热解炭去除污水中Cr(VI)
Türkan Altun, Yakup Kar
(1.Department of Chemical Engineering, Selcuk University, Campus, 42031Konya, Turkey;2.Department of Petroleum & Natural Gas Engineering, skenderun Technical University, 31200Hatay, Turkey)
热解炭去除污水中Cr(VI)
Türkan Altun1, Yakup Kar2

以胡桃壳在450 ℃裂解得到生物质炭BC450,以胡桃壳与20%沥青砂在450 ℃裂解得到BCTS20,与商业活性炭(CAC)进行对比研究去除污水中Cr(VI)的能力。与BC450相比,BCTS20具有更丰富的表面官能团。在适当条件下,BC450、BCTS20、 CAC对Cr(VI)的去除率分别为80.47%、90.01%、95.69%。采用Langmuir、Freundlich、D-R 模型研究吸附等温线,其中Langmuir模型最佳。BC450、BCTS20、CAC的最大 Langmuir吸附容量分别为36.55、49.76、51.94 mg/g。这些炭材料对Cr(VI)的吸附可能归因于由离子交换、静电作用与螯合作用引起的化学过程。
热解炭;胡桃壳;Cr(VI)
1 Introduction
Heavy metals are an important group of pollutants in wastewater and treated water because they have various deleterious effects on health, living organisms, and the environment. Within the heavy metal pollutant groups, Cr(VI) is one of the most dangerous inorganic water pollutants, due to its carcinogenic, mutagenic, and toxic effects[1-4]. Cr(VI) ion in discharge effluents results from many industrial activities, such as electroplating, leather tanning, cement manufacture, textile dyeing, metal processing, and the production of paint, pigments and wood preservatives[5-7]. Such industries incur significant costs in reducing the concentrations of Cr(VI) ion in industrial effluents to a permissible level of 0.05 mg/L before discharge into surface waters[8].
The treatment technologies commonly applied for removal of Cr(VI) ion from wastewater include chemical precipitation, oxidation/reduction, filtration, ion exchange, membrane separation, and adsorption[9-11]. Among these, adsorption onto commercial activated carbons is the most common technique, owing to its advantages such as high efficiency, simplicity of design and ease of operation[12,13]. However, the process is relatively expensive because of the use of high-cost adsorbent. There is an increasing interest in lignocellulosic materials, primarily formed by various agricultural by-products such as nut shells, oily seed cakes and sawdust because such materials are renewable, abundant, and cheap. In the majority of previous studies, the biomass materials used were evaluated directly either as low-or-no-cost alternative adsorbents or as precursors for activated carbon production and bio-oil production by pyrolysis.
With the increasing importance of bio-oil production from biomasses, it is expected that there will be abundant sources of bio-chars as by-products from pyrolysis of biomasses[14]. Bio-chars can be directly used as adsorbents for the removal of pollutants[15]owing to the abundance of oxygen/nitrogen functional groups on their surface[16], and they also have been used as precursors for activated carbon production[17,18]. However, to the best of our knowledge, very few studies have been reported on Cr(VI) removal from aqueous solutions by bio-chars obtained from pyrolysis of agricultural by-products. Mohan et al. reported that bio-chars of oak origin could remove similar amounts of Cr(VI) as activated carbons[19]. Wang et al.[20]stated that charcoal bio-char (Fe-BC) had an high adsorption capacity for Cr(VI) ion. The usability of bio-char obtained from the pyrolysis of almond shell as an alternative adsorbent in the removal of heavy metals ions ( Ni(II) and Co(II) ) by adsorption process was studied by Klet al.[21]. As a result, they reported that the bio-char can be used as an adsorbent for the removal of heavy metals from aqueous solutions. The detailed literatures on the sorption of various contaminants originated organic and inorganic by different bio-chars were summarized by Mohan et al.[22]as a critical review. Zhou et al.[23]stated that the iron modified biochar has an excellent performance for the removal of various contaminants such as heavy metals Pb(II), Cr(VI), and As(V), phosphate and methylene blue from aqueous solutions. Deveci[24]reported that the bio-chars obtained from catalytic pyrolysis of oily seeds ofPistaciaterebinthusL. with natural zeolite and alumina could be used as effective and low cost adsorbents in the removal of Cr(VI) ion from aqueous solutions.
Walnut shell is an agricultural by-product that is suitable for the production of bio-oil by pyrolysis[25], it has a high calorific value of 20.18 MJ/kg, and potential annual availability in Turkey is estimated to be about 150 000 tons[26]. Furthermore, Nowicki et al.[27]reported that walnut shells were suitable as a precursor for producing cheap activated carbons with good adsorption properties.
Bio-chars generated during bio-oil production via pyrolysis have useful properties in the remediation process of metals from water[17,19]. Furthermore, bio-char yields obtained from the pyrolysis of walnut shells vary between 23 wt% and 34 wt%[25]. Therefore, the evaluation of bio-chars obtained from the pyrolysis of walnut shell either as direct adsorbent or as precursor for activated bio-chars in the removal of heavy metals from aqueous media will increase the range of applications and add value to the country's economy.
The objective of this study is to investigate the adsorption capacity of bio-chars in the removal Cr(VI) ion from aqueous solution. The adsorption capacities of bio-chars were compared with that of commercial activated carbon via batch studies by a systematic evaluation of effective parameters such as pH, contact time, chromium concentration, adsorbent dose, and temperature. Freundlich, Langmuir, and D-R isotherms were used to examine the kinetics of adsorption and to calculate isotherm parameters.
2 Experimental
2.1 Materials
All chemicals and reagents used in the present study were of analytical grade (Merck KGaA, Darmstadt, Germany). Stock solution Cr (VI) of 1 000 mg/L was prepared by dissolving the exact quantity of K2Cr2O7in 1 000 mL of deionized-distilled water. The Cr (VI) solution of 55 mg/L used as adsorbate was prepared by diluting the stock solution immediately prior to use. The pH value of the solution was adjusted by 0.1 mol/L NaOH or 0.1 mol/L HNO3.
Bio-chars (abbreviated as BC450 and BCTS20) were obtained from pyrolysis of walnut shell alone and in combination with tar sand (20 wt%) in a previous study, respectively[25], and also CAC (commercial activated carbon) were used for comparison. The bio-chars were used directly, without being subjected to any upgrading.Before use, the bio-chars samples were stored in glass bottles.
2.2 Apparatus
The surface morphology of the BC450 and BCTS20 samples were examined by scanning electron microscopy (LEO EVO 40 SEM). The FT-IR spectra of the bio-chars were recorded using a FT-IR Spectrometer (Perkin Elmer Spectrum 100). An elemental analyzer (LECO CHNS 932) was used to determine the elemental contents of the bio-chars. The pH values for the test solution of Cr(VI) were adjusted with an Orion pH meter (model 900S2). A thermostatic shaker (model GFL 3033) and magnetic stirrer (model IKAMAG-RO15) were used in the adsorption experiments. Cr(VI) concentration after adsorption was determined by a UV-vis Spectrophotometer (Shimadzu UV-1700) at 540 nm.
2.3 Batch adsorption experiments
Batch adsorption experiments were performed according to an established method described in detail in previous studies[4,28〗[29]. The amounts of Cr(VI) ions loaded on the particles of BC450, BCTS20, and CAC were calculated by conducting a mass balance on the solute before and after each experiment. For each adsorbent, all experiments were performed in triplicate and average results were reported.
The effects of the different initial pH values (range 2-9) on adsorption were studied under 0.05 g of adsorbent, 25 mL of test solution at a concentration of 55 mg/L, temperature of 25±1 ℃, and contact time of 60 min.To examine the effects of the contact time (range 10-240 min), the experiments were performed at pH 2, with all other variables identical above. Adsorption isotherms were studied at different initial Cr(VI) concentrations between 10 and 65 mg/L by holding other variables constant (0.05 g of adsorbent, 25 mL of test solution, pH 2, 25±1 ℃, and contact time of 60 min). The fourth series of experiments were performed to examine the effects of adsorbent dosage, varying from 0.002 to 0.12 g, with other parameters as defined in the previous series. To determine the effect of temperature on the adsorption process, the last series experiments was carried out at temperature of 25, 35, and 65±1 ℃, with other conditions as in the third group.
3 Results and discussion
3.1 Characterization of the bio-chars
Elemental analysis of C, H, N, S, and O (by difference) was performed for the BC450 and BCTS20 bio-chars. The elemental analysis results were then used to calculate the H/C molar ratios of the bio-chars. All results are shown in Table 1. When the elemental contents of the bio-chars are compared with those of the walnut shell and tar sand reported in a previous study[25], it was seen that the bio-chars (BC450 and BCTS20) are richer in carbon content, at 76.93wt% and 68.57wt%, respectively. The H/C molar ratio of BCTS20 was slightly higher than that of BC450. The higher H/C ratio of BCTS20 suggests it will have a greater adsorption capacity[30].

Table 1 Elemental contents and molar ratios for the bio-chars.
*Calculated by difference.
In order to determine the functional groups involved in Cr (VI) adsorption, the FT-IR spectra of the bio-chars are compared (Fig. 1). Comparison of the spectra in Fig. 1 shows that the surface chemistries of bio-chars are quite different in terms of the functional groups on their surfaces. BCTS20 includes important active groups like —CH, N—H, and C—O, which are involved in the adsorption of Cr(VI)[13]. The spectrum indicates that these functional groups correspond to the bands occurring in range of 2 937-2 967, 1 462, and 1 085 cm-1, respectively. The results reveal that the addition of tar sand to the pyrolysis process produces a significant effect on the surface properties of char product.

Fig. 1 FT-IR spectra of pyrolytic charcoals: BC450 and BCTS20.
Morphological analysis of the surface of the bio-chars was carried out by scanning electron microscopy. Scanning electron micrographs of bio-chars BC450 and BCTS20 are illustrated in Fig. 2 a,b. The SEM micrograph of BC450 clearly shows that the BC450 has an amorphous and heterogeneous structure, with many rudimentary and randomly distributed fine pores within its structure. In contrast, Fig. 2(b) reveals that the BCTS20 has a highly homogeneous structure with a predominance of mesopores, which have a major role in many liquid-solid adsorption processes[19].
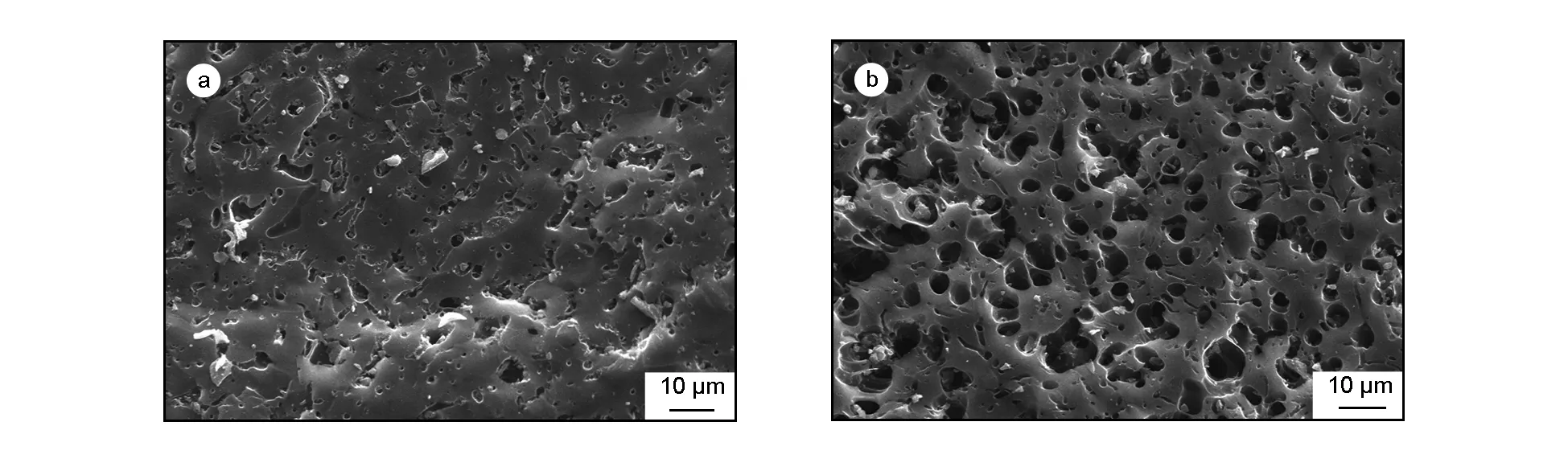
Fig. 2 SEM micrographs of (a) BC450 and (b) BCTS20.
3.2 Effect of pH on Cr(VI) ion removal
To determine the effect of pH on the removal of Cr(VI) from aqueous media by the bio-chars (BC450 and BCTS20) and commercial activated carbon (CAC, Merck), a series of adsorption experiments were performed. The results in Fig. 3 show that the maximum Cr (VI) adsorption values for BC450, BCTS20, and CAC are 80.47%, 90.01%, and 95.69% respectively at about pH 2.0. Adsorption percentages of all adsorbents decreases sharply when pH was increased from 2.0 to 5.0, then levelled off with a further increase of pH values.
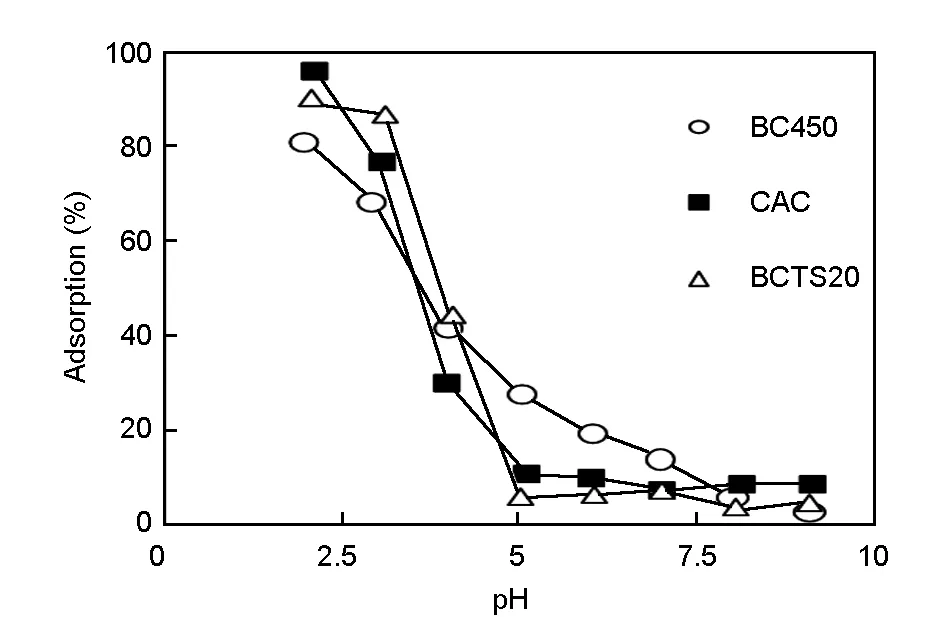
Fig. 3 Effect of pH value on Cr (VI) removal by the charcoals.
The effect of pH on the removal of chromium ion from aqueous media is associated with the interactions between the chromium ion and the bio-char walls and also the complexes present on the sorbent surface[19]. As reported by many studies[32,33], the high chromium removal attained at low pH values can be explained by strong acidic conditions that cause a highly protonated adsorbent surface, which results in a high uptake of Cr (VI) in the anionic form. This is because a more protonated surface produces stronger electrostatic attraction between the positively-charged sorbent surface and negatively-charged chromate ion. The decrease in adsorption efficiency at pH>5.0 may be attributed to the weakening of electrostatic attraction between the sorbents and the sorbate ions owing to the dual competition of chromate and hydroxyl ions to be adsorbed on the surface of the sorbents[31-35]. Therefore, subsequent adsorption studies of the optimal values for other operating parameters were carried out at the optimal pHintof 2.0. This agrees with many earlier studies of the removal of Cr(VI) ion by different bio-chars, in which the optimal pH value was reported as 2.0[19,32,34,36].
3.3 Effect of contact time on Cr(VI) ion removal
Optimal contact time is a significant parameter in terms of the total cost of the adsorption process, because the shaking process used during adsorption involves energy consumption. A series of contact-time experiments was carried out at optimal pH 2 with a contact time ranging from 10 to 240 min, and all other conditions held constant (Fig. 4).
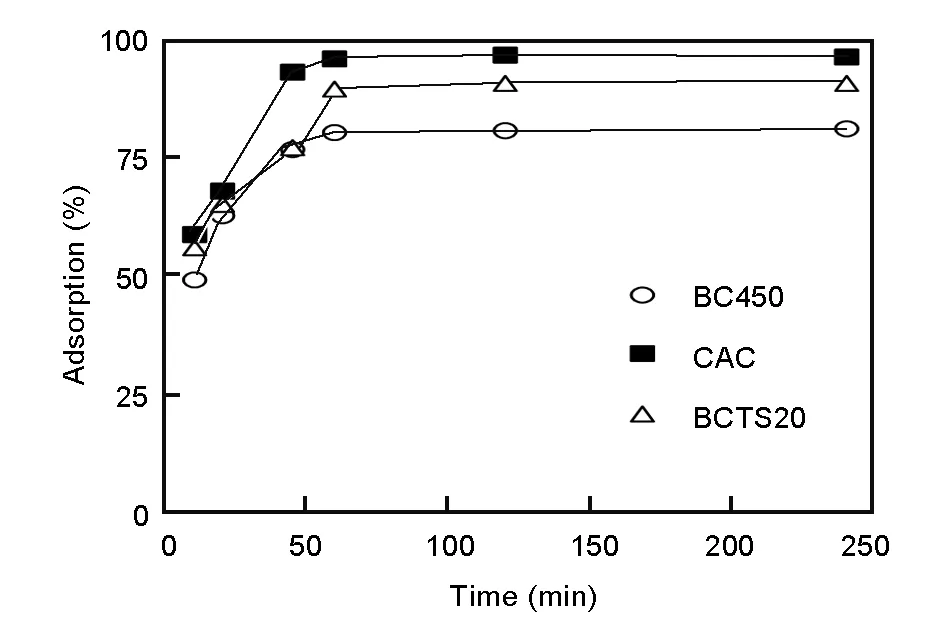
Fig. 4 Effect of contact time on Cr (VI) removal capacity by the charcoals.
For all adsorbents, the percentage removal of Cr(VI) ion increased with contact time from 10-60 min, and reached a maximum value at a contact time of 60 min. There was a negligible increase in the adsorption percentage above 60 min. At the optimal contact time of 60 min, the adsorption percentages were 80.51% for BC450, 96.25% for CAC, and 89.80% for BCTS20. On the basis of these results, a contact time of 60 min was adopted as the optimal shaking period in the subsequent studies.
High adsorption rates were recorded up to the optimal contact time of 60 min because of the abundance of free binding sites. During the initial stage of adsorption, mesopores are largely saturated with chromium ions. During later stages, the adsorption rate was very slow because Cr (VI) ions are forced to migrate deep into the micropores, resulting in much a great resistance[37].
3.4 Effect of sorbent dose on Cr(VI) ion removal
The relationship was established between the sorbent dose and the percentage removal of Cr(VI) ion. A series of adsorption experiments were performed on the treatment of required sorbents (BC450, CAC and BCTS20) in mass range of 0.002-0.130 g with 25 mL of Cr(VI) solution in 55 ppm. All adsorption experiments were carried out at the two optimized conditions in previous series (pHint2 and contact time of 60 min). The results are given in Fig. 5. For all sorbents, Cr (VI) adsorption percentage increased with sorbent dose, reached a maximum at 0.064 g/25 mL sorbent dose, and then remained almost constant for a further increase in sorbent dose.
The results can be attributed to the occurrence of great surface area and an increase in the number of binding sites[36-38]. In this stage, the highest Cr(VI) removal efficiencies obtained at the optimal sorbent dose were 81.82%, 95.64% and 92.15% for BC450, CAC, and BCTS20, respectively. The difference in the Cr(VI) removal capacities of the sorbents could be attributed to the diversity of active groups on the sorbent surface and the variation in their concentration[39].

Fig. 5 Effect of adsorbent concentration on Cr (VI) removal.
3.5 Influence of initial concentration on Cr (VI) removal
To determine the effect of initial concentration of Cr(VI) solution on the removal of Cr(VI) ion by bio-chars, a series of adsorption experiments were performed on each sorbent (BC450, CAC, and BCTS20) with initial solution concentrations ranging from 10 to 65 mg/L under the optimal conditions (2.65 g/L sorbent dose, pHint2, and 60 min of contact time). With an increase in initial concentration of Cr (VI) solution (Ci, Mm), the Cr(VI) uptake (per their mass at equilibrium (qe, mmol/g)) of all the sorbents increased, their percentage adsorption values decreased. The increase inqevalues of all the sorbents was significant up toCi= 55 mg/L (1.058 mM) due to the great availability of active sites on the sorbent at low initial solution concentrations, resulting from a high driving force[37], but was then negligible with a further increase of the concentrations.
3.6 Adsorption isotherms
Adsorption isotherms or capacity studies are very important in calculating the amounts of metal ions partitioned between sorbent and liquid phase at equilibrium as a function of increase in metal ion concentration[40].
Isotherm models represent the best fitting to the adsorption of Cr(VI) ions by each sorbent (BC450, CAC, and BCTS20). The isotherms were analyzed using Freundlich, Langmuir, and D-R (Dubinin-Radushkevich) models. The Langmuir model is given in Eq. (1).
(1)
where,Ceandqeare the solute concentration and uptake value of metal ion of sorbent per its mass respectively;As(mol/g) andKb(L/mol) are the coefficients.
Another common isotherm is the Freundlich model described in Eq. (2).
(2)
wherekandnare constants.
The D-R isotherm model is expressed in Eq. (3).
lnqe=lnX'm-K'×ε2
(3)
whereX'm(mmol/g) andK'(mol2/kJ2) are the maximum adsorption capacity of sorbent and a constant related to the adsorption energy, respectively;εis the Polanyi potential, which is expressed as follows:
whereR(kJ/mol×K) is the gas constant andTis absolute temperature. The magnitude of mean adsorption energy (E, kJ/mol) is useful to estimate the type of adsorption process, and can be determined using the following equation:
For all the sorbents, Table 2 shows the model parameters and correlation coefficients (R2) . As seen in Table 2, theR2of the Langmuir isotherm for each of the sorbents was greater than the other models, which indicates that the Langmuir isotherm represents best the adsorption of Cr(VI) onto the bio-chars. This result is an indication of the adsorption of Cr(VI) ions onto a monolayer of the surfaces of BC450 and BCTS20 under the experimental conditions. From the fitted Langmuir model, the maximum adsorption capacities are 36.55, 49.76, and 51.94 mg/g of BC450, BCTS20, and CAC, respectively.
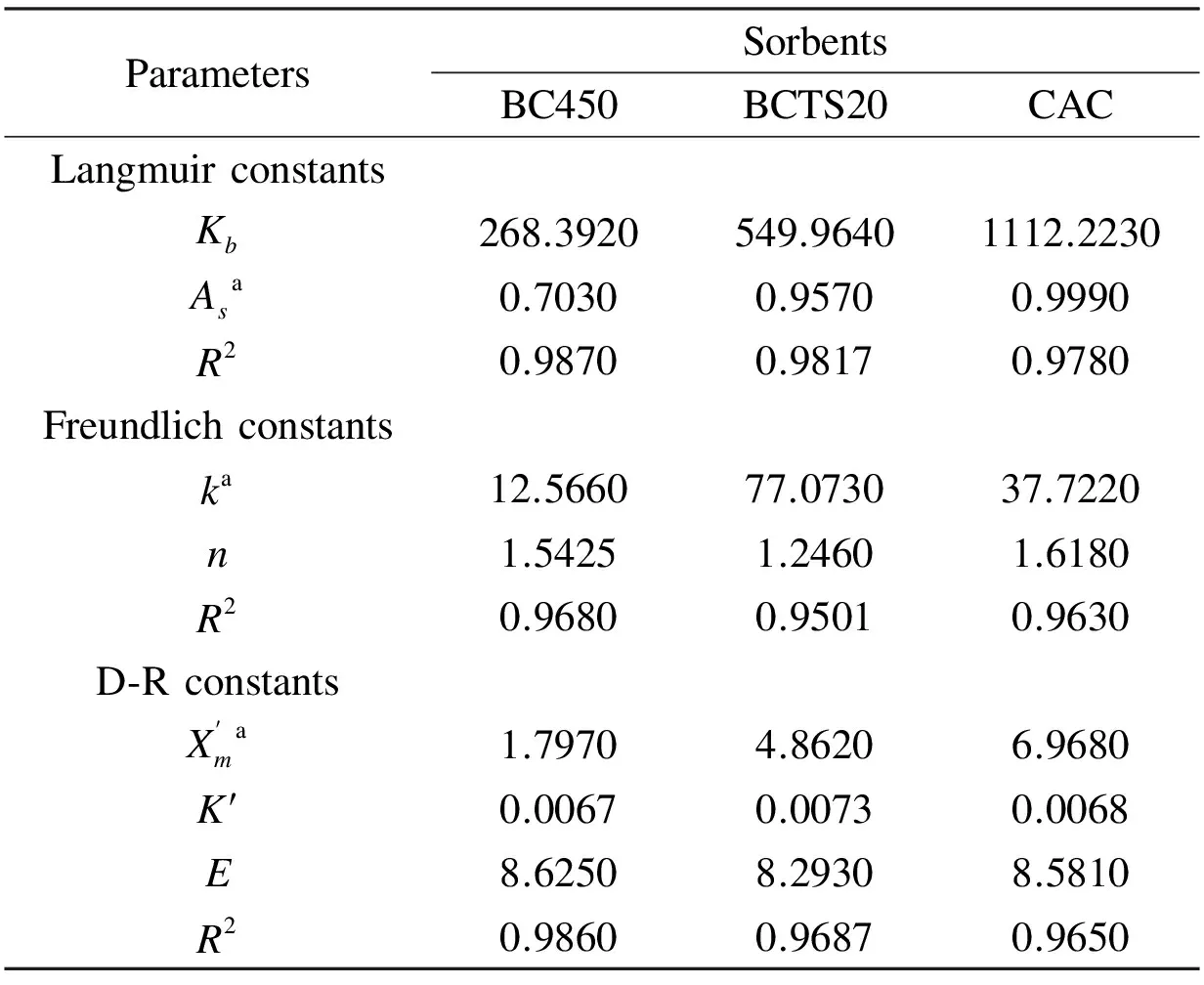
Table 2 Isotherms model constants for BC450, BCTS20 and CAC.
a: mmol/g dry sorbent.
The favorability of a adsorption process can be expressed according to the magnitude ofRLfrom Langmuir constant (Kb), which is determined from the following equation[13,36,42]and called as the dimensionless separation factor.
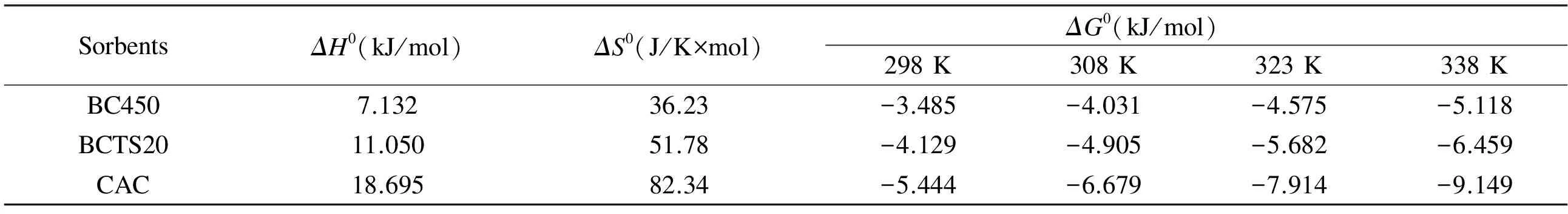
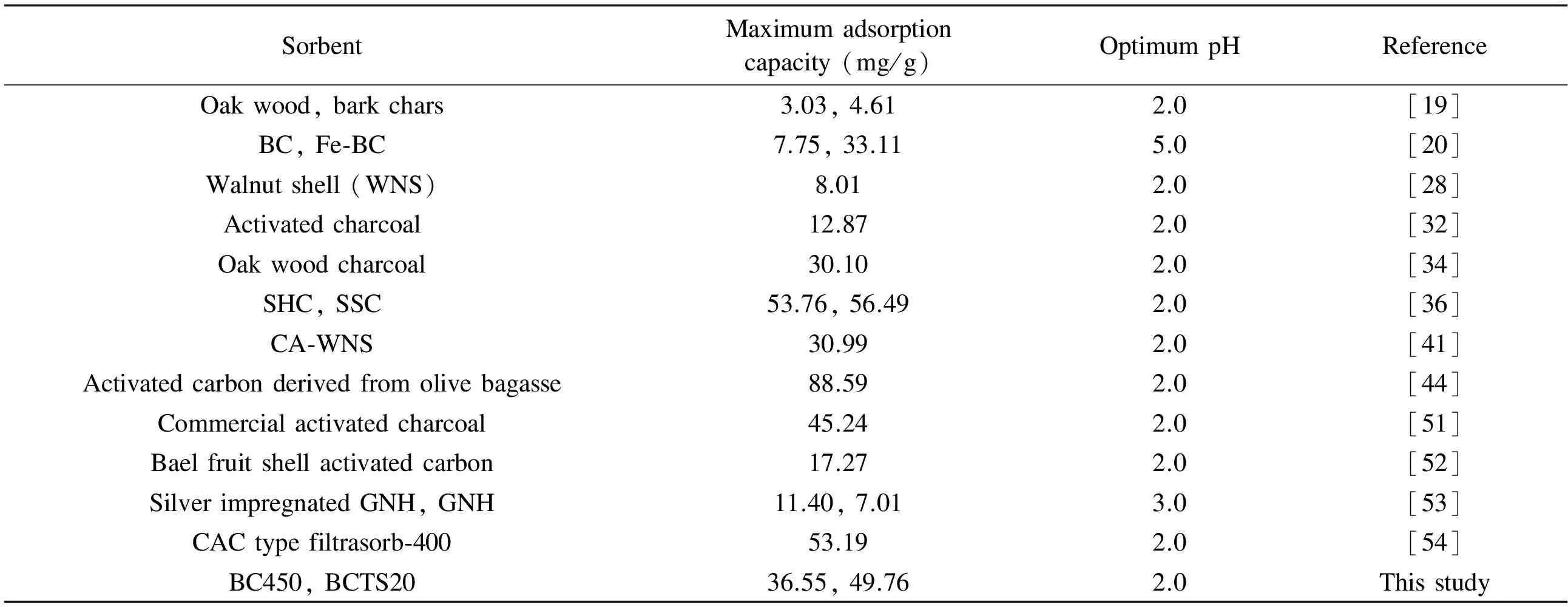
For 55 mg/L (1.058 Mm of the optimalCi), theRLvalues for BC450, BCTS20, and CAC were calculated as 0.00351, 0.00172 and 0.00085, respectively. These results indicate that the adsorption process was favorable, because 0 Langmuir isotherm constants are not sufficient to explain whether the type of adsorption is either physical or chemical[41]. However, the magnitude of mean adsorption energy (E); which is calculated from the D-R isotherm, is a useful indicator of the type of adsorption process[44]. From Table 2, it is seen that the values ofEfor all sorbent were within the range 8-16 kJ/mol, which indicates a chemical adsorption, dominated by ion-exchange, electrostatic attraction and chelation[41]. 3.7 Adsorption kinetics Adsorption kinetics is important in the modeling and design of adsorption process[45]. Many kinetic studies using experimental data for the removal of Cr(VI) by various carbonaceous materials have previously reported that the kinetic of the adsorption process was compatible with the pseudo-second-order model[13,31,36]. Therefore, only the suitability of experimental data to the pseudo-second-order model was examined, using the pseudo-second order equation[46]given in Eq. (4). (4) wherek2(g/mg×min) andqt(mg/g) are the rate constant of the pseudo-second-order kinetic equation and the amount of Cr(VI) removed at any time (t, min), respectively. Table 3 shows the results obtained for the adsorption kinetics, showing that the values ofqecalculated from the pseudo-second-order kinetics model (termedqe,cal,) were nearly equal to the experimental values ofqe(marked asqe,exp). Also, theR2values were very close to 1.0, indicating that the adsorption kinetic is consistent with the pseudo-second-order kinetic model. As a result, the compatibility of experimental data with the pseudo-second-order kinetics model confirms that chemisorption is likely dominant in the removal of Cr(VI) by BC450, BCTS20, and CAC. This result is in accordance with other studies[13,19,47]. 3.8 Effect of temperature and the adsorption thermodynamics In order to determine the effect of temperature on the adsorption process, a series of adsorption experiments were carried out within the temperature range 25-65 ℃ with other parameters held constant at the optimal values already determined above. The thermodynamic parameters, including standard Gibbs free energy change (ΔG0), standard enthalpy change (ΔH0), and standard entropy change (ΔS0) were calculated using the following equations[41,48]: ΔG0=-RTlnKc (5) (6) (7) whereCAeandKcare the adsorbent concentration at equilibrium and the equilibrium constant, respectively. Table 3 Results of the pseudo-second order kinetic model for the removal of Cr(VI) by BC450, BCTS20 and CAC. The magnitudes of thermodynamic parameters (ΔH0andΔS0) were calculated from the slope and intercept of the linear plots of LogKcversus 1/T, respectively. The values of thermodynamic parameters calculated for the removal of Cr(VI) by the sorbents are given in Table 4. In general, the negative value ofΔG0is an indicator for the feasibility and spontaneous nature of adsorption process[36,41,44]. From the results in Table 4, it was seen that there was a continuous increase in the negative values ofΔG0with increasing temperature from 25 to 65 ℃. As a result, it may be suggested that the process of Cr(VI) on the sorbents was spontaneous, and that the degree of spontaneity increased with temperature, resulting in a more negative value ofΔG0at a higher temperature. The more negative value indicates a more energetically favorable adsorption process[39]. Table 4 Thermodynamic parameters for the removal of Cr(VI) by the bio-chars and CAC. 3.9 Comparison of adsorption capacities of bio-chars with other sorbents An increasing number of studies have examined the removal of Cr(VI) ion from aqueous medium by various low-cost materials such as biomass, bio-char, and activated bio-char. Table 5 compares the adsorption capacities reported for the various sorbents used for removal of Cr(VI)[19,20,28,32,34,36,41,44,51-54]. Table 5 Comparison of the maximum adsorption capacities(Langmuir isotherm) of bio-chars with other sorbents for Cr(VI) removal. The positive values forΔH0andΔS0for all sorbents show that the adsorption process carried out under experimental conditions is endothermic. Moreover, the positive values of bothΔH0andΔS0indicate that chemisorption is the dominant mechanism of Cr(VI) adsorption on the bio-chars and CAC. In addition, the positive value ofΔS0indicates that the adsorption process is irreversible and stable[49,50]. These results are similar to those previously reported for the removal of Cr(VI) by various carbonaceous materials[36,44]. Compared to the values reported in the literatures, the bio-chars used in the present study appear to be a highly efficient sorbent for the removal of Cr(VI) ion from aqueous solutions. The SEM and FT-IR results reveal that the use of tar sand as an additive during pyrolysis led to the formation of a large amount of surface functional groups of the resulting bio-chars. The removal of Cr(VI) from aqueous solution by the bio-chars was dependent on pH, initial Cr(VI) concentration, bio-char dose, contact time. And the maximum removal of Cr(VI) achieved by the bio-chars was 80.47% for BC450, 90.01% for BCTS20 and 95.69% for CAC at about pH 2. The bio-chars prepared in the present study are superior to many sorbents that were previously reported for the removal of Cr(VI) in terms of adsorption capacity. The bio-chars tested in the present study (BC450 and BCTS20) can be used effectively for the remediation of chromium-polluted wastewater streams. [1] Liu W, Zhang J, Zhang, C, et al. Adsorptive removal of Cr (VI) by Fe-modified activated carbon prepared from trapa natans husk[J]. Chemical Engineering Journal, 2010, 162: 677-684. [2] Sikaily E A, Nemr A E, Khaled A, et al. Removal of toxic chromium from waste water using green alga ulva lactuca and its activated carbon[J]. Journal of Hazardous Materials, 2007, 148: 216-228. [3] Li H, Li Z, Liu T, et al. A novel technology for biosorption and recovery of hexavalent chromium in wastewater by bio-functional magnetic beads[J]. Bioresource Technology, 2008, 99: 6271-6279. [4] Arslan G, Edebali S, Pehlivan E. Physical and chemical factors affecting the adsorption of Cr(VI) via humic acids extracted from brown coals[J]. Desalination, 2010: 255, 117-123. [5] Kim S D, Park K S, Gu M B. Toxicity of hexavalent chromium to daphnia magna: influence of reduction reaction by ferrous iron[J]. Journal of Hazardous Materials, 2002, A93: 155-164. [6] Dönmez G, Aksu Z. Removal of chromium (VI) from salina wastewaters by dunaliella species[J]. Process Biochemistry, 2002, 38: 751-762. [7] Gottipati R, Mishra S. Process optimization of adsorption of Cr (VI) on activated carbons prepared from plant precursors by a two-level full factorial design[J]. Chemical Engineering Journal, 2010, 160: 99-107. [8] Baral A, Engelken R D. Chromium-based regulations and greening in metal finishing industries in the USA[J]. Environmental Science and Policy, 2002, 5: 121-133. [9] Terry P A. Characterization of Cr ion exchange with hydrotalcite[J]. Chemosphere, 2004, 57: 541-546. [10] Ölmez T. The optimization of Cr(VI) reduction and removal by electrocoagulation using response surface methodology[J]. Journal of Hazardous Materials, 2009, 162: 1371-1378. [11] Alguacil F J, Caravaca C, Martin M I. Transport of chromium(VI) through a cyanex 921-supported liquid membrane from HCI solutions[J]. Journal of Chemical Technology and Biotechnology, 2003, 78: 1048-1053. [13] Moussavi G, Barikbin B. Biosorption of chromium (VI) from industrial wastewater onto pistachio hull waste biomass[J]. Chemical Engineering Journal, 2010, 162: 893-900. [14] Liu Z, Zhang F S. Removal of copper (II) and phenol from aqueous solution using porous carbons derived from hydrothermal chars[J]. Desalination, 2011, 267: 101-106. [15] Liu W J, Zeng F X, Jiang H, et al. Preparation of high adsorption capacity bio-chars from waste biomass[J]. Bioresource Technology, 2011, 102: 8247-8252. [16] Cheng H N, Wartelle L H, Klasson K T, et al. Solid-state NMR and ESR studies of activated carbons produced from pecan shells[J]. Carbon, 2010, 48: 2455-2469. [17] Mohan D, Pittman J C U, Bricka M, et al. Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production[J]. Journal of Colloid Interface Science, 2007, 310: 57-73. [18] Cao N, Darmstadt H, Roy C. Activated carbon produced from charcoal obtained by vacuum pyrolysis of softwood bark residues[J]. Energy Fuels, 2001, 15: 1263-1269. [19] Mohan D, Rajput S, Singh V K, et al. Modeling and evaluation of chromium remediation from water using low cost bio-char, a green adsorbent[J]. Journal of Hazardous Materials, 2011, 188: 319-333. [20] Wang X J, Wang Y, Wang X, et al. Microwave-assisted preparation of bomboo charcoal-based iron-containing adsorbents for Cr(VI) removal[J]. Chemical Engineering Journal, 2011, 174: 326-332. [22] Mohan D, Sarswat A, Ok Y S, et al. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent-A critical review[J]. Bioresource Technology, 2014, 160: 191-202. [23] Zhou Y, Gao B, Zimmerman A R, et al. Biochar-supported zerovalent iron for removal of various contaminants from aqueous solutions[J]. Bioresource Technology, 2014, 152: 538-542. [24] Deveci H, Kar Y. Adsorption of hexavalent chromium from aqueous solutions by bio-chars obtained during biomass pyrolysis[J]. Journal of Industrial and Engineering Chemistry, 2013, 19: 190-196. [25] Kar Y. Co-pyrolysis of walnut shell and tar sand in a fixed-bed reactor[J]. Bioresource Technology, 2011, 102: 9800-9805. [27] Nowicki P, Pietrzak R, Wachowska H. Sorption properties of activated carbons obtained from walnut shells by chemical and physical activation[J]. Catalysis Today, 2010, 150: 107-114. [28] Pehlivan E, Altun T. Biosorption of chromium (VI) ion from aqueous solutions using walnut, hazelnut and almond shell[J]. Journal of Hazardous Materials, 2008, 155: 378-384. [29] APHA. Standard methods for the examination of water and wastewater[S]. 17thed, APHA-WWA-WPCF, vol. 3, 1989. [30] Raveendran K, Ganesh A. Adsorption characteristics and pore-development of biomass-pyrolysis char[J]. Fuel, 1998, 77: 769-781. [31] Nameni M, Alavi Moghadam M R, Arami M. Adsorption of hexavalent chromium from aqueous solutions by wheat bran[J]. International Journal of Environmental Science and Technology, 2008, 5: 161-168. [32] Mor S, Ravindra K, Bishnoi N R. Adsorption of chromium from aqueous solution by activated alumina and activated charcoal[J]. Bioresource Technology, 2007, 98: 954-957. [33] Karthikeyan T, Rajgopal S, Miranda L R. Chromium (VI) adsorption from aqueous solution by hevea brasilinesis sawdust activated carbon[J]. Journal of Hazardous Materials, 2005, B124: 192-199. [34] Pehlivan E, Kahraman H, Pehlivan E. Sorption equilibrium of Cr(VI) ions on oak wood charcoal (Carbo Ligni) and charcoal ash as low-cost adsorbents[J]. Fuel Processing Technology, 2011, 92: 65-70. [35] Baral S S, Dasa S N, Rath P. Hexavalent chromium removal from aqueous solution by adsorption on treated sawdust[J]. Biochemical Engineering Journal, 2006, 31: 216-222. [36] Jain M, Garg V K, Kadirvelu K. Adsorption of hexavalent chromium from aqueous medium onto carbonaceous adsorbents prepared from waste biomass[J]. Journal of Environmental Management, 2010, 91: 949-957. [37] Wang F Y, Wang H, Ma J W. Adsorption of cadmium (II) ions from aqueous solution by a new low-cost adsorbent-bamboo charcoal[J]. Journal of Hazardous Materials, 2010, 177: 300-306. [38] Bansal M, Singh D, Garg V K. A comparative study for the removal of hexavalent chromium from aqueous solution by agricultural wastes’carbons[J]. Journal of Hazardous Materials, 2009, 171: 83-92. [39] Dakiky M, Khamis M, Manassra A, et al. Selective adsorption of chromium(VI) in industrial wastewater using low-cost abundantly available adsorbents[J]. Advances in Environmental Research, 2002, 6: 533-540. [40] Karatas M. Removal of Pb(II) from water by natural zeolitic tuff: kinetics and thermodynamics[J]. Journal of Hazardous Materials, 2012, 199-200: 383-389. [41] Altun T, Pehlivan E. Removal of Cr(VI) from aqueous solutions by modified walnut shells[J]. Food Chemistry, 2012, 132: 693-700. [42] Kadirvelu K, Thamaraiselvi K, Namasivayam C. Adsorption of nickel (II) from aqueous solution onto activated carbon prepared from coir pith[J]. Separation and Purification Technology, 2001, 24: 497-505. [43] Babel S, Kurniawan T A. Cr(VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan[J]. Chemosphere, 2004, 54: 951-967. [46] Ho Y S, Mckay G. Pseudo-second order model for sorption processes[J]. Process Biochemistry, 1999, 34: 451-465. [47] Wang X S, Chen L F, Li F Y, et al. Removal of Cr(VI) with wheat-residue derived black carbon: Reaction mechanism and adsorption performance[J]. Journal of Hazardous Materials, 2010, 175: 816-822. [48] Romero-Gonzalez J, Peralta-Videa J R, Rodriguez E. Determination of thermodynamic parameters of Cr(VI) adsorption from aqueous solution onto Agave lechuguilla biomass[J]. The Journal of Chemical Thermodynamics, 2005, 37: 343-347. [49] Panda L, Das B, Rao D S, et al. Application of dolochar in the removal of cadmium and hexavalent chromium ions from aqueous solutions[J]. Journal of Hazardous Materials, 2011, 192: 822-831. [50] Singh D B, Prasad G, Rupainwar D C. Adsorption technique for the treatment of As (V) rich effluents[J]. Colloids and Surfaces, 1996, A 111: 49-56. [51] Seyf-laye Alfa-Sika M, Liu F, Chen H. Optimization of key parameters for chromium (VI) removal from aqueous solutions using activated charcoal[J]. Journal of Soil Science and Environmental Management, 2010, 1(3): 55-62. [52] Anandkumar J, Mandal B. Removal of Cr(VI) from aqueous solution using bael fruit (Aegle marmelos correa) shell as an adsorbent[J]. Journal of Hazardous Materials, 2010, 168: 633-640. [53] Dubey S P, Gopal K. Adsorption of chromium (VI) on low cost adsorbents derived from agricultural waste material: a comparative study[J]. Journal of Hazardous Materials, 2007, 145: 465-470. [54] Hamadi N K, Chen X D, Farid M M, et al. Adsorption kinetics for the removal of chromium (VI) from aqueous solution by adsorbent derived from used tyres and sawdust[J]. Chemical Engineering Journal, 2001, 84: 95-105. Removal of Cr(VI) from aqueous solution by pyrolytic charcoals Türkan Altun1, Yakup Kar2 Bio-chars produced by the pyrolysis of walnut shells at 450 ℃ (BC450) and theco-pyrolysis of walnut shells and 20 wt% tar sand (BCTS20)at the same temperature, were investigated as potential adsorbents for the removal of Cr(VI) ions from aqueous solutions using batch experiments. The BCTS20 has more abundant surface functional groups than BC450. The Cr(VI) removal percentages under optimal conditions were 80.47and 95.69% for BC450 and BCTS20, respectively. Langmuir, Freundlich and D-R models were used to fit the adsorption isotherms and the Langmuir model described the adsorption isotherms best. The adsorption of Cr(VI) was by a chemical process dominated by ion-exchange, electrostatic attraction and chelation. The maximum Langmuir adsorption capacities were 36.55 and 49.76 mg per g of BC450 and BCTS20, respectively. The maximum Langmuir adsorption capacity of BCTS20 is comparable to that of some reported commercial activated carbons. Pyrolytic charcoal; Walnut shell; Hexavalent chromium Yakup Kar. E-mail: karyakup@gmail.com Yakup Kar. E-mail: karyakup@gmail.com 1007-8827(2016)05-0501-09 TQ127.1+1 A 10.1016/S1872-5805(16)60028-8 Receiveddate: 2016-07-01;Reviseddate: 2016-09-15 English edition available online ScienceDirect ( http:www.sciencedirect.comsciencejournal18725805 ).
4 Conclusions