Boron-doped α-Ni(OH)2 for electro-catalytic oxidation of ureain in alkaline medium
YANG Duo, YU Qingtao, MAO Liqun, YANG Jinghe, GAO Li
(Engineering Center for Clean Chemical Process and Technology, College of Chemistry and Chemical Engineering,Henan University, Kaifeng 475004, Henan, China)
Boron-dopedα-Ni(OH)2for electro-catalytic oxidation of ureain in alkaline medium
YANG Duo, YU Qingtao, MAO Liqun, YANG Jinghe, GAO Li*
(EngineeringCenterforCleanChemicalProcessandTechnology,CollegeofChemistryandChemicalEngineering,HenanUniversity,Kaifeng475004,Henan,China)
Boron-dopedα-Ni(OH)2nanoflowers(B-α-Ni(OH)2) were synthesized by a liquid-phase method using P123 (EO20PO70EO20) as a template and NaBH4as alkali and boron sources. The average size of these B-α-Ni(OH)2nanoflowers is in a range of 200-500 nm with many porous. The B-α-Ni(OH)2nanoflowers were developed as electrocatalysts for urea electro-oxidation in alkaline media with more than 10 times enhancement in current density compared to bulk nickel hydroxide powders. Results demonstrated that the obtained B-α-Ni(OH)2electrode exhibited high activity and good stability. The positive correlation between the scan rates and the anodic currents implied a single diffusion-controlled kinetic process. The enhanced electro-catalytic oxidation of urea using B-α-Ni(OH)2nanoflowers has promising applications in urea-rich wastewater remediation, hydrogen production, and fuel cells.
boron-dopedα-Ni(OH)2; urea; electro-catalytic oxidation electro-catalysts; hydrogen production; fuel cells
Article ID: 1008-1011(2016)05-0548-06
Urea (CO(NH2)2) is widely used in the agricultural industry as an animal feed additive and nitrogen-release fertilizer, which produced a large amount of waste water with differing urea concentrations[1]. Urea management has been becoming a major environmental and health issue. Moreover, urea has been identified as a good hydrogen carrier for long-term sustainable energy supply[2]. Therefore, it is necessary for urea removal/decomposition during wastewater treatment. Electrocatalytic oxidation has been used as a method for treatment of urea water due to no need of extra instruments equipment[1,3]. However, this method requires expensive noble metal catalysts for urea decomposition[3-4].
Catalyst development plays an important role in the cost reduction of urea oxidation. Recently, nickel and nickel-based modified electrodes were developed as catalysts for urea oxidation electrocatalytic activity[1,5-7]. BOGGS et al. successfully demonstrated urea oxidation to produce hydrogen using Ni electrodes in alkaline medium[1]. Furthermore, several Ni based catalysts have been developed to efficiently electro-oxidize urea in alkaline medium[8-10]. BOGGS et al. proposed that the electro-oxidation of urea on a Ni catalyst follows a direct oxidation mechanism, and the redox transition between Ni(OH)2and NiOOH contributes to the electro-catalytic oxidation of urea molecules, according to the following reactions (Eqs.(1)-(4))[1].
(1)
(2)
(3)
(4)
Reactions (1) and (2) take place at the anodic compartment of the electrolyzer, while reaction (3) takes place at the cathodic compartment of the cell apparatus. The overall reaction is given by reaction (4). DARAMOLA et al. presented a mechanism for urea oxidation in the presence of nickel oxyhydroxide (NiOOH) including elementary steps using density functional theory (DFT) methods[11]. In alkaline medium, it was suggested that Ni2+oxidation to its active Ni3+form mediates the urea oxidation reaction, as Eqs. (1)-(2)[12]. During the urea electro-oxidation in alkaline media process, the catalytic active NiOOH is chemically reduced to the inactive Ni(OH)2by urea, at the same time, urea is chemically oxidized to its products[1]. During the urea oxidation in alkaline medium process, the rate-limiting step was found to be desorption of CO2. They reported that the NiOOH catalyst surface could be deactivated by the surface blockage due to CO groups[11]. Nickel and nickel hydroxides catalysts can promote electrochemical redox reactions and alleviate diffusion resistance. Properties of Ni(OH)2can be improved by providing special nanostructure. The doping other atoms modification is also an important way to improve its properties.
In this work, we synthesized B-α-Ni(OH)2nanoflowers by a liquid-phase method as electro-catalysts for urea electro-catalytic oxidation. Results demonstrated that the obtained B-α-Ni(OH)2electrode exhibited high activity and good stability.
1 Experimental section
1.1Reagents
Nafion (5% ethanol solution, mass fraction) was purchased from Alfa Aesar, and diluted to 0.1% with doubly distilled water in use. Pluronic P123 (surfactant copolymer poly (ethylene oxide)-poly (propylene oxide)-poly (ethylene oxide, EO20-PO70-EO20) was purchased from Sigma-Aldrich. Ni(NO3)2·6H2O was a product of Tianjin Kermel Chemical Co. in analytical grade. Commercial bulk nickel hydroxide powders and sodium borohydride were obtained from Sinopharm Chemical Reagent Co., Ltd. Urea (99.7%) was obtained from Tianjin De'en Chemical Reagent Co., Ltd. Other chemicals were all analytical reagents from Beijing Chemical Company.
1.2Synthesis of materials
The synthesis of B-α-Ni(OH)2was based on a self-assembly between a triblock copolymer template P123 and two precursors(sodium borohydride and nickel species) in a flask. Put Ni(NO3)2·6H2O (8.6 g) into 40 mL water, and then stirring at ambient temperature after ultrasonic dispersion. Then, the nickel solution was put into the 400 mL solution of P123 (4 g), and then slow stirring at 313 K for more than 3 h. When the temperature drops to ambient temperature, the 20 mL sodium borohydride (1.5 g) solution was added into the mixture. After 2 h, the same quantity of sodium borohydride solution was added into the mixture. After 210 min, light green sediments were formed. The mixture was filtered and washed many times with water and ethanol to remove organics, ions, and surfactant P123 and dried under vacuum at 333 K, then we got B-α-Ni(OH)2[13-14].
1.3Preparation of nickel electrodes and electrochemical measurements
A three electrode system was used. Ag/AgCl electrode filled with saturated potassium chloride was used as reference electrode and platinum wire was used as counter electrode with 1 mol/L aqueous solution of KOH as electrolyte. The working electrode was modified glassy carbon electrode (GCE) (3 mm in diameter). Electrochemical experiments were tested on a CHI760E electrochemical workstation. Cyclic voltammetry (CV), linear sweep voltammetry (LSV) were conducted at a rate of 50 mV·s-1in alkaline aqueous solution. Chronoamperograms curve was recorded in alkaline aqueous solution at 0.5 V for 1 000 s. The amperometrici-tcurve was measured in alkaline aqueous solution at 0.5 V for 600 s.
A preparation method of the modified electrode process is as follows: The bare GCE was polished with 1.0, 0.3, and 0.05 μm alumina slurry, rinsed thoroughly with deionized water, and dried by N2blowing. 5 mg catalyst was added into 1 mL 0.1% nafion solution to form homogeneous mixtures under ultrasonication. Next, the various suspensions of 10 μL were drop in the surface of GCE or modified GCE. Finally, the as-prepared catalyst film was dried at room temperature or under the infrared lamp.
1.4Characterization
The samples crystalline structure were examined by X-ray diffraction (XRD) by X-ray D8 Advance (Bruker, Germany) instrument with Cu Kαradiation (λ= 0.15418 nm). Samples microstructure was determined using a scanning electron microscope (SEM, XL30S-FEG, 5 kV). The morphology and dispersion of the samples were observed using Transmission electron microscopy (TEM, FEI Tecnai G2T20). Fourier transform infrared (FT-IR) spectra were measured by transmission on a Bruker Vertex 80 FT-IR Spectrometer on KBr pellets with 2 cm-1resolution.
2 Results and discussion
The structures and composition of the obtained B-Ni(OH)2nano-catalyst were studied by XRD and FT-IR spectrum. Fig. 1A shows the XRD pattern of Ni(OH)2material. The diffractive peaks at 2θ=11.4°, 22.7°, 33.5° and 60.0° could be assigned to the (003), (006), (101) and (110) planes, respectively, which fit well with rhombohedral type ofα-Ni(OH)2layered structure[15-17]. No other obvious diffraction peaks were monitored, demonstrating the high quality of the sample.
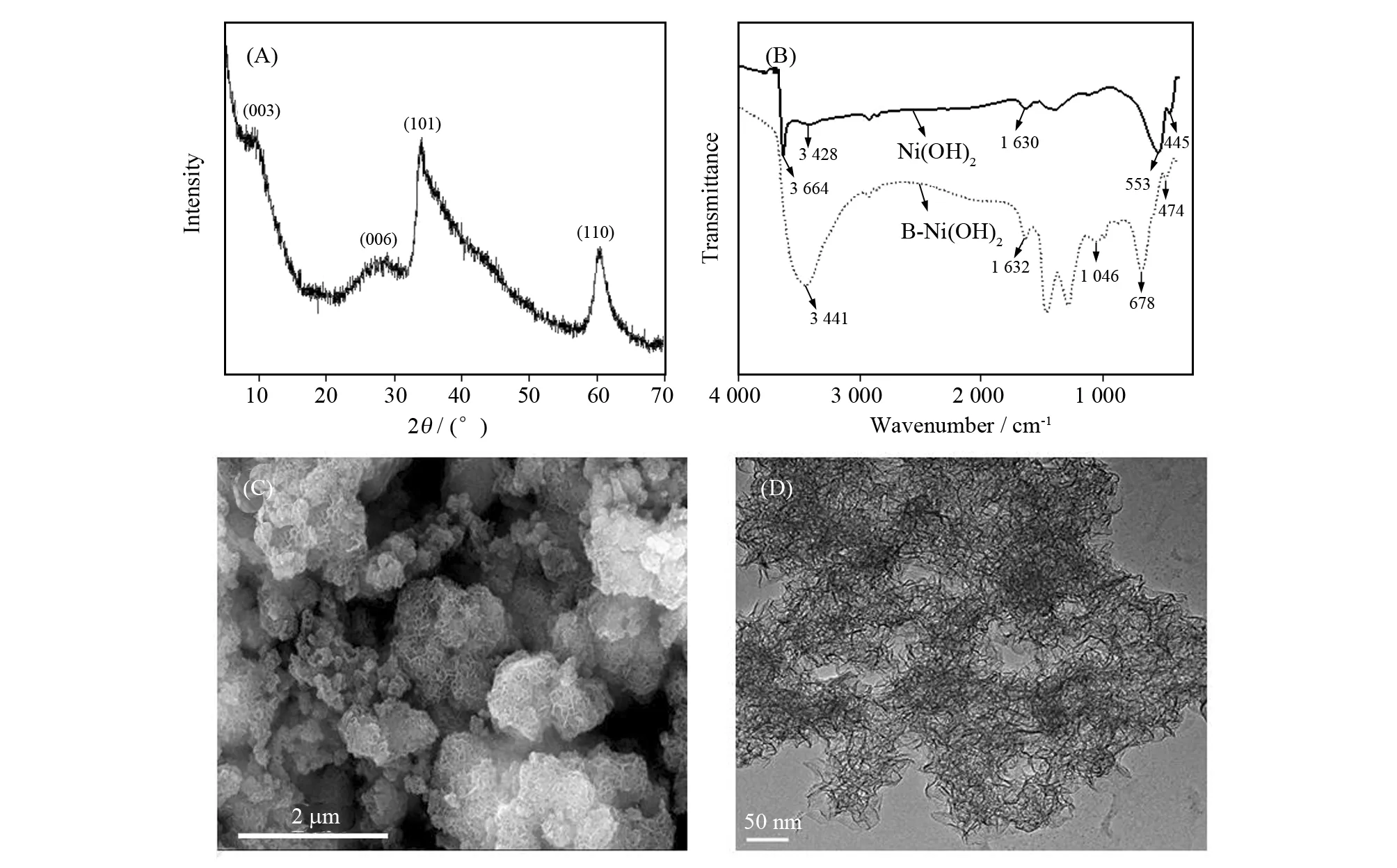
Fig.1 XRD pattern (A), FT-IR spectra (B), SEM (C) and TEM (D) characterization of B-α-Ni(OH)2
FT-IR spectra of B-α-Ni(OH)2and Ni(OH)2is shown in Fig.1B. As shown in the B-Ni(OH)2FT-IR spectrum, the strong and broad band around 3 441 cm-1is assigned to the stretching vibration of the adsorbed water while the band around 1 632 cm-1is assigned to the bending vibration of the adsorbed water molecules[5]. Peak of B-α-Ni(OH)2at 474 cm-1is due to the Ni-O stretching vibration. Peak of B-α-Ni(OH)2at 678 cm-1assigned to the stretching vibration of non-hydrogen-bonded OH groups[18]. Peak of B-α-Ni(OH)2at 1 387 cm-1assigned to C-O-C groups, while the peak at 1 462 cm-1assigned to the stretching vibration of CH2- in the molecule of P123. FT-IR spectrum of Ni(OH)2is similar to B-α-Ni(OH)2.
As shown in Fig. 1C and 1D, the morphology and structure of the B-α-Ni(OH)2samples were investigated by SEM and TEM. The B-α-Ni(OH)2samples are flower-like nanospheres comprising of densely packed irregular sheets (Fig. 1C).The average size of these B-α-Ni(OH)2nanoflowers is in the range of 200-500 nm. As shown in Fig. 1D, the size of the nanosheets is about 50 nm and the thickness of the nanosheets was approximately 5 nm[13].
The electro-oxidation properties of B-α-Ni(OH)2and bulk-Ni(OH)2catalysts toward urea oxidation were investigated. Fig. 2A shows cyclic voltammetry urea electro-oxidation of 0.33 mol/L urea in 1 mol/L KOH solution catalyzed by B-α-Ni(OH)2and bulk-Ni(OH)2at a scan rate of 50 mV·s-1. The curve of B-α-Ni(OH)2shows exhibited oxidation current density starting at 0.41 V and a peak current density at 0.55 V vs. Ag/AgCl in the KOH solution containing 0.33 mol/L urea. In 1 mol/L KOH solution, the CV curve illustrates an anodic and cathodic peak related to the Ni(OH)2/NiOOH redox couple. As a comparison, the urea oxidation peak current density of bulk Ni(OH)2is very weak. The B-α-Ni(OH)2nanoflowers were developed as electrocatalysts for urea electro-oxidation in alkaline media more than 10 times enhancement in current density compared to bulk nickel hydroxide powders. The excellent catalytic property was also been verified by LSV (Fig. 2B).
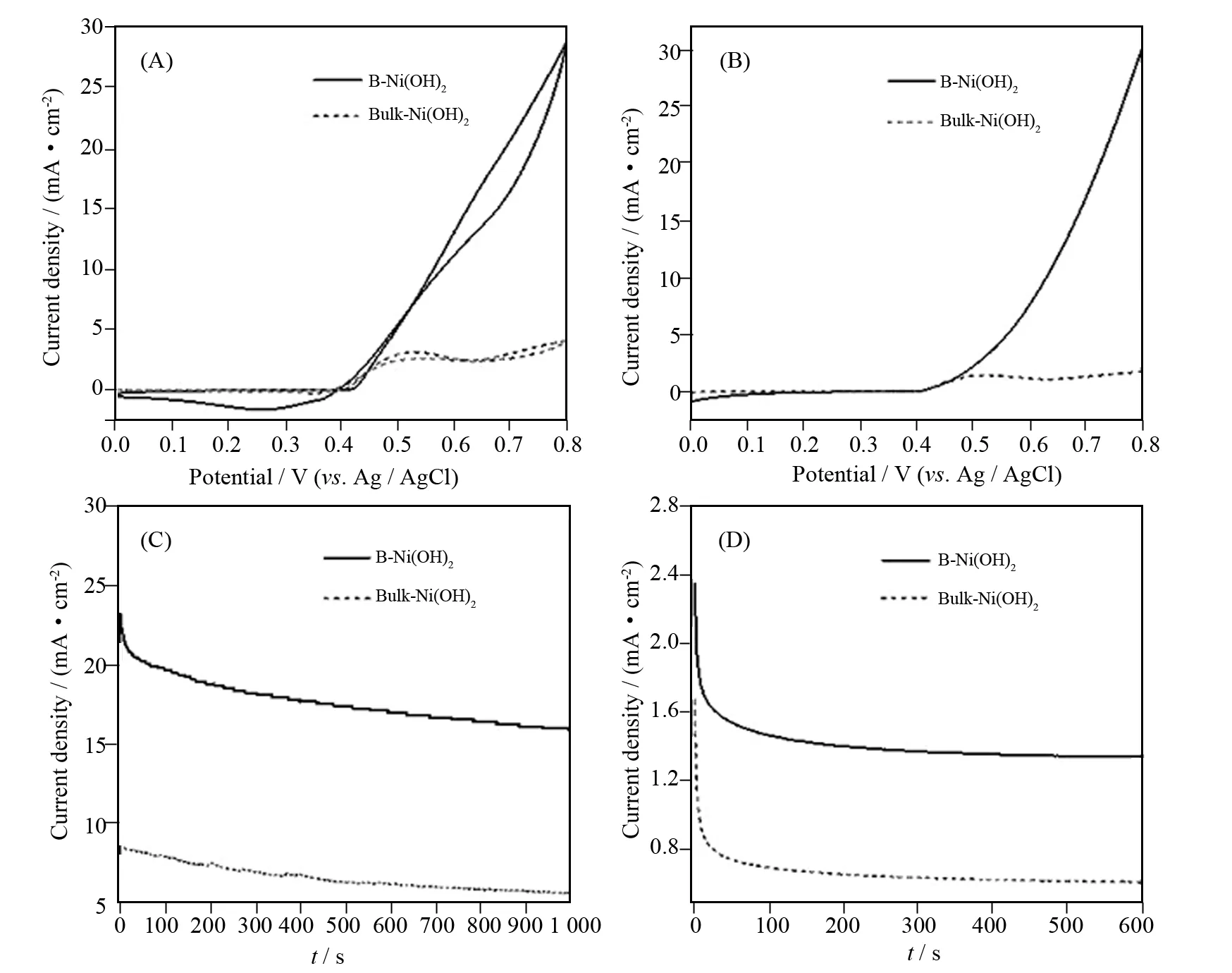
Fig.2CV (A) and LSV (B) for urea electro-oxidation of 0.33 mol/L urea in 1 mol/L KOH solution catalyzed by B-α-Ni(OH)2, Bulk-Ni(OH)2at 50 mV·s-1scan rate. Chronoamperograms curve (C) and amperometrici-tcurve (D) for urea electro-oxidation >of 0.33 mol/L urea in 1 mol/L KOH solution catalyzed by B-α-Ni(OH)2, Bulk-Ni(OH)2at 0.50 V vs. Ag/AgCl
To evaluate the stability of nanocatalysts, as shown in Fig.2C chronoamperograms curve experiments were used to further evaluate the electrocatalytic activities of B-α-Ni(OH)2nanoflowers and bulk-Ni(OH)2catalysts/electrodes for urea oxidation. CA curves (performed at 0.5 V vs. Ag/AgCl for 1000 s) exhibits that the B-α-Ni(OH)2nanoflowers have a higher reaction current density and a slower current degradation over time compared with the bulk nickel hydroxide powders for the entire time course. Actually, the B-α-Ni(OH)2nanoflowers exhibited better electrocatalytic stability performance[6,9].
As shown in Fig. 2D, amperometrici-tcurve experiments were used to further evaluate the electrocatalytic activities of B-α-Ni(OH)2and bulk-Ni(OH)2catalysts for urea oxidation. The experiments were performed at a constant voltage of 0.50 V vs. Ag/AgCl for 600 s. As shown in Fig. 2D, the decay was slow and the current density finally reached a stable current density. B-α-Ni(OH)2showed the higher activity and higher stability for urea oxidation. The urea oxidation current catalyzed by B-α-Ni(OH)2was more than 3 times higher than that of bulk nickel hydroxide powders. The result of amperometrici-tcurve was consistent with that of CV, LSV and CA.
As shown in Fig. 3A, the effect of scan rate was investigated. It can be seen that oxidation peak current density (Ip) for methanol oxidation become larger with the increase of the scan rate. TheIpwas linear with the square root of scan rate(v1/2) in the range of 20-140 mV·s-1, suggesting that the scan rates and the anodic currents density implied the electrochemical reaction controlled by a single diffusion-controlled kinetic process (Fig. 3A inset).
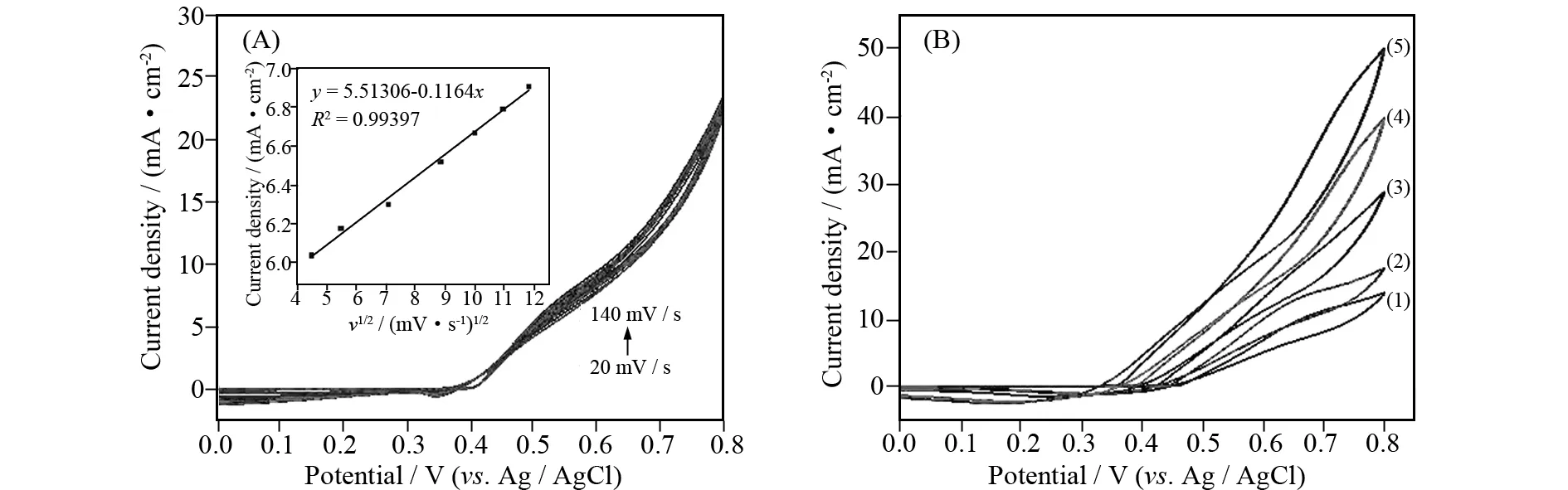
Fig.3 (A) CVs for urea electro-oxidation on B-α-Ni(OH)2 in 1 mol/L KOH solution containing 0.33 mol/L urea at different scan rates (20 mV·s-1< scan rate < 140 mV·s-1); Inset: the line relation between peak current density and the square root of the scan rate. (B) Effect of different concentrations of KOH on the urea electro-oxidation onB-α-Ni(OH)2 electrode at a scan rate of 50 mV·s-1
As shown in Fig. 3B, effect of different concentrations of KOH was also investigated. The experiments implied an improvement in the oxidation current density with increasing concentrations of KOH. Since concentrations of OH-are dependence on the formation of NiOOH that catalyzes the urea oxidation process reaction, it is prospective to see an increase in the anodic current density with increasing KOH concentration. At the same time, the onset potential for the urea oxidation shifts to more negative values with increasing concentrations of KOH. This explains that the urea oxidation reaction becomes thermodynamically more favorable at higher concentrations of KOH[10].The redox transition between Ni(OH)2and NiOOH contributes to the electro-catalytic oxidation of urea molecules, which is consisted with previous reports[1,10,19-20].
3 Conclusions
The electrocatalyic oxidation urea on B-α-Ni(OH)2electrode and nickel hydroxide electrode in alkaline medium was investigated. The B-α-Ni(OH)2nanoflowers were synthesized through a liquid-phase method and used as electro-catalysts for catalytic oxidation of urea. XRD, FT-IR, SEM, TEM, spectroscopy, cyclic voltammetry, linear sweep voltammetry, chronoamperograms, amperometrici-ttechniques were used to characterize the B-α-Ni(OH)2nanoflowers. The electrochemical analysis showed that the urea oxidation current of the B-α-Ni(OH)2nanoflowers is more than 10 times higher than that of the bulk nickel hydroxide powders. The catalytic process on the B-α-Ni(OH)2nanoflowers modified electrode is a di-ffusion controlled process. The experiments implies an improvement in the oxidation current density with increasing concentrations of KOH. Urea oxidation occurs after the formation of NiOOH on the electrode surface. The enhanced electro-catalytic oxidation of urea using B-α-Ni(OH)2synthesized by this method reveals great potential for future applications in urea-rich wastewater remediation, hydrogen production, and fuel cells.
[1] BOGGS B K, KING R L, BOTTE G G. Urea electrolysis: direct hydrogen production from urine [J]. Chem Commun, 2009, 32(32): 4859-4861.
[2] ROLLINSON A N, JONES J, DUPONT V, et al. Urea as a hydrogen carrier: a perspective on its potential for safe, sustain and long-term energy supply [J]. Energ Environ Sci, 2011, 4(4): 1216-1224.
[3] SIMKA W, PIOTROWSKI J, ROBAK A, et al. Electrochemical treatment of aqueous solutions containing urea [J]. J Appl Electrochem, 2009, 39(7): 1137-1143.
[4] MILLER A T, HASSLER B L, BOTTE G G. Rhodium electrodeposition on nickel electrodes used for urea electrolysis [J]. J Appl Electrochem, 2012, 42(11): 925-934.
[5] WANG D, YAN W, VIJAPUR S H, et al. Enhanced electrocatalytic oxidation of urea based on nickel hydroxide nanoribbons [J]. J Power Sources, 2012, 217: 498-502.
[6] WANG D, YAN W, BOTTE G G. Exfoliated nickel hydroxide nanosheets for urea electrolysis [J]. Electrochem Commun, 2011, 13(10): 1135-1138.
[7] GUO F, YE K, CHENG K, et al. Preparation of nickel nanowire arrays electrode for urea electro-oxidation in alkaline medium [J]. J Power Sources, 2015, 278: 562-568.
[8] YAN W, WANG D, BOTTE G G. Nickel and cobalt bimetallic hydroxide catalysts for urea electro-oxidation [J]. Electrochim Acta, 2012, 61(2): 25-30.
[9] JI R Y, CHAN D S, JOW J J, et al. Formation of open-ended nickel hydroxide nanotubes on three-dimensional nickel framework for enhanced urea electrolysis [J]. Electrochem Commun, 2013, 29(10): 21-24.
[10] VEDHARTHINAM V, BOTTE G G. Understanding the electro-catalytic oxidation mechanism of urea on nickel electrodes in alkaline medium [J]. Electrochim Acta, 2012, 81(11): 292-300.
[11] DARAMOLA D A, SINGH D, BOTTE G G. Dissociation rates of urea in the presence of NiOOH catalyst: a DFT analysis [J]. J Phys Chem A, 2010, 114(43): 11513-11521.
[12] VEDHARTHINAM V, BOTTE G G. Direct evidence of the mechanism for the electro-oxidation of urea on Ni(OH)2catalyst in alkaline medium [J]. Electrochim Acta, 2013, 108: 660-665.
[13] YANG J H, WANG C, YANG D, et al. Boron-dopedα-Ni(OH)2nanoflowers with high specific surface area as electrochemical capacitor materials [J]. Mater Lett, 2014, 128: 380-383.
[14] YANG J H, YU Q, LI Y, et al. Batch fabrication of mesoporous boron-doped nickel oxide nanoflowers for electrochemical capacitors [J]. Mater Res Bull, 2014, 59(16): 382-386.
[15] SOLER-LLLIA G J D A A, JOBBGY M, REGAZZONI A E, et al. Synthesis of nickel hydroxide by homogeneous alkalinization. precipitation mechanism [J]. Chem Mater, 1999, 11(11): 3140-3146.
[16] JEEVANANDAM P, KOLTYPIN Y, GEDANKEN A. Synthesis of nanosizedα-nickel hydroxide by a sonochemical method [J]. Nano Lett, 2001, 1(5): 263-266.
[17] XIAO J, CHEN B, LIANG X, et al. NiO microspheres with tunable porosity and morphology effects for CO oxidation [J]. Catal Sci Technol, 2011, 1(6): 999-1005.
[18] OLIVA P, LEONARDI J, LAURENT J F, et al. Review of the structure and the electrochemistry of nickel hydroxides and oxy-hydroxides [J]. J Power Sources, 1982, 8(2): 229-255.
[19] MAJDI S, JABBARI A, HELI H. A study of the electrocatalytic oxidation of aspirin on a nickel hydroxide-modified nickel electrode [J]. J Solid State Electr, 2007, 11(5): 601-607.
[20] HELI H, JAFARIAN M, MAHJANI M G, et al. Electro-oxidation of methanol on copper in alkaline solution [J]. Electrochim Acta, 2004, 49(27): 4999-5006.
[责任编辑:吴文鹏]
碱性介质中硼掺杂的α-Ni(OH)2电催化氧化尿素
杨朵,郁清涛,毛立群,杨敬贺,高丽*
(河南大学 化学化工学院,化工与清洁技术工程中心,河南 开封 475004)
以P123(EO20PO70EO20)为模板剂,NaBH4为碱及硼源,采用液相法合成了硼掺杂的α-Ni(OH)2纳米花. 该纳米花平均尺寸在200~500 nm之间,呈多孔状. 研究结果表明,该B-α-Ni(OH)2电极具有良好的电催化作用,活性高,稳定性好,在电极上对尿酸的氧化动力学过程为扩散控制过程. 与Ni(OH)2相比,其在碱性介质中电催化氧化尿素的电流密度提高了10倍以上. B-α-Ni(OH)2纳米花在富含尿素的废水处理、制氢和燃料电池上具有潜在的应用前景.
硼掺杂的α-Ni(OH)2; 尿素; 电催化氧化; 制氢; 燃料电池
date: 2016-05-13.
Supported by the National Natural Science Foundation of China (21403053, U1404503).
, E-mail: gaoli@henu.edu.cn.
O627.12 Document code: A
Biography: YANG Duo(1990-), female, master, majoring in electrochemical catalysis.*

