋白,序列高度保守。MFS 成员二级结构一致,含有十二个跨膜结构域。中间面向细胞质的部分由一个大的胞质环,将蛋白分为各含六个跨膜结构域的两个半区。虽然跨膜域具有很高的一致性,但在一些重要结构域内,部分保守氨基酸存在差异,且这几个氨基酸的差异与底物特异性相关[52]。植物源单糖转运蛋白的数量随物种的不同呈现差异。迄今,拟南芥有至少五十三个单糖转运蛋白被鉴定分离,蓖麻与灰绿藜中各发现八个与七个单糖转运蛋白[53-54]。AM 真菌Glomussp(DAOM 19789)基因组预测其不具备编码蔗糖裂解酶的能力,但其至少编码三个单糖转运蛋白[15]。真菌源单糖转运蛋白的数量是否因种的不同而变化?另外,蔗糖转运蛋白担负碳水化合物转运过丛枝前体质膜的任务,但植物细胞内的单糖是否作为蔗糖的补充形式导入双膜间隙,进而进入 AM 真菌也有待进一步研究。
3.2物质交换的过程
AM 真菌与共生植物间矿质养分和碳水化合物的交换同时进行,两者相互耦连,互相促进。如图1所示,诸如氮、 磷等矿质养分,从真菌一侧经丛枝膜上的真菌源转运蛋白[图1中(a)、 (d),多数为未知蛋白]导入丛枝前体质膜与真菌膜之间的间隙,再由定位于丛枝前体质膜上的植物源矿质养分转运蛋白[图1中(c)、 (e),多数为已知蛋白]转运至共生植物[18, 54]。与矿质养分相对应,蔗糖经定位于丛枝前体质膜上的蔗糖转运蛋白转运至双膜间隙[图1中(g)],而后于双膜间隙内酶解为单糖,再由丛枝膜上的真菌源单糖转运蛋白导入胞内菌丝。无论矿质养分由双膜间隙导入共生植物还是单糖由双膜间隙导入 AM 真菌,都包括与 H+的同向共转运。而 H+离子浓度梯度的驱动则由 H+-ATPase[图1中(b)]所供给,即 AM 真菌与共生植物间矿质养分和碳水化合物的交换为涉及能量的主动运输过程[22]。矿质养分、 碳水化合物转运蛋白与 H+-ATPases 共表达,H+-ATPases 活性被抑制后新的丛枝双膜结构无法形成,即便是已经发育完好的丛枝也会慢慢消解[55]。丛枝结构内,质膜 H+-ATPase 产生 H+梯度,驱动 H+与 Pi,H+与单糖同向转运的结论已在多种植、 菌共生体上得以证实[56-57]。因 H+的同向运输,矿质养分与碳水化合物的转运相互关联。高土壤磷条件下,真菌单糖转运蛋白基因MST2 与蒺藜苜蓿PT4 (AM 真菌侵染特异诱导的磷转运蛋白)同时下调,且 RNAi 抑制MST2 表达时,菌根结构(尤其是丛枝)发育缓慢,PT4 表达水平下降[15]。与此相印证,高土壤磷条件下,根系中持续表达SUT1 糖转运蛋白基因的土豆株系拥有较高的菌根侵染率[58]。上述事实证明植物碳水化合物与真菌氮、 磷等矿质养分的交换相耦连,AM 真菌吸收的矿质养分与共生植物供给的碳水化合物实行“等价交换”,四个跨膜运输过程缺一则难以为继[59-61]。
4 植、 菌间碳水化合物与矿质养分交换研究展望
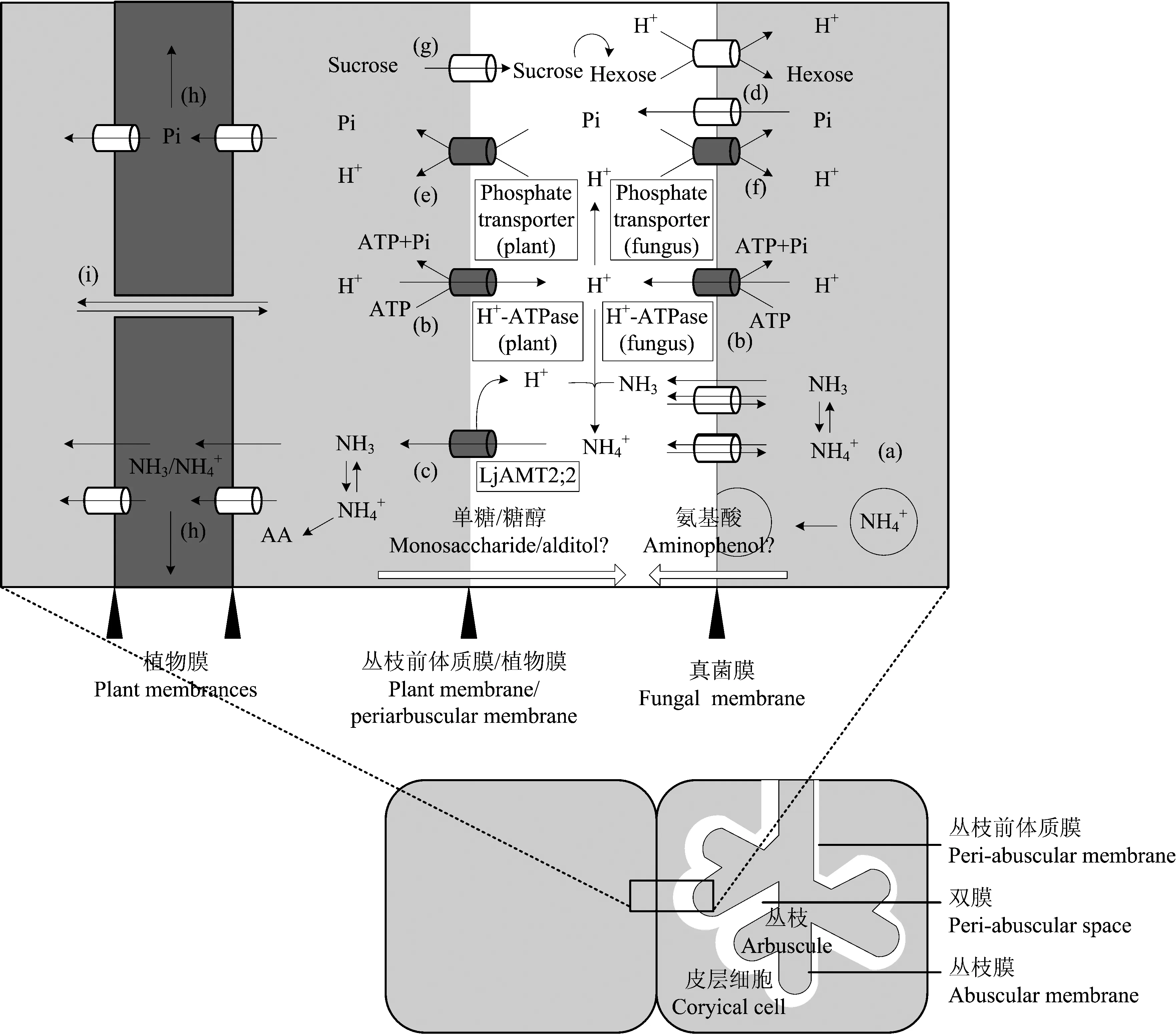
图1 真菌氮(N)、 磷(P)与共生植物碳水化合物在双膜界面上的物质交换示意图Fig.1 The scheme illustrates N, P, and carbohydrate exchanges at the mycorrhizal interface(改编自 Adopted from Harrison[18], Casieri[22], Guether[60])
AM 真菌与共生植物间矿质养分、 碳水化合物的物质交换广泛存在于自然界。研究植、 菌间物质交换的过程,对了解菌根类植物的养分吸收具有重要意义。交换场所方面,AM 真菌与植物共生关系形成后二者物质交换的主要场所为根内丛枝。然而根内菌丝与丛枝具有一致的双膜结构,且真菌源的磷转运蛋白在根内菌丝上亦有表达。笔者推测根内菌丝亦为 AM 真菌与共生植物间物质交换的场所之一,其物质交换的作用可能主要表现于丛枝形成前的早期侵染阶段。除根内菌丝外,在 AM 真菌发育的多个时期,根外菌丝能够吸收诸如木糖,甘露糖等单糖[15]。因此,根外菌丝能否吸收土壤中植物根系分泌的某些碳源(诸如脂肪酸类)作为 AM 真菌与共生植物间物质交换的补充,有待进一步研究。同时,栽培生产上是否能通过外源单糖,加强 AM 真菌碳源强度以此促进菌根效应的提升,可作为 AM 真菌应用研究的一个方向。交换形式方面,AM 真菌与共生植物间物质双向交换,真菌供给共生植物氮、 磷的交换形式较为明晰而其他诸如钾(K)、 锌(Zn)等元素的交换形式有待研究。进一步,因部分矿质养分转运蛋白的转运功能存在多样性(磷转运蛋白只能转运磷酸根离子,氮、 硫转运蛋白能转运氨基酸)。AM 真菌与共生植物间物质交换形式是否随环境条件(如逆境胁迫)而变化仍需要进一步研究(图1); 目前,共生植物反馈 AM 真菌碳水化合物的研究相对较少,葡萄糖是共生植物供给 AM 真菌的主要碳源。但菌根植物种类繁多,其光合产物亦存在多样性(蔗糖、 山梨醇、 甘露醇、 木糖醇),当 AM 真菌与植物共生关系形成后以糖醇类物质作为光合产物的共生植物是否能将糖醇类物质直接供给 AM 真菌(图1)?如若不能,此类物质在碳水化合物及矿质养分交换过程中的代谢途径有待进一步明晰。交换过程方面,AM 真菌、 共生植物间矿质养分、 碳水化合物的物质交换过程相互耦连、 相互促进。而涉及此过程的信号分子、 调控模式、 代谢通路犹未可知,明晰二者交换耦连的机理对 AM 真菌与植物共生的研究同样具有理论与应用意义。
[1]Remy W, Taylor T N, Hass H,etal. Four hundred-million-year-old vesicular arbuscular mycorrhizae[J]. Proceedings of the National Academy of Sciences, 1994, 91: 11841-11843.
[2]Redecker D, Kodner R, Graham L E. Glomalean fungi from the Ordovican[J]. Science, 2000, 289: 1920-1921.
[3]Smith S E, Read D J. Mycorrhizal symbioses (3rd ed.)[M]. London: Academic, 2008.
[4]Jakobsen I, Rosendahl L. Carbon flow into soil and external hyphae from roots of mycorrhizal cucumber plants[J]. New Phytologist, 1990, 115(1): 77-83.
[5]Douds Jr D D, Pfeffer P E, Shachar-Hill Y. Application of in vitro methods to study carbon uptake and transport by AM fungi[J]. Plant and Soil, 2000, 226: 255-261.
[6]Bago B, Pfeffer P E, Abubaker J,etal. Carbon export from arbuscular mycorrhizal roots involves the translocation of carbohydrate as well as lipid[J]. Plant Physiology, 2003, 131(3): 1496-1507.
[7]Bago B. Putative sites for nutrient uptake in arbuscular mycorrhizal fungi[J]. Plant and Soil, 2000, 226: 263-274.
[8]刘进法, 夏仁学, 王明元, 等. 接种丛枝菌根真菌对枳吸收利用磷酸铝的影响[J]. 应用生态学报, 2008, 19(10): 2155-2160.
Liu J F, Xia R X, Wang M Y,etal. Effects of inoculation with arbuscular mycorrhizal fungi on AlPO4uptake byPoncirustrifoliata[J]. Chinese Journal of Applied Ecology, 2008, 19(10): 2155-2160.
[9]Shu B, Wang P, Xia R X. Effects of mycorrhizal fungi on phytate-phosphorus utilization in trifoliate orange (PoncirustrifoliataL. Raf.) seedlings[J]. Acta Physiologiae Plantarum, 2014, 36(4): 1023-1032.
[10]Maldonado-Mendoza I E, Dewbre G R, Harrison M J. Expression of aGlomusintraradicesphosphate transporter gene (GiPT) in the extra-radical mycelium of an arbuscular mycorrhiza: regulation in response to phosphate[J]. Molecular Plant-Microbe Interactions, 2001, 14: 1140-1148.
[11]Benedetto A, Magurno F, Bonfante P, Lanfranco L. Expression profiles of a phosphate transporter gene (GmosPT) from the endomycorrhizal fungusGlomusmosseae[J]. Mycorrhiza, 2005, 15: 620-627.
[12]Neumann E, George E. Extraction of arbuscular mycorrhiza mycelium from compartments filled with wet sieved soil and glass beads[J]. Mycorrhiza, 2005, 15: 533-537.
[13]Neumann E, Schmid B, Romheld V, George E. Extraradical development and contribution to plant performance of an arbuscular mycorrhizal symbiosis exposed to complete or partial rootzone drying[J]. Mycorrhiza, 2009, 20: 13-23.
[14]Koltai H, Kapulnik Y. Arbuscular mycorrhizas: physiology and function[M]. Berlin: Springer, 2010.
[15]Helber N, Wippel K, Sauer N,etal. A versatile monosaccharide transporter that operates in the arbuscular mycorrhizal fungusGlomusspis crucial for the symbiotic relationship with plants[J]. The Plant Cell, 2011, 23: 3812-3823.
[16]Bouwmeester H J, Roux C, Lopez-Raez J A, Bécard G. Rhizosphere communication of plants, parasitic plants and AM fungi[J]. Trends in Plant Science, 2007, 12(5): 224-230.
[17]Smith S E, Read D J. Mycorrhizal symbiosis[M]. London: Academic Press, 1996.
[18]Harrison M J, Dewbre G R, Liu J Y. A phosphate transporter fromMedicagotruncatulainvolved in the acquisiton of phosphate released by arbuscular mycorrhizal fungi[J]. The Plant Cell, 2002, 14: 2413-2429.
[19]Rékangalt D, Pépin R, Verner M C,etal. Expression of the nitrate transporternrt2 gene from the symbiotic basidiomycete Hebeloma cylindrosporum is affected by host plant and carbon sources[J]. Mycorrhiza, 2009, 19(3): 143-148.
[20]Thomson B D, Clarkson D T, Brain P. Kinetics of phosphorus uptake by the germ-tubes of the vesicular-arbuscular mycorrhizal fungus,Gigasporamargarita[J]. New Phytologist, 1990, 116(4): 647-653.
[21]Harrison M J, van Buuren M L. A phosphate transporter from the mycorrhizal fungusGlomusversiforme[J]. Nature, 1995, 378: 626-629.
[22]Casieri L, Lahmidi N A, Doidy J,etal. Biotrophic transportome in mutualistic plant-fungal interactions[J]. Mycorrhiza, 2013, 23(8): 597-625.
[23]Xu G H, Chague V, Melamed-Bessudo C,etal. Functional characterization ofLePT4: a phosphate transporter in tomato with mycorrhiza-enhanced expression[J]. Journal of Experimental Botany, 2007, 58(10): 2491-2501.
[24]Nagy R, Karandashov V, Chague V,etal. The characterization of novel mycorrhiza-specific phosphate transporters fromLycopersiconesculentumandSolanumtuberosumuncovers functional redundancy in symbiotic phosphate transport in solanaceous species[J]. The Plant Journal, 2005, 42(2): 236-250.
[25]Chen A, Chen X, Wang H,etal. Genome-wide investigation and expression analysis suggest diverse roles and genetic redundancy of Pht1 family genes in response to Pi deficiency in tomato[J]. BMC Plant Biology, 2014, 14(1): 61.
[26]Liu J, Versaw W K, Pumplin N,etal. Closely related members of theMedicagotruncatulaPHT1 phosphate transporter gene family encode phosphate transporters with distinct biochemical activities[J]. Journal of Biological Chemistry, 2008, 283(36): 24673-24681.
[27]Grunwald U, Guo W, Fischer K,etal. Overlapping expression patterns and differential transcript levels of phosphate transporter genes in arbuscular mycorrhizal, Pi-fertilised and phytohormone-treatedMedicagotruncatularoots[J]. Planta, 2009, 229(5): 1023-1034.
[28]Watts-Williams S J, Jakobsen I, Cavagnaro T R,etal. Local and distal effects of arbuscular mycorrhizal colonization on direct pathway Pi uptake and root growth inMedicagotruncatula[J]. Journal of Experimental Botany, 2015, 66(13): 4061-4073.
[29]Paszkowski U, Kroken S, Roux C, Briggs S P. Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscular mycorrhizal symbiosis[J]. Proceedings of the National Academy of Sciences, 2002, 99(20): 13324-13329.
[30]Glassop D, Godwin R M, Smith S E, Smith F W. Rice phosphate transporters associated with phosphate uptake in rice roots colonised with arbuscular mycorrhizal fungi[J]. Botany, 2007, 85(7): 644-651.
[31]Shu B, Xia R X, Wang P. Differential regulation of Pht1 phosphate transporters from trifoliate orange (PoncirustrifoliataL. Raf.) seedlings[J]. Scientia Horticulturae, 2012, 146: 115-123.
[32]Misson J, Thibaud M C, Bechtold N,etal. Transcriptional regulation and functional properties ofArabidopsisPht1;4, a high affinity transporter contributing greatly to phosphate uptake in phosphate deprived plants[J]. Plant Molecular Biology, 2004, 55(5): 727-741.
[33]Shin H, Shin H S, Dewbre G R, Harrison M J. Phosphate transport inArabidopsis:Pht1; 1 andPht1; 4 play a major role in phosphate acquisition from both low- and high- phosphate environments[J]. The Plant Journal, 2004, 39(4): 629-642.
[34]Ai P, Sun S, Zhao J,etal. Two rice phosphate transporters,OsPht1; 2 andOsPht1; 6, have different functions and kinetic properties in uptake and translocation[J]. The Plant Journal, 2009, 57(5): 798-809.
[35]Doidy J, van Tuinen D, Lamotte O,etal. TheMedicagotruncatulasucrose transporter family: characterization and implication of key members in carbon partitioning towards arbuscular mycorrhizal fungi[J]. Molecular Plant, 2012, 5: 1346-1358.
[36]Shachar-Hill Y, Pfeffer P E, Douds D,etal. Partitioning of intermediary carbon metabolism in vesicular-arbuscular mycorrhizal leek[J]. Plant Physiology, 1995, 108: 7-15.
[37]Solaiman M D Z, Saito M. Use of sugars by intraradical hyphae of arbuscular mycorrhizal fungi revealed by radiorespirometry[J]. New Phytologist, 1997, 136: 533-538.
[38]Govindarajulu M, Pfeffer P E, Jin H R,etal. Nitrogen transfer in the arbuscular mycorrhizal symbiosis[J]. Nature, 2005, 435: 819-823.
[39]Jin H, Pfeffer P E, Douds D Detal. The uptake, metabolism, transport and transfer of nitrogen in an arbuscular mycorrhizal symbiosis[J]. New Phytologist, 2005, 168: 687-696.
[40]Cruz C, Egsgaard H, Trujillo C,etal. Enzymatic evidence for the key role in arginine in nitrogen translocation by arbuscular mycorrhizal fungi[J]. Plant Physiology, 2007, 144: 782-792.
[41]Allen J W, Shachar-Hiil Y. Sulfur transfer through an arbuscular mycorrhiza[J]. Plant Physiology, 2009, 149: 549-560.
[42]Smith S E, Jakobsen I, Gronlund M, Smith F A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition[J]. Plant Physiology, 2011, 156: 1050-1057.
[43]Chalot M, Blaudez D, Brun A. Ammonia: a candidate for nitrogen transfer at the mycorrhizal interface[J]. Trends in Plant Science, 2006, 11: 263-266.
[44]Heinemeyer A, Ineson P, Ostle N, Fitter A H. Respiration of the external mycelium in the arbuscular mycorrhizal symbiosis shows strong dependence on recent photosynthates and acclimation to temperature[J]. New Phytologist, 2006, 171: 159-170.
[45]Blee K A, Anderson A J. Transcripts for genes encoding soluble acid invertase and sucrose synthase accumulate in root tip and cortical cells containing mycorrhizal arbuscules[J]. Plant Molecular Biology, 2002, 50(2): 197-211.
[46]Garcia-Rodriguez S, Azcon-Aguilar C, Ferrol N. Transcriptional regulation of host enzymes involved in the cleavage of sucrose during arbuscular mycorrhizal symbiosis[J]. Physiologia Plantarum, 2007, 129: 737-746.
[47]Javot H, Penmetsa R V, Terzaghi N,etal. AMedicagotruncatulaphosphate transporter indispensable for the arbuscular mycorrhizal symbiosis[J]. Proceedings of the National Academy of Sciences, 2007, 104(5): 1720-1725.
[48]Schüβler A, Martin H, Cohen D,etal. Characterization of a carbohydrate transporter from symbiotic glomeromycotan fungi[J]. Nature, 2006, 444(7121): 933-936.
[49]Raghothama K G. Phosphate acquisition[J]. Annual Review of Plant Biology, 1999, 50(1): 665-693.
[50]Reizer J, Reizer A, Saier Jr M H. A functional superfamily of sodium/solute symporters[J]. Biochimica et Biophysica Acta, 1994, 1197(2): 133-166.
[51]Daram P, Brunner S, Persson B L,etal. Functional analysis and cell-specific expression of a phosphate transporter from tomato[J]. Planta, 1998, 206(2): 225-233.
[52]Büttner M, Sauer N. Monosaccharide transporters in plants: structure, function and physiology[J]. Biochimica et Biophysica Acta, 2000, 1465(1): 263-274.
[53]Büttner M. The monosaccharide transporter (-like) gene family inArabidopsis[J]. Febs Letters, 2007, 581(12): 2318-2324.
[54]Klepek Y S, Geiger D, Stadler R,etal.ArabidopsisPOLYOL TRANSPORTER5, a new member of the monosaccharide transporter-like superfamily, mediates H+-symport of numerous substrates, including myo-inositol, glycerol, and ribose[J]. The Plant Cell, 2005, 17(1): 204-218.
[55]Wang E, Yu N, Bano S A,etal. A H+-ATPase that energizes nutrient uptake during mycorrhizal symbioses in rice andMedicagotruncatula[J]. The Plant Cell, 2014, 26(4): 1818-1830.
[56]Mimura T. Physiological control of phosphate uptake and phosphate homeostasis in plant cells[J]. Functional Plant Biology, 2001, 28(7): 655-660.
[57]Williams L E, Lemoine R, Sauer N. Sugar transporters in higher plants-a diversity of roles and complex regulation[J]. Trends in Plant Science, 2000, 5(7): 283-290.
[58]Gabriel-Neumann E, Neumann G, Leggewie G, George E. Constitutive overexpression of the sucrose transporterSoSUT1 in potato plants increases arbuscular mycorrhiza fungal root colonization under high, but not under low, soil phosphorus availability[J]. Journal of Plant Physiology, 2011, 168(9): 911-919.
[59]Fellbaum C R, Gachomo E W, Beesetty Y,etal. Carbon availability triggers fungal nitrogen uptake and transport in arbuscular mycorrhizal symbiosis[J]. Proceedings of the National Academy of Sciences, 2012, 109(7): 2666-2671.
[60]Guether M, Neuhäuser B, Balestrini R,etal. A mycorrhizal-specific ammonium transporter fromLotusjaponicusacquires nitrogen released by arbuscular mycorrhizal fungi[J]. Plant Physiology, 2009, 150(1): 73-83.
[61]Kiers E T, Duhamel M, Beesetty Y,etal. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis[J]. Science, 2011, 333(6044): 880-882.
Progress on material exchange between arbuscular mycorrhizal(AM) fungi and host plant: A review
SHU Bo, LI Wei-cai, LIU Li-qin, WEI Yong-zan, SHI Sheng-you*
(SouthSubtropicalCropsResearchInstitute,CATAS,Zhanjiang/KeyLaboratoryofTropicalFruitBiology,MinistryofAgriculture,Zhanjiang,Guangdong524091,China)
Arbuscular mycorrhizal (AM) fungi can form symbiosis with 80% of terrestrial plant species. The exchange between mineral nutrients of AM fungi and carbohydrate of host plant is important for material cycle in whole ecosystem. Nowadays, there are many studies on the AM fungi promoting host plant mineral nutrient absorption. The AM fungi can enhance availability of mineral nutrients by small diameter of extraradical hyphae, activate soil nutrients by releasing organic acids and soil enzymes, ensure the efficiency of soil nutrition transport into extraradical hyphae by the lower value ofKmnutrient transporter on extraradical hyphae, ensure the rate of nutrition transport in intraradical hyphae by converting nutrients ions to suitable forms, and promote the efficiency of the nutrition transport into host plant by inducing symbiotic plant nutrients transporters. However, the progress of plant feedback carbohydrate to fungi is few. As the important role of AM symbiosis in whole ecosystem, researches about the locations (arbuscule, intraradical hyphae and extraradical hyphae), the forms (ionic forms, polymer, amino acid, sucrose and monosaccharide) and the process (active transport) of the mineral nutrients and carbohydrate exchange are significant. This review summarizes the characteristics of membrane system of arbuscule and intraradical hyphae, the forms of nitrogen (N), phosphate (P) and carbohydrate within the exchange, and the process of exchange which relates to transmembrane, energy expenditure and N or P coupling with carbohydrate. Finally, the prospect of AM fungi and host plant materials exchanges is proposed.
mineral nutrients; carbohydrate; membrane system; transporter
2014-11-25接受日期: 2015-05-25网络出版日期: 2015-07-17
国家自然科学基金(31401818); 中央级公益性科研院所基本科研业务费专项(1630062014006)资助。
舒波(1985—), 男, 四川广安人, 博士, 助理研究员, 主要从事果树菌根方面的研究。E-mail: bshbest@163.com
E-mail: ssy7299@163.com
Q945.12; S154.34
A
1008-505X(2016)04-1111-07

