Effects of transcranial direct current stimulation (tDCS) for auditory hallucinations: A systematic review
Haibin LI, Yiran WANG, Jiangling JIANG, Wei LI, Chunbo LI,
•Systematic review and meta-analysis•
Effects of transcranial direct current stimulation (tDCS) for auditory hallucinations: A systematic review
Haibin LI1, Yiran WANG1, Jiangling JIANG1, Wei LI1, Chunbo LI1,2*
transcranial direct current stimulation, auditory hallucination, schizophrenia
1. Background
Auditory hallucination (AH) is a common symptom in patients with schizophrenia and its average prevalence is 60% (range 25–94%).[1]Even worse is that 70% of patients have serious consequences due to AH, such as suicide.[2,3]In most patients, AH can be alleviated using antipsychotics, however, 25 to 30% of patients have persistent auditory hallucinations which are not responsive to antipsychotics.[2,4-5]
Transcranial direct current stimulation (tDCS)is a non-invasion brain stimulation which has been suggested as a safe and promising treatment for antipsychotic-refractory auditory hallucinations in patients with schizophrenia.[6]The exact mechanism underlying tDCS has not been demonstrated yet,however tDCS is believed to cause a sub-threshold alteration of the resting membrane potential and has prolonged effects after efficient simulation.[7]It modulates membrane potential selectively, relying on the current polarity, duration or strength, and is able to induce after effect excitability changes in the motor cortex.[8]The continuous direct current is low (eg.1-3mA) through electrodes always placed over point midway between F3 and FP1 (left dorsolateral prefrontal cortex) and the cathode located over a point midway between T3 and P3 (left temporo-parietal junction).[9-12]The time of stimulation can be set from 5 to 20 minutes.[13-16]There have been some concerns about tDCS despite more than half a century of use.[9]Toxins and electrode dissolution products at the electrodetissue interface(17)are the only risks tDCS presents for skin contact.
tDCS has been used for over half a century.[6]Some case reports have shown that tDCS benefited patients with AH.[12,18-19]However, some reports indicated that the opposite was true.[14]In addition, several trials have also been published. The conclusions of these trials were also inconsistent. The reports from Brunelin and Ganesan found tDCS reduced AH(20-21),but the trial by Fitzgerald and colleagues claimed there was no evidence showing patients with AH could benefit from tDCS.[14]The aim of the current study is to conduct a systematic review to explore the effects of tDCS on AH in patients with schizophrenia.
2. Methods
2.1 Search strategy and study selection
The process of study selection is shown in Figure 1.First, we searched relevant randomized controlled trials from PubMed, EMBASE, the Cochrane Library,Chinese National Knowledge Infrastructure, Chongqing VIP database for Chinese Technical Periodicals,WANFANG DATA, Chinese Biological Medical Literature Database, and Taiwan Electronic Periodical Services(TEPS) published before February 13, 2016. The search keywords were: (“Transcranial direct current stimulation” or "tDCS" or “Transcranial direct current stimulus” or “Transcranial Electrical Stimulation”) and(“Auditory Hallucination” or “Auditory Hallucinations”or “Verbal Auditory Hallucination”, “phonism” or”voice*” ). The review was also conducted in Chinese with the following search terms: “Jing Lu Zhi Liu Dian Ci Ji (经颅直流电刺激 )”, “Jing Shen Fen Lie Zheng (精 神 分 裂 症 )”, “Huan Ting (幻 听 )”, “Ting Huan Jue( 听 幻 觉 )”. Secondly, we imported identif i ed original articles into Note Express (version 3.2.0) and removed duplicates. Two authors (Haibin Li, Yiran Wang)independently screened the titles, abstracts and fulltext. Only when they both judged an article as suitable for this review was the article included. If they couldn’t reach an agreement, a third person (Jiangling Jiang)decided whether the article was suitable for inclusion.Third, we hand-searched the references of included articles for additional potential papers.
2.2 Inclusion and exclusion criteria
Inclusion criteria for studies was the following: (a)randomized controlled trials (RCTs). We placed no restrictions on language or publication status, (b) any patients suffering from auditory hallucinations with a primary diagnosis of schizophrenia or other type of schizophrenia-spectrum type disorders irrespective of gender, race or nationality, were eligible for inclusion, (c)diagnostic systems included DSM-IV (APA 1994), DSM-5 (APA 2013), ICD-10 (WHO 1992), CCMD-3 and other validated diagnostic instruments, (d) no restrictions on setting, (e) studies using tDCS as the intervention group,(f) studies using pharmacological therapy, other therapy,sham stimulus, or no intervention as the control group.Exclusion criteria were the following: (a) studies were not RCTs, (b) auditory hallucinations were caused by specificother non-mental illness.
2.3 Outcome measures
The primary outcome of this study was improvement in auditory hallucination, which was measured by Auditory Hallucination Rating Scale (AHRS)[22-23], Positive and Negative Syndrome Scale (PANSS)[24]and other measures of auditory hallucination.
The secondary outcomes were psychiatric symptoms and adverse effects as measured by PANSS,Scale for Assessment of Negative Symptoms (SANS),[25]scores on the treatment emergent symptom scale(TESS),and drop-outs.
2.4 Evaluation of the quality of included studies
The quality of included studies was assessed by The Cochrane Risk of Bias tool in the 5.1.0 revision of the Cochrane Handbook for Systematic Reviews of Interventions[26]and the Grading of Recommendations Assessment, Development and Evaluation (GRADE)tool.[27]Two authors independently conducted the quality evaluation.
If three or more of these items were rated as having a “high risk of bias”, the overall rating of risk of bias for the article was also rated as “high”; if less than three items were rated as “high” the overall risk of bias for the article was classified as “low”. We categorized the level of evidence into high, medium, low, and very low according to the GRADE criteria (https://gradepro.org)based on characteristics of each study.
2.5 Data extraction
Data was extracted using standard, pre-designed forms. Where disputes arose, HL and YW discussed the disagreement until agreement was reached. Where it was not possible to resolve disagreement by discussion,a third author (JJ) made the final decision.
The data extraction form included: (a) general characteristics of the study (e.g. author’s name, study year); (b) characteristics of demography (e.g. sample size, average age, gender, type of intervention, inclusion and exclusion criteria); (c) study data (e.g. primary outcome, secondary outcome). More details are given in tables 1 and 2.

Table 1. Characteristics of the 3 randomized controlled trials
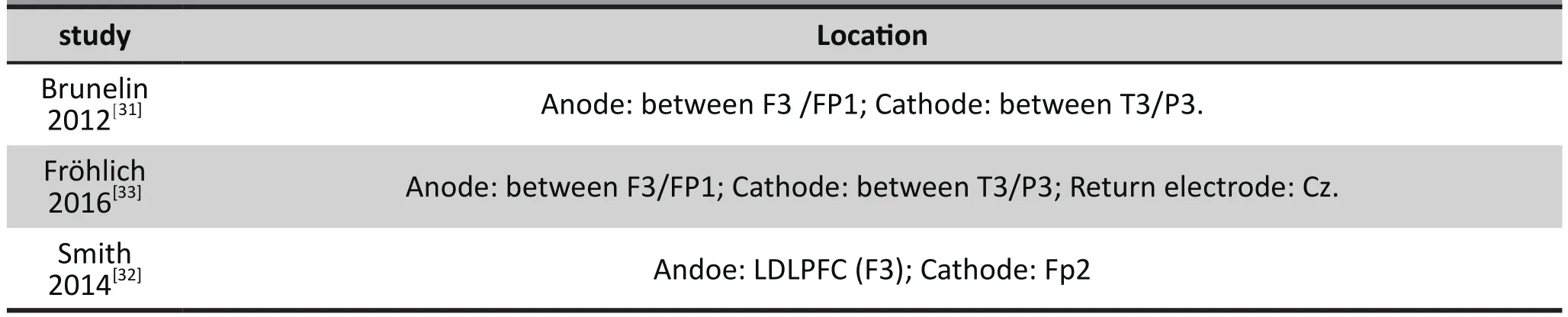
Table 2. Supplemental information provided for the 3 studies included in the systematic review
2.6 Statistical analysis
For binary outcomes we calculated a standard estimation of the risk ratio (RR) and its 95% conf i dence interval (CI). It has been shown that the RR is more intuitive[28]than odds ratios and that odds ratios tend to be interpreted as RRs by clinicians (Deeks 2000). For continuous outcomes we estimated the mean difference(MD) between groups with 95% CIs.
We used the Cochrane RevMan 5.3 and R 3.1.0 for data analysis. P value and I2were used to assess the heterogeneity. If p>0.1 and I2<50%, the heterogeneity of studies was not signif i cant and we would use a fi xedeffect model to estimate the result. If p≤ 0.1 or I2≥50%,the heterogeneity was significant, and we used a random effect model. Reasons for heterogeneity were explored by subgroup analysis and sensitivity analysis.The heterogeneity may be caused by different electrode location, time of stimulation, and current intensity.
3. Results
3.1 Results of the search
There were 304 potential articles published before 13 February 2016 identified with a standardized search strategy and reference search. By screening abstracts and titles, 287 articles were excluded. After reading the full-text of 17 articles, 14 articles were excluded. The sample from Mondino 2015a and Mondino 2015b[13,29]overlapped and could not be distinguished. As a result,these studies were excluded. Brunelin 2014[30]was a secondary analysis of Brunelin 2012[31], so we only used the data from Brunelin 2012. Fitzgerald 2014(14]did not report demographic data for each group, and was therefore excluded. Eventually, we included 3 articles.Selection process is shown in Figure 1.
The three studies (Smith 2014[32], Brunelin 2012[31]and Fröhlich 2016[33]) were included with 89 participants(45 in the active group, 44 in the sham group). For full details, please see the characteristics of included studies (Table 1).
All the studies used a randomized double-blind sham-controlled design. Two studies (Smith 2014,Fröhlich 2016) conducted treatments in 5 consecutive days (except for weekends and holidays). The other(Brunelin 2012) used stimulation twice a day.
Sample sizes in included studies were all small: 24 participants were in Fröhlich 2016, 30 participants were in Brunelin 2012 and 33 participants were included in Smith 2014. Fröhlich 2016 and Smith 2014 also included schizoaffective psychosis and Brunelin 2012 only enrolled schizophrenia
Active tDCS: All the electrodes used 10/20 placement system. Smith 2014 placed the anode over the LDLPFC (F3) and the cathode over the contralateral supraorbital ridge (Fp2). Brunelin 2012 and Fröhlich 2016 both placed the anode over theF3/FP1 (left dorsolateral prefrontal cortex), and the cathode over the T3/P3 (left temporo-parietal junction), while Fröhlich 2016 had a third electrode: a return electrode over Cz(posterior midline). The current was set at 2mA for 20 minutes.

Figure 1. Identification of included studies
Sham tDCS: The sham group had stimulation with 2 mA lasting only 40 seconds, though the electrodes remained in place for 20 minutes.
All the studies reported PANSS, and the auditory items of PANSS were used to assess auditory hallucinations. Fröhlich 2016 and Brunelin 2012 also reported AHRS to assess auditory hallucination. Smith 2014 assessed the auditory hallucination by the PSYCHRATS hallucination scale, however we did not find the data for this scale, so we did not include the scale. Fröhlich 2016 used an adverse effects stimulation questionnaire for assessment of adverse effects. Smith 2014 used the MATRICS Consensus Cognitive Battery(MCCB) to explore the cognitive effects. Smith 2014 reported that 1 participant dropped out from Sham tDCS group, 3 participants dropped out from Active tDCS group. Other studies reported no missing data.
The heterogeneity of primary outcome was substantial (p=0.09, I2=59%), so we adopted a randomeffect model. There may be the following reasons for heterogeneity: 1) the location of the electrodes was different, especially in Smith 2014. 2) the sample of each study was small. 3) the stimulation frequency was different. We did not use subgroup analysis and sensitivity analysis because of the small number of articles that met inclusion criteria.
3.2 Primary outcomes
3.2.1 Auditory hallucination
Brunelin 2012 and Fröhlich 2016 measured auditory hallucinations using AHRS, however P3 in the PANSS was used in Smith 2014. In the Brunelin 2012, the mean (sd) AHRS scores were reduced from 28.3(4.1)to 19.9(5.8) in the active tDCS group (n=15) and from 27.2(6.9) to 25.1(7.7) in the sham tDCS group (n=15)after stimulation (d=1.58, p<0.001). The study also explored the maintenance effect after a month and three months, however, they did not provide the data.We did not include these data. In Fröhlich 2016, they did not find a signif i cant difference between active tDCS and sham tDCS. The mean (sd) AHRS scores reduced from 27.00(6.90) to 20.62(8.13) in the tDCS group, and from 26.69(6.30) to 18.15(10.77) in the sham group.Smith 2014 found no significant difference between active tDCS and sham tDCS.
The results showed in Figure 2 were considered heterogeneous (I2=59%, p=0.09). A random-effect model was used. The results indicated that auditory hallucinations in patients treated with tDCS were not signif i cantly relieved compared to non-tDCS.
3.3 Secondary outcomes
3.3.1 PANSS
In Brunelin 2012, Fröhlich 2016 and Smith 2014, other schizophrenia symptoms were assessed by PANSS.Mean(sd) total PANSS score was significantly different in Brunelin 2012, from 76.9(16.4) to 66.9(15.0) in the active tDCS group while it went from 82.8(15.4)to 80.5(12.0) in the sham tDCS group (d=0.98; 95%CI=0.22–1.73, p=0.01). In Fröhlich 2016 (mean(sd) PANSS scores: active tDCS group 73.1(12.9)to 73.38(14.24), sham tDCS group 66.92(17.17) to 63.85(14.25) and; Smith 2014 mean(sd) PANSS scores in the active tDCS group 65.14(18.38) to 65.71(16.96),sham tDCS 73.93(14.61) to 70.80(13.14). There were no signif i cant differences in either of these studies.
The results shown in Figure 3 were considered heterogeneous (I2=78%, p=0.008). A random-effect model was conducted. TDCS was not shown to be signif i cantly effective compared to non-tDCS.
3.3.2 Side effects
Brunelin 2012 did not report side effects. The study described tDCS as “an easy-to-use, low-cost stimulation tool with few side effects”. Smith 2014 assessed side effects with an experimenter administered open-ended questionnaire at each session. The results showed participants tolerated the treatment well. There were no significant differences in side effects between the two groups. Fröhlich 2016 administered an adverse effects stimulation questionnaire for their study. No differences between the tDCS and sham groups were found.
3.4 Risk of biases and level of evidence
The risks of biases are reported in Table 3. Two independent raters reached an agreement in all 7 items. All 3 studies claimed they used randomization,and concealment of allocation. However, only Smith 2014 described randomization and concealment of allocation in full detail, and Brunelin 2012 did not show randomization and concealment of allocation clearly,so the risk for these two items was unclear. Fröhlich 2016 did not report randomization clearly, therefore the risk for this item was unclear. We did not identify other potential sources of bias.
Based on the GRADE criteria, the quality of evidence for the primary outcome was low. Brunelin 2012 drew a conclusion that tDCS was useful for reduction of auditory hallucinations, however no significant difference was found between tDCS group and sham group in Fröhlich 2016 and Smith 2014. So the results of the studies were inconsistent. The amount of imprecision was high, because of the wide confidence interval. And only 3 studies met inclusion criteria,therefore the publication bias is also unclear.
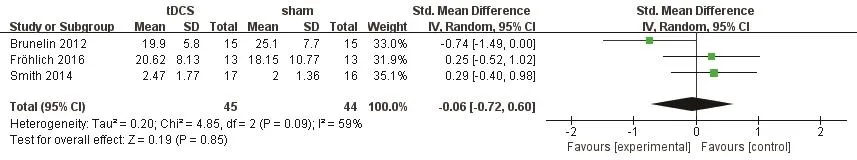
Figure 2. Comparison of Auditory scores at the end of the intervention between the tDCS group and the sham group

Figure 3. Comparison of mean PANSS scores at the end of the intervention between the tDCS group and the sham group

Table 3. Evaluation of risk of bias in the 3 included studies based on the seven items in the Cochrane Risk of Bias (RoB) tool
4. Discussion
4.1 Main findings
After a review of available databases we identified 3 RCTs. The number of published articles on this topic was small, therefore it is difficult to draw any strong conclusions. The scarcity of RCTs examining the effect of tDCS on auditory hallucinations in individuals with schizophrenia implies that there is much room for further research in this area. In addition, we did not identify any studies from China and other low-and middle-income countries. This is a clear indicator of the lack of high-quality research.
Only Brunelin 2012 showed signif i cant differences in their trial. It was the fi rst RCT on tDCS as a treatment for auditory hallucinations in schizophrenia.[34]There was a lack of effect for tDCS on hallucinations or symptoms in both Smith 2014 and Fröhlich 2016. The outcomes were inconsistent. So we cannot draw any strong conclusions from these studies.
In addition, using the GRADE criteria the overall level of evidence was classified as low, which means further study is likely to have an important impact on our estimate and change it. Large sample RCTs will be needed in the future.
4.2 Limitations
There were several limitations that should be considered: 1) Only 89 participants were in 3 studies,the samples were too small therefore statistical power was low. 2) The level of evidence was very low and the results were inconsistent, so the efficiency of tDCS for auditory hallucination could not be proven.3) Smith 2014 placed the electrode in different area from Brunelin 2012 and Fröhlich 2016, which may inf l uence results. 4) In these studies, they did not limit antipsychotic medications. Different antipsychotic medications may inf l uence the effect of tDCS differently.5) Data is lacking from low- and middle-income countries.
4.3 Implications
Given the current state of research on this topic it is clear that large sample RCTs on the effect of tDCS towards auditory hallucinations will be needed before any strong conclusions can be drawn. There are several case reports showing a positive effect from tDCS on auditory hallucinations[34]and some published reports also show tDCS alleviating negative symptoms of schizophrenia and improving cognitive functioning.[35]At the same time several studies also found that there was no improvement in cognitive functioning from tDCS and there were additional side effects. Though tDCS offers the possibility of being a promising treatment for auditory hallucinations, much evidence is still needed in favor of its efficacy.
Funding
This study was funded by Shanghai Shen Kang Hospital Development Center (SHDC12014111), the Science and Technology Commission of Shanghai Municipality(13z2260500, 14411961400) and the Shanghai Health System Leadership in Health Research Program(XBR2011005). The funding agencies played no role in the design, analysis, or write up of the results of the study.
Conflict of interest
The authors report no conflict of interest related to this manuscript.
Acknowledgment
We thank the reviewers of this analysis for their useful comments.
Author’s contributions
HL and CL designed the study. CL supervised the assessment and analysis. HL and YW conducted the search, selection, data extraction and analysis. JJ and WL checked the assessment of risk of Bias and GRADE.HL wrote the draft manuscript. All authors contributed to writing of this manuscript.
1. Slade PD, Bentall RP. Sensory Deception: A Scientific Analysis of Hallucination. Johns Hopkins University Press;1988
2. Falloon IR, Talbot RE. Persistent auditory hallucinations:coping mechanisms and implications for management.Psychol Med. 1981; 11(2): 329-339
3. Upthegrove R, Broome MR, Caldwell K, Ives J, Oyebode F,Wood SJ. Understanding auditory verbal hallucinations:A systematic review of current evidence. Acta Psychiatr Scand. 2016; 133(5): 352-367
4. Meltzer HY. Treatment of the neuroleptic-nonresponsive schizophrenic patient. Schizophr Bull. 1992; 18(3): 515-542
5. Slotema CW, Blom JD, de Weijer AD, Diederen KM,Goekoop R, Looijestijn J, et al. Can Low-frequency repetitive transcranial magnetic stimulation really relieve medication-resistant auditory verbal hallucinations?Negative results from a large randomized controlled trial.Biol Psychiatry. 2011; 69(5): 450-456. doi: http://dx.doi.org/10.1016/j.biopsych.2010.09.051
6. Andrade C. Transcranial direct current stimulation for refractory auditory hallucinations in schizophrenia. J Clin Psychiatry. 2013; 74(11): e1054-8. doi: http://dx.doi.org/10.4088/JCP.13f08826
7. Shin YI, Foerster A, Nitsche MA. Reprint of: Transcranial direct current stimulation (tDCS) - Application in neuropsychology. Neuropsychologia. 2015; 74: 74-95. doi:http://dx.doi.org/10.1016/j.neuropsychologia.2015.06.021
8. Liebetanz D, Nitsche MA, Tergau F, Paulus W.Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002; 125(Pt 10): 2238-2247
9. Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N,Antal A, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008; 1(3): 206-223. doi:http://dx.doi.org/10.1016/j.brs.2008.06.004
10. Nawani H, Bose A, Agarwal SM, Shivakumar V, Chhabra H,Subramaniam A, et al. Modulation of corollary discharge dysfunction in schizophrenia by tDCS: preliminary evidence. Brain Stimul. 2014; 7(3): 486-468. doi: http://dx.doi.org/10.1016/j.brs.2014.01.003
11. Shenoy S, Bose A, Chhabra H, Dinakaran D, Agarwal SM,Shivakumar V, et al. Transcranial direct current stimulation(tDCS) for auditory verbal hallucinations in schizophrenia during pregnancy: A case report. Brain Stimul. 2015; 8(1):163-164. doi: http://dx.doi.org/10.1016/j.brs.2014.10.013
12. Praharaj SK, Behere RV, Sharma PS. Cathodal transcranial direct current stimulation over left temporoparietal area for treatment-refractory delusions and auditory hallucinations in schizophrenia: A case study. J ECT. 2015; 31(4): 277-278.doi: http://dx.doi.org/10.1097/YCT.0000000000000237
13. Mondino M, Jardri R, Suaud-Chagny MF, Saoud M, Poulet E, Brunelin J. Effects of fronto-temporal transcranial direct current stimulation on auditory verbal hallucinations and resting-state functional connectivity of the left temporoparietal junction in patients with schizophrenia. Schizophr Bull. 2016; 42(2): 318-26. doi: http://dx.doi.org/10.1093/schbul/sbv114
14. Fitzgerald PB, McQueen S, Daskalakis ZJ, Hoy KE. A negative pilot study of daily bimodal transcranial direct current stimulation in schizophrenia. Brain Stimul. 2014; 7(6): 813-816. doi: http://dx.doi.org/10.1016/j.brs.2014.08.002
15. Yang YB, Xiao N, Li MY, Song WQ. [Comparation between transcraniai magnetic stimulation and transcranial direct current stimulation(review)]. Zhongguo Kang Fu Li Lun Yu Shi Jian. 2011; 17(12): 1131-1135. Chinese. doi: http://dx.chinadoi.cn/10.3969/j.issn.1006-9771.2011.12.010
16. Zeng BT, Wang JJ, Li CB. [Research situation of transcranial stimulation of direct current in the treatment of mental diseases]. Zhongguo Shen Jing Ji Bing Za Zhi. 2015; 41(4):250-253. Chinese. doi: http://dx.chinadoi.cn/10.3936/j.issn.1002-0152.2015.04.014
17. Agnew WF, McCreery DB. Considerations for safety in the use of extracranial stimulation for motor evoked potentials.Neurosurgery. 1987; 20(1): 143-147
18. Bose A, Sowmya S, Shenoy S, Agarwal SM, Chhabra H,Narayanaswamy JC, et al. Clinical utility of attentional salience in treatment of auditory verbal hallucinations in schizophrenia using transcranial direct current stimulation(tDCS). Schizophr Res. 2015; 164(1-3): 279-280. doi: http://dx.doi.org/10.1016/j.schres.2015.01.040
19. Palm U, Keeser D, Blautzik J, Pogarell O, Ertl-Wagner B,Kupka MJ, et al. Prefrontal transcranial direct current stimulation (tDCS) changes negative symptoms and functional connectivity MRI (fcMRI) in a single case of treatment-resistant schizophrenia. Schizophr Res.2013; 150(2): 583-585. doi: http://dx.doi.org/10.1016/j.schres.2013.08.043
20. Brunelin J, Mondino M, Jardri R, Poulet E. Effects of transcranial direct current stimulation on treatmentresistant pyschotic symptoms and brain functionalconnectivity in patients with schizophrenia. Schizophr Res.2014; 153: S70--S71. doi: http://dx.doi.org/10.1016/S0920-9964(14)70229-4
21. Venkatasubramanian G, Bose A, Agarwal SM, Chhabra H, Shivakumar V, Shenoy S, et al. Clinical Utility of Add-On tDCS in Schizophrenia: An Open Label Study of 50 Patients. Brain Stimul. 2015; 8(2): 370. doi: http://dx.doi.org/10.1016/j.brs.2015.01.189
22. Hoffman RE, Hawkins KA, Gueorguieva R, Boutros NN,Rachid F, Carroll K, et al. Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Arch Gen Psychiatry. 2003; 60(1):49-56
23. Hoffman RE, Gueorguieva R, Hawkins KA, Varanko M,Boutros NN, Wu Y, et al. Temporoparietal transcranial magnetic stimulation for auditory hallucinations: Safety,efficacy and moderators in a fifty patient sample. Biol Psychiatry. 2005; 58(2): 97-104. doi: http://dx.doi.org/10.1016/j.biopsych.2005.03.041
24. Kay SR, Flszbein A, Opfer LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull.1987; 13(2): 261
25. Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Br J Psychiatry Suppl. 1989; 7: 49-58
26. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011. Available from:www.handbook.cochrane.org
27. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008; 336(7650): 924-926. doi:http://dx.doi.org/10.1136/bmj.39489.470347.AD
28. Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. [The problem of therapeutic efficacy indices. 3.Comparison of the indices and their use]. Therapie. 1999;54(4): 405-411. French
29. Mondino M, Haesebaert F, Poulet E, Suaud-Chagny MF,Brunelin J. Fronto-temporal transcranial Direct Current Stimulation (tDCS) reduces source-monitoring def i cits and auditory hallucinations in patients with schizophrenia.Schizophr Res. 2015; 161(2-3): 515-516. doi: http://dx.doi.org/10.1016/j.schres.2014.10.054
30. Brunelin J, Mondino M, Jardri R, Poulet E. Effects of transcranial direct current stimulation on treatmentresistant pyschotic symptoms and brain functionalconnectivity in patients with schizophrenia. Schizophr Res.2014; 153(1): S70-S71. doi: http://dx.doi.org/10.1016/S0920-9964(14)70229-4
31. Brunelin J, Mondino M, Gassab L, Haesebaert F, Gaha L,Suaud-Chagny MF, et al. Examining transcranial directcurrent stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am J Psychiatry. 2012; 169(7): 719-724.doi: http://dx.doi.org/10.1176/appi.ajp.2012.11071091
32. Smith RC, Tobe RH, Boules S, Mattiuz S, Ravishankar H,Youssef M, et al. Effects of Transcranial Direct Current Stimulation (Tdcs) on Cognition, symptoms, and smoking in schizophrenia: a randomized controlled study. New York:ELSEVIER Science Inc; 2014. p. 20S--20S
33. Fröhlich F, Burrello TN, Mellin JM, Cordle AL, Lustenberger CM, Gilmore JH, et al. Exploratory study of oncedaily transcranial direct current stimulation (tDCS) as a treatment for auditory hallucinations in schizophrenia.Eur Psychiatry. 2016; 33: 54-60. doi: http://dx.doi.org/10.1016/j.eurpsy.2015.11.005
34. Mondino M, Brunelin J, Palm U, Brunoni AR, Poulet E,Fecteau S. Transcranial direct current stimulation for the treatment of refractory symptoms of schizophrenia.Current evidence and future directions. Curr Pharm Des.2015; 21(23): 3373-3383
35. Levasseur-Moreau J, Brunelin J, Fecteau S. Non-invasive brain stimulation can induce paradoxical facilitation. Are these neuroenhancements transferable and meaningful to security services? Front Hum Neurosci. 2013; 7: 449. doi:http://dx.doi.org/10.3389/fnhum.2013.00449

Haibin Li is currently a doctoral student at the Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine. His main research interests are psychiatry and evidence-based medicine.
经颅直流电刺激治疗精神分裂症幻听症状的有效性及安全性:一项系统综述研究
李海滨,王怡然,蒋江灵,李伟,李春波
经颅直流电刺激,幻听,精神分裂症
Background:Transcranial direct current stimulation (tDCS) is a non-invasion brain stimulation, which has been suggested as a safe and promising treatment for auditory hallucinations, however, no systematic review has been conducted to evaluate the effects of tDCS on auditory hallucinations (AH).Objective:To investigate the efficacy and safety of tDCS for auditory hallucinations among patients with schizophrenia.Methods:We searched relevant randomized controlled trials (RCTs) from PubMed, EMBASE, the Cochrane Library, Chinese National Knowledge Infrastructure, Chongqing VIP database for Chinese Technical Periodicals, WANFANG DATA, Chinese Biological Medical Literature Database, and Taiwan Electronic Periodical Services (TEPS) before February 13, 2016. Studies were selected based on pre-def i ned inclusion and exclusion criteria. The quality of each included study was assessed by the risk of bias table. The levels of evidence of primary outcomes were evaluated using GRADE criteria. Data synthesis was conducted using RevMan 5.3.Results:304 papers were screened. Finally, three studies with a combined sample size of 87 patients were included in the meta-analysis. Two studies were classif i ed as having ‘low risk of bias’, one study was classif i ed as having 'unclear'. Inconsistent results and the overall level of evidence of primary outcome was graded as‘low’.Conclusions:The sample sizes of the published studies were small and the results were inconsistent. We could not draw any strong conclusions from these trials. Further high quality RCTs with large sample sizes are needed to assess the efficacy of tDCS for auditory hallucinations in patients with schizophrenia.
[Shanghai Arch Psychiatry. 2016; 28(6): 301-308.
http://dx.doi.org/10.11919/j.issn.1002-0829.216121]
1Shanghai Key Laboratory of Psychotic Disorders, Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai, China
2Brain Science and Technology Research Center, Shanghai Jiao Tong University, Shanghai, China
*correspondence: Professor Chunbo Li. Mailing address: 600 South Wan Ping RD, Shanghai, China. Postcode: 200030. E-Mail: licb@smhc.org.cn
背景:经颅直流电刺激(transcranial direct current stimulation, tDCS)是一项非侵入的脑刺激技术。它被认为是一项安全而有前景的精神分裂症幻听症状的治疗方法。然而尚无系统综述对tDCS治疗幻听的效果进行评价。目标:探索tDCS对于有幻听精神分裂症患者的有效性及安全性。方法:我们从以下数据库搜索了相关的临床对照试验:PubMed、EMBASE、the Cochrane Library、中国知网、维普、万方、中国生物医学文献和台湾电子期刊服务网等数据库,时间截止于2016年2月13日。根据预先定好的纳入/排除标准筛选研究文献。纳入文献的质量经过偏倚风险评估,主要结局的证据等级水平采用GRADE评定,应用RevMan5.3进行数据分析。结果:总共检索到415篇文献。最终3篇文献纳入meta分析,合计样本量87。其中2篇的研究评价为“低偏倚风险”, 1个研究为“无法判断”。3个纳入研究均为小样本量、主要结局指标结果不一致,主要结局指标的证据等级被评为“低水平”。结论:已发表的文献样本量均小,而且主要结局指标的结果不一致。我们无法从这些研究中得出一致结论。关于tDCS对于精神分裂症患者幻听症状的疗效评价,我们需要进一步的大样本、高质量的临床随机对照试验来验证。
- 上海精神医学的其它文章
- Effect of repetitive transcranial magnetic stimulation on cigarette smoking in patients with schizophrenia
- Effect of clonazepam co-administered with clozapine on the serum clozapine and norclozapine concentration of patients with schizophrenia: A Retrospective Survey
- Eye movement indices in the study of depressive disorder
- Comparative analysis of results from a cognitive emotion regulation questionnaire between international students from West Asia and Xinjiang college students in China
- Why is diagnosing MDD challenging?
- Psychogenic blepharospasm: a diagnostic dilemma

