Effect of clonazepam co-administered with clozapine on the serum clozapine and norclozapine concentration of patients with schizophrenia: A Retrospective Survey
Ping JIANG, Zhiguang LIN, Yi JIN, Juanjuan REN, Hongmei LIU, Huiru CUI, Jijun WANG,Chunbo LI*
Effect of clonazepam co-administered with clozapine on the serum clozapine and norclozapine concentration of patients with schizophrenia: A Retrospective Survey
Ping JIANG1, Zhiguang LIN1, Yi JIN2, Juanjuan REN1, Hongmei LIU1, Huiru CUI1, Jijun WANG1,Chunbo LI1*
serum concentration; co-medication; clozapine; norclozapine ; clonazepam
1. Introduction
Clozapine (CLZ) is an atypical antipsychotic used in treating schizophrenia. It is generally accepted that CLZ is an effective medication for the treatment of schizophrenia. CLZ has no significant extrapyramidal reactions. It not only has effect on positive/negative symptoms, impulsive aggressive behavior, but also improves cognitive function in those with schizophrenia.The efficacy of CLZ has been shown to be about 30 to 60% for those with refractory schizophrenia.[1,2]
CLZ is absorbed quickly in the body, and if its serum concentration exceeds a certain range, significant side effects (leukopenia, fewer granulocytes) will occur.The potentially permanent movement disorder tardive dyskinesia occurs in about 5% schizophrenia patients with CLZ. Its mechanism of action is not entirely clear.Therefore, therapeutic drug monitoring is necessary to monitor serum CLZ concentration and to reduce side effects.[1]
CLZ is often combined with other psychotropic drugs according to the patient’s clinical condition, and therefore CLZ sometimes has drug–drug interactions with other psychotropic drugs in the treatment of comorbid physical and mental illnesses. Most pharmacokinetic interactions with CLZ and other psychotropic drugs occur at the metabolic level and usually involve changes in the activity of the major drug-metabolizing enzymes involved in their biotransformation.
Clonazepam (CLNAZ), as a tranquilizer of the benzodiazepine class and a broad-spectrum antiseizure drug, also can be used to treat sleep disorders,the movement disorder known as akathisia, reduce anxiety, and improve sleep quality.[3]CLNAZ binds to GABAAreceptors and increases the effect of the neurotransmitter GABA.
Depending on the circumstances, doctors sometimes prescribe CLZ alongt with CLNAZ. However research into whether co-medication with CLNAZ has an impact on the serum concentration of CLZ and its major metabolite norclozapine (N-CLZ) in schizophrenia is relatively scarce. Our retrospective survey is to investigate the effects of co-medication with CLNAZ and other factors on the serum levels of CLZ, andN-CLZ concentrations in 341 patients with schizophrenia. We aim to provide further evidence for the improvement of therapeutic drug monitoring and individualizing of dosages of CLZ for individuals with schizophrenia who are also on CLNAZ.[4]
2. Methods
2.1 Participants
A retrospective survey of serum CLZ and N-CLZ concentrations of in patients with schizophrenia was conducted at Shanghai Mental Health Centre, Shanghai,China from December 2007 to December 2010.This study was approved by the Institutional Ethics Committee of the Shanghai Mental Health Center (IORG Number: IORG0002202, FWA Number: FWA00003065).The Institutional Ethics Committee, Shanghai Mental Health Center also approved the consent procedure. As shown in fi gure 1, 387 patients were initially chosen and 341 were included in the final analysis.
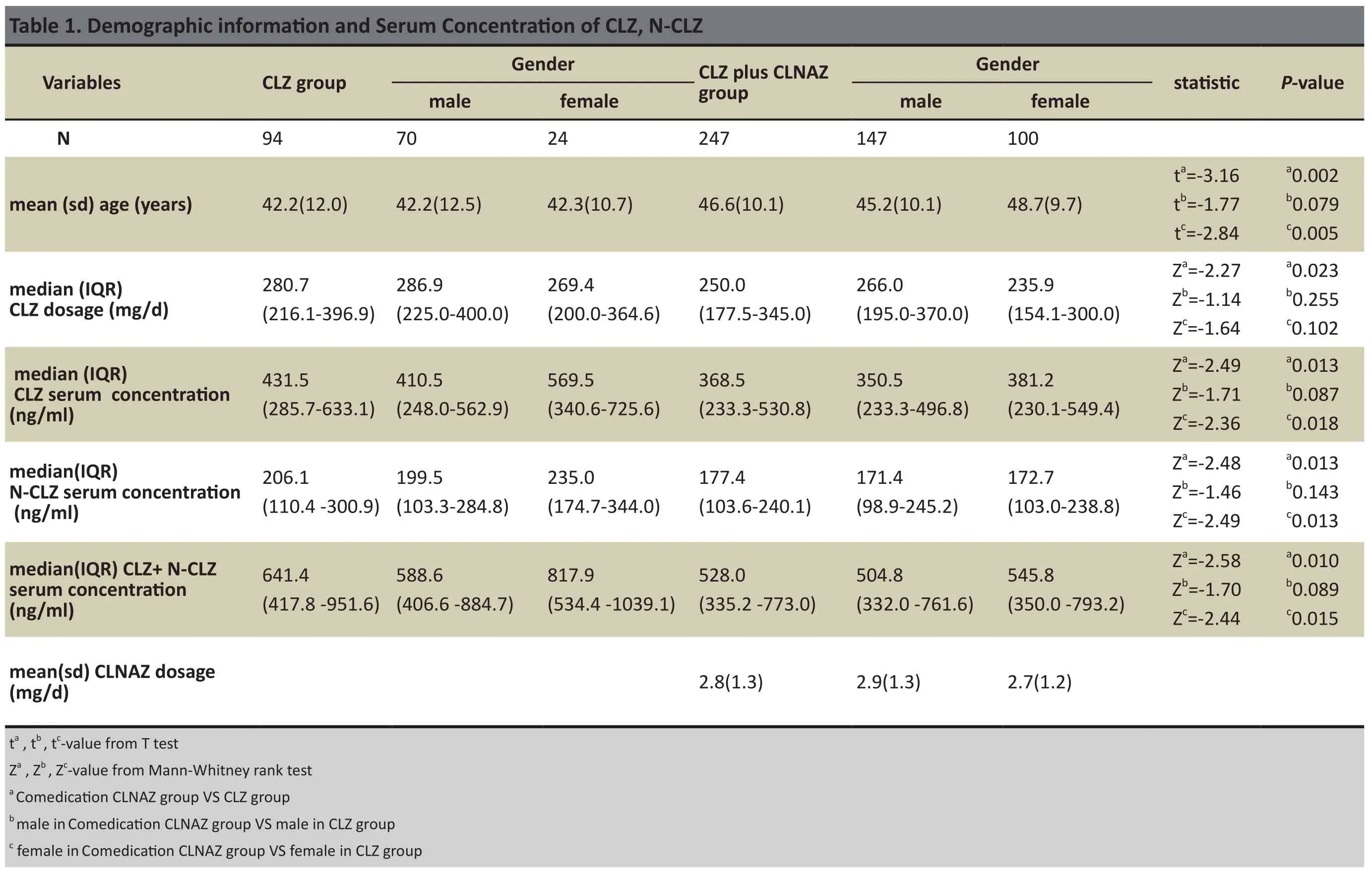
There were in total 341 patients (217 male,124 female; mean(sd) age of 45.4(10.8) years).We analyzed patients’ CLZ dosage, serum CLZ and N-CLZ concentrations and CLNAZ co-medication. We determined patients’ serum concentrations of CLZ and N-CLZ 3 times in the previous 3 months. The mean CLZ dose for 341 patients was 254.2 (189.1-354.2) mg/d. Within this group, 94 of the patients went through monotherapy using CLZ (CLZ group:70 males, 24 females; mean (sd) age, 42.2(12.0) years; mean CLZ dose 280.7 (216.1-396.9) mg/d).The other 247 patients were treated with CLNAZ in addition to CLZ (comedication CLNAZ group: 147 males, 100 females; mean(sd) age, 46.6(10.1) years; mean CLZ dose, 250.0(177.5-345.0) mg/d). See table 1 for demographic information.In this retrospective survey, all data (serum CLZ and N-CLZ concentrations, demographic information) were collected from medical archives.
The inpatients who had been taking CLZ for at least two weeks had serum extracted from the cubital vein 5ml from 6-7 pm (11-12 hours after the last medication).Serum samples were analyzed by high performance liquid chromatography (HPLC) to determine serum concentration of CLZ and N-CLZ, its metabolite.
2.2 Sample process
A solution system composed of 0.5mL serum, 0.1mL alcohol of 2 moL/L NaOH, 15µl alcohol of 10 ng/µL diazepam (internal standard) and 3mL ethylether was used. By vortexing for 1 min, the organic layer was separated into the tapered tube, and was evaporated at 60°C. The residue was dissolved into a 100µL flow.Agilent 1200 HPLC analyzer was used in this study.Conditions of chromatography: Chromatographic column was the C18 column. The mobile phase was composed of methanol: glacial acetic acid :tetramethylethylenediamine : water (2800:10:9:1440).The detection wavelength was 254nm. CLZ, N-CLZ and diazepam (internal standard) were purchased from Sigma Company.
2.3 Statistics
Data was analyzed by stepwise regression using SPSS 21.0 software. Independent variables included the following: Gender(X1)(male=0, female=1), Age(X2),CLZ dosage(X3), Co-medication dosage of CLNAZ (X4)(No=0,Yes=1). Normally distributed data (e.g. age, comedication CLNAZ dosage) was expressed as mean(sd); non-normally distributed data (e.g. CLZ dosages,CLZ serum concentration, N-CLZ serum concentration)was expressed as the median (IQR25-IQR75). The t-test was used for comparing 2 groups’ normally distributed data and Mann-Whitney rank sum test was used for comparing the 2 groups non-normally distributed data.Statistical signif i cance level was set at p<0.05.
3. Results
3.1 Demographic Data
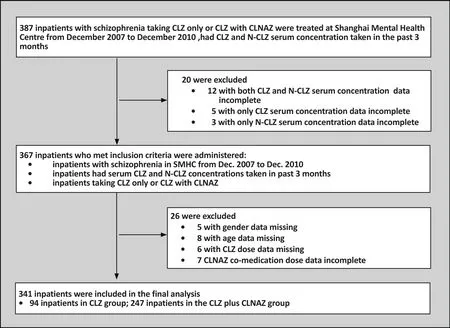
Figure 1. Flow chart of the study
Of the 341 patients included for analysis in this study,27.6% were taking CLZ (n=94:M70 and F24; mean (sd)age:42.2(12.0) years; median CLZ dosage: 280.7(216.1-396.9) mg/d). Patients who were taking CLNAZ in addition to CLZ accounted for 72.4% of all patients in this analysis (n=247:M147 and F100; mean (sd) age:46.6(10.1) years; median CLZ dosage: 250.0(177.5-345.0) mg/d). See table 1.
3.2 CLZ group and CLZ plus CLNAZ group
The mean (sd) age of the CLZ plus CLNAZ group(43.5[11.1] years) was significantly higher than the CLZ group’s (42.1[12.1] years) ( p=0.002). The median CLZ dosage (250.0 (177.5-345.0) mg/d) in the CLZ plus CLNAZ group was signif i cantly lower than that of the CLZ group (280.7(216.1-396.9) mg/d) (p= 0.023). Median CLZ and N-CLZ serum concentrations in the CLZ plus CLNAZ group (368.5(233.3-530.8) ng/ml,177.4(103.6-240.1) ng/ml) were significanty lower than in the CLZ group (431.5(285.7-633.1) ng/ml, 206.1(110.4 -300.9)ng/ml)(p=0.013; p=0.013) respectively. Median CLZ+ N-CLZ serum concentrations in the CLZ plus CLNAZ group (528.0(335.2-773.0) ng/ml) were significantly lower than those of the CLZ group (641.4(417.8-951.6)ng/ml)( P = 0.010), as shown in Table 1.
3.3 Differences by gender in the CLZ plus CLNAZ group
The mean (sd) age of those in the CLZ plus CLNAZ group (48.7[9.7] years) was significantly higher than that of the CLZ only group (42.3[10.7] years) (p=0.005).Median CLZ and N-CLZ serum concentrations of females in the CLZ plus CLNAZ group (381.2[230.1-549.4] ng/ml,172.7[103.0-238.8] ng/ml) were significantly lower than those of the CLZ group (569.5[340.6-725.6] ng/ml, 235.0 [174.7-344.0] ng/ml)(p=0.018; p=0.013)respectively. CLZ + N-CLZ serum concentrations of females in the CLZ plus CLNAZ group (545.8[350.0-793.2]ng/ml) was significantly lower than that of the CLZ group (817.9[534.4-1039.1] ng/ml)(p=0.010), as shown in Table 1.
?
3.4 Stepwise Regression Analyses
SPSS 21.0 was used to perform stepwise regression analysis. We gradually removed or selected factors affecting the serum concentration of CLZ and N-CLZ.The results showed: Gender (X1), CLZ dosage (X3)and Co-medication of CLNAZ with CLZ (X4), these three factors were significantly associated with CLZ serum concentration (p=0.009, p<0.001, p=0.010). Age(X2) was not significantly associated with CLZ serum concentration (p=0.625). CLZ serum concentration was set as the dependent variable (Y) and the regression equation was: y=366.25+71.01 X1+0.39 X3-74.51 X4. Therefore, the CLZ serum concentration (Y) was negatively correlated with co-medication of CLNAZ with CLZ (X4), and positively correlated with gender (X1) and CLZ dosage (X3). See table 2.
CLZ dosage (X3) and co-medication with CLNAZ (X4)were two factors that were signif i cantly associated with N-CLZ serum concentration (p<0.001, p=0.020). Gender(X1) and age (X2) were not signif i cantly associated with N-CLZ serum concentration (p=0.196, p=0.894). N-CLZ serum concentration was set as the dependent variable(Y) and the regression equation was: Y=156.85 + 0.20 X3- 35.43 X4. That means that co-medication with CLNAZ is associated with a lower N-CLZ serum concentration,while a higher CLZ dosage is associated with a higher N-CLZ serum concentration (see table 3).
4. Discussion
4.1 Main findings
In this study, we retrospectively analyzed the serum CLZ and N-CLZ concentrations, co-medication with CLNAZ,and demographic data for 341 Chinese patients with schizophrenia. We further explored the effects of CLNAZ co-medication and other related factors on serum CLZ, N -CLZ concentration in Chinese patients with schizophrenia. In our retrospectively study, serum CLZ, N-CLZ and CLZ + N-CLZ concentrations in the CLZ plus co-medication with CLNAZ group were both statistically signif i cant lower than that of the CLZ group respectively.The regression analysis results showed that serum CLZ and N-CLZ concentration were significantly different for those patients who were being co-medicated withCLNAZ and that co-medication with CLNAZ is negatively related to serum CLZ and N-CLZ concentration.
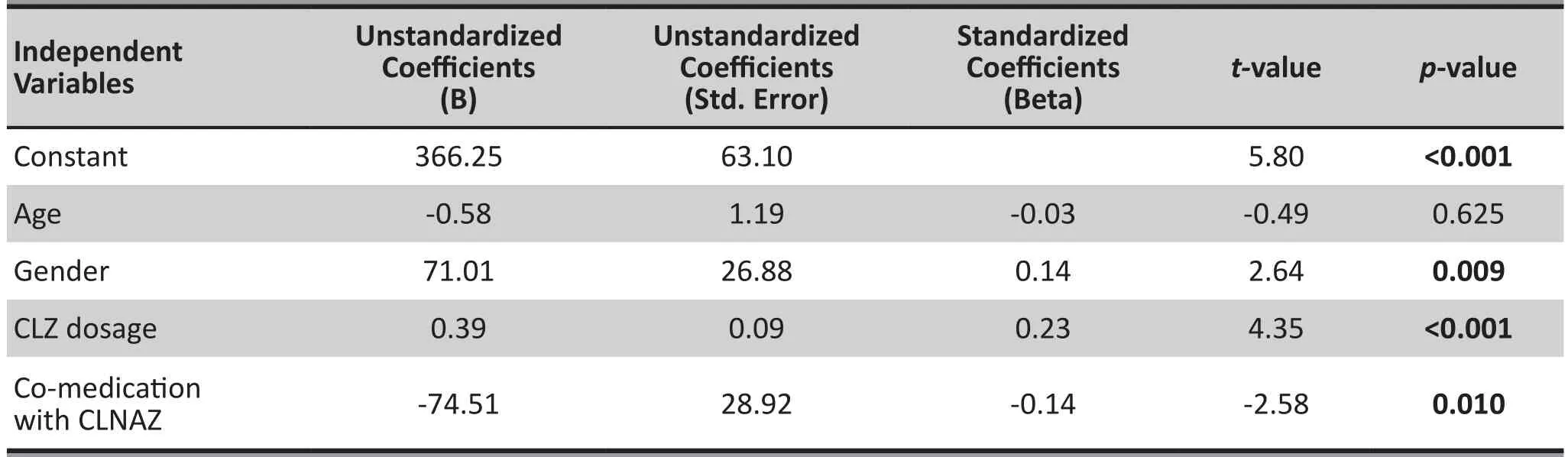
Table 2. Stepwise regression analysisa for factors related to CLZ serum concentration in patients with schizophrenia (n=341)
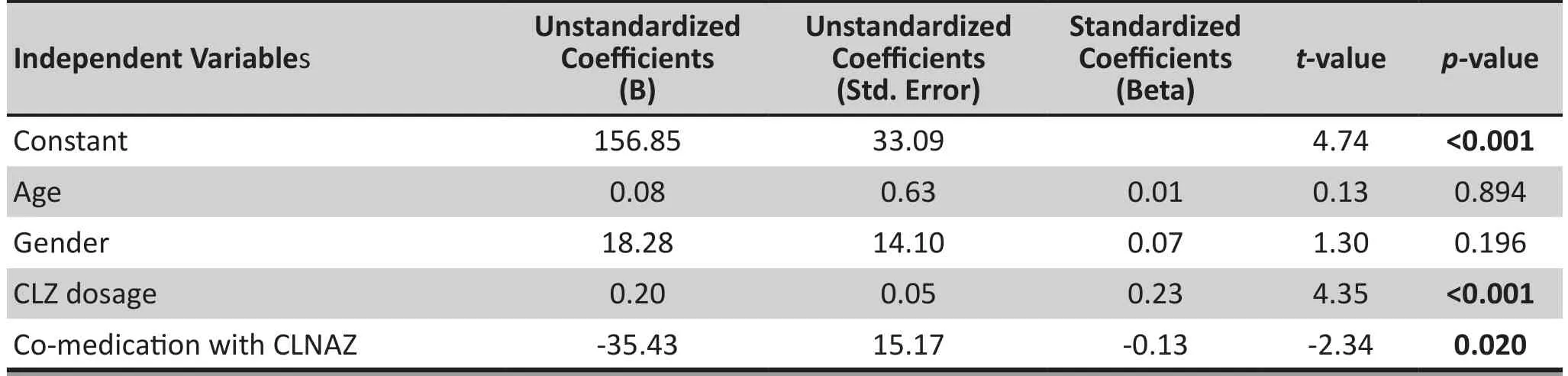
Table 3. Stepwise regression analysisa for factors related to N-CLZ serum concentration in patients with schizophrenia (n=341)
About 23% of antipsychotic-treated (more than 1 year) schizophrenic patients have Tardive Dyskinesia(TD) symptoms. TD is an involuntary movement disorder characterized by complex repetitive neck, buccolingual and sometimes generalized stereotypic hyperkinetic features. Moreover, TD may even be life-threatening owing to such complications as dysphagia, respiratory disturbances, and renal failure from dehydration or rhabdomyolysis caused by muscle breakdown from increased motor activity. The mechanism of TD is still unclear,[5]therefore this condition is difficult to treat and there is no single drug considered 100% effective in rapidly reducing TD symptoms. According to the patient’s condition, CLZ and CLNAZ are often concomitant medications, which means both antipsychotic drugs CLZ and CLNAZ with advance-rapid onset, effective sedation and long half-life will have effect. Kimiagar concurrently used three drugs (CLZ, CLNAZ, tetrabenazine) to treat six severe TD patients with psychotic disorders (5 had schizophrenia and 1 had bipolar disorder with psychotic features). After treatment, there were fewer incidences of patients having convulsions. Four patients’ symptoms markedly improved after 1 week, and all six patients’ TD symptoms disappeared completely after 4 weeks.[6,7]
There are several possible mechanisms for comedication of CLZ and CLNAZ improving the symptoms of TD for those with schizophrenia. Patients whose NE activity is increased via the atypical antipsychotic CLZ inhibiting norepinephrine (NE) systems or patients whose presynaptic membrane NE receptor activity is increased have a tendency to have TD. CLNAZ belongs to the benzodiazepine class of drugs. Benzodiazepine receptors with gamma aminobutyric acid (GABA)receptors constitute GABA-benzodiazepine-Cl-channel complex, and CLNAZ strengthens GABA energy nerve conduction in the GABA-benzodiazepine-Cl-channel complex in TD patients or patients have a tendency to have TD. Therefore, in a way, co-medication with CLNAZ increases serum CLZ concentration.[8,9]
Excessive serum CLZ concentration may trigger a series of toxic, even life-threatening physical reactions.Clinicians will lower CLZ dosages to maintain serum CLZ and N-CLZ concentration within a safe range that avoids toxicity side effects.[8,9]This may explain why both CLZ dosage and serum CLZ,N-CLZ,CLZ+N-CLZ concentrations in co-medication with CLNAZ group were significantly lower than those of the CLZ group. Meanwhile, this may at least partly be the reason that co-medication with CLNAZ was negatively correlated with serum concentration of CLZ and N-CLZ. Another result shows that CLZ dose was signif i cantly and positively correlated with CLZ and N-CLZ serum concentration in both the CLZ only group and co-medication with CLNAZ group.This result is consistent with a study by Chang and colleagues. CLZ serum concentration in CLZ group,431.5(285.7-633.1) ng/ml, was just within the effective blood concentration range (300-600) ng/ml. The serum CLZ concentration in the co-medication with CLNAZ group is 368.5(233.3-530.8) ng/ml, was slightly lower that the effective blood concentration range. This showed that the patients included in this study had relatively safe and effective use of CLZ.[9,10]
Female CLZ serum concentration was relatively higher in this study, which is consistent with Lin’s study.[11]The main metabolic pathway of CLZ in vivo is demethylation. CLZ is catalyzed into norclozapine (N-CLZ)in the liver by CYP1A2 and other enzymes. CYP1A2 activity directly affects serum concentration of CLZ and N-CLZ, and indirectly affects the improvement of psychopathological symptoms. Gender and other factors(e.g. smoking) may have lead to the female patients’serum CLZ concentration being higher than those seen among the male patients in this study. There can be up to a 70 times greater difference in CYP1A2 between individuals which may partly account for the differences in serum CLZ concentration (max difference was 3.4 higher) among participants in this study.
An interesting result is all the serum CLZ , N-CLZ,CLZ+N-CLZ concentrations of females in the comedication with CLNAZ group were significantly lower than those of the CLZ group while CLZ dosage was not significantly different. There is a possibility that CLZ metabolism was improved by co-medication with CLNAZ to a greater extent in female patients than male patients. However this question requires further investigation.
4.2 Limitations
In this retrospective study, the co-medication group and control group had different sample sizes, and the factors for state, type, course and complications of schizophrenia were not controlled. Given these limitations, the conclusions need to be further verified. In the future, prospective experiments should be designed with this consideration in mind. The sample size and the state, type, course and complications of schizophrenia in groups need to be controlled. Future studies should also have scales to evaluate patients’ outcomes.
4.3 Implications
CLZ co-medicated with CLNAZ is an effective treatment method for patients with schizophrenia. Therapeutic drug monitoring of CLZ in patients with schizophrenia is very important. CLZ doses should be adjusted in a timely manner to achieve the best therapeutic effect when comedication with CLNAZ is being considered.[5,9,12,13]
Funding
Funding for this study was provided by: The National Natural Science Fund (No.81171267); National Basic Research Program of China (No.2007CB512306);Shanghai Key Laboratory of Psychotic Disorders(No.13dz2260500); Shanghai Program for Fostering ScientificLeaders in Health (No. XBR2011005).
Conflict of interest statement
The authors report no conflict of interest related to this study
Ethical approval
The study has been approved by The Institutional Ethics Committee, Shanghai Mental Health Center (IORG Number: IORG0002202, FWA Number: FWA00003065).
Authors’ contributions
Jiang and Li conceived the design and drafted the manuscript. Lin and Ren took part in the data collection by HPLC. Jin and Cui performed the statistical analyses.Liu and Wang helped to draft the manuscript. All authors read and approved the final manuscript. We thank Jiayi Cui who provided language assistance.
1. Mercolini L, Grillo M, Bartoletti C, Boncompagni G, Raggi MA. Simultaneous analysis of classical neuroleptics,atypical antipsychotics and their metabolites in human plasma. Anal Bioanal Chem. 2007; 388: 235-243. doi:http://dx.doi.org/10.1007/s00216-007-1195-1
2. Couchman L, Morgan PE, Spencer EP, Johnston A,Flanagan RJ. Plasma clozapine and norclozapine in patients prescribed different brands of clozapine (Clozaril,Denzapine, and Zaponex). Therapeutic drug monitoring.2010; 32: 624-627. doi: http://dx.doi.org/10.1097/FTD.0b013e3181f0a1a2
3. Faulkner MA, Singh SP. Neurogenetic disorders and treatment of associated seizures. Pharmacotherapy 2013;33:330-343. doi: http://dx.doi.org/10.1002/phar.1201
4. Stanworth D, Hunt NC, Flanagan RJ. Clozapine--a dangerous drug in a clozapine-naive subject. Forensic Sci Int. 2012; 214: e23-25.doi: http://dx.doi.org/10.1016/j.forsciint.2011.07.032
5. Rana AQ, Chaudry ZM, Blanchet PJ. New and emerging treatments for symptomatic tardive dyskinesia. Drug Des Devel Ther. 2013; 7: 1329-1340. doi: http://dx.doi.org/10.2147/DDDT.S32328
6. Kimiagar I, Dobronevsky E, Prokhorov T, Miniovitz A,Rabey JM. Rapid improvement of tardive dyskinesia with tetrabenazine, clonazepam and clozapine combined: a naturalistic long-term follow-up study. J Neurol. 2012; 259:660-664. doi: http://dx.doi.org/10.1007/s00415-011-6235-2
7. Sheng X, Hua K, Yan H, Luo L. [The relationship of clozapine plasma concentrations with dose, efficacy, and side reaction in schizophrenia]. Zhongguo Shen Jing Jing Shen Ji Bing Yan Jiu. 1990; 16(2): 90-92. Chinese
8. Nielsen J, Damkier P, Lublin H, Taylor D. Optimizing clozapine treatment. Acta Psychiatr Scand. 2011;123: 411-422. doi: http://dx.doi.org/10.1111/j.1600-0447.2011.01710.x
9. Rajkumar AP, Poonkuzhali B, Kuruvilla A, Jacob M,Jacob KS. Clinical predictors of serum clozapine levels in patients with treatment-resistant schizophrenia. Int Clin Psychopharmacol. 2013; 28: 50-56. doi: http://dx.doi.org/10.1097/YIC.0b013e32835ac9da
10. Castberg I, Skogvoll E, Spigset O. Quetiapine and drug interactions: evidence from a routine therapeutic drug monitoring service. J Clin Psychiatry. 2007; 68: 1540-1545
11. Lin Z, Weng Y, Zhuang D, Zhu L, Jiang J, Li H. [The plasma concentration of clozapine and its correlated]. Shanghai Arch Psychiatry. 2001; 4: 210-213. Chinese
12. Domingues DS, Pinto MA, de Souza ID, Hallak JE, Crippa JA, Queiroz ME. Determination of drugs in plasma samples by high-performance liquid chromatography-tandem mass spectrometry for therapeutic drug monitoring of schizophrenic patients. J Anal Toxicol. 2016; 40: 28-36. doi:http://dx.doi.org/10.1093/jat/bkv107
13. Pinninti NR, Faden J, Adityanjee A. Are Second-Generation Antipsychotics Useful in Tardive Dystonia? Clin Neuropharmacol. 2015; 38: 183-197. doi: http://dx.doi.org/10.1097/WNF.0000000000000106

Dr. Ping Jiang obtained a PhD from the Department of Biochemistry and Molecular Biology, the Second Military Medical University in 2005. She has been working in the Department of Biochemical Pharmacology of Shanghai Mental Health Center since December 2010. Her current research interest is monitoring the concentration of psychotropic drugs in the blood.
联用氯硝西泮对精神分裂症患者氯氮平、N-去甲氯氮平血药浓度影响的回顾性研究
蒋平,林治光,金一,任娟娟,刘红梅,崔慧茹,王继军,李春波
血药浓度;联合用药;氯氮平;去甲氯氮平;氯硝西泮
Background:For patients with schizophrenia clozapine (CLZ) is sometimes co-prescribed with clonazepam(CLNAZ). However, the impact of administration of CLZ along with CLNAZ on the serum concentration of CLZ and its major metabolite N-CLZ in schizophrenia is not well understood.Aim:To investigate the effects of CLNAZ co-medication, patient gender, age and CLZ dosage on serum concentration of CLZ and norclozapine (N-CLZ) in individuals with schizophrenia.Methods:Serum CLZ and N-CLZ concentrations and demographic data were retrospectively analyzed for 341 patients with schizophrenia. We used SPSS 21.0 to perform stepwise regression to analyze the concentration data and demographics. Variables included in the analysis were: serum concentration of CLZ, N-CLZ, and CLZ dosage, gender, age and CLNAZ co-medication.Results:(1) CLNAZ co-medication signif i cantly affects serum CLZ and N-CLZ concentration in schizophrenics(p=0.010, p=0.020); (2) CLNAZ co-medication, gender and CLZ dosage significantly affect serum CLZ concentration in patients with schizophrenia (p=0.010, p=0.009, p<0.001). Serum CLZ concentration is negatively correlated with CLNAZ co-medication, and is positively correlated with being female and CLZ dosage; (3) CLNAZ co-medication and CLZ dosage were signif i cantly related to serum N-CLZ concentration in participants (p=0.020, p<0.001). Serum N-CLZ concentration was negatively correlated with CLNAZ comedication, and positively correlated with CLZ dosage.Conclusion:CLNAZ co-medication is associated with changes in serum CLZ and N-CLZ concentration. It is indicated that gender and/or CLZ dosage are also related to serum CLZ and N-CLZ concentration. Therapeutic drug monitoring and dosage regulation of CLZ should be performed for patients with schizophrenia who are also taking CLNAZ to maintain a safe and effective serum concentration of CLZ and N-CLZ.
[Shanghai Arch Psychiatry. 2016; 28(6): 318-325.
http://dx.doi.org/10.11919/j.issn.1002-0829.216066]
1Shanghai Key Laboratory of Psychotic Disorders,Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai, China;Postcode: 200030
2Institute of Psychology, Chinese Academy of Sciences, Beijing, China; Postcode: 100101
*correspondence: Professor Li Chunbo; Mailing address: Shanghai Institute of Mental Health,Shanghai Jiao Tong University School of Medicine,600 Wan Ping Nan Road, Shanghai P.R.China; Postcode: 200030; E-mail: chunbo_li@163.com
背景:对精神分裂症患者,氯氮平(CLZ)有时是与氯硝西泮(CLNAZ)联合使用的。然而,联用氯硝西泮(CLNAZ)和氯氮平(CLZ)对氯氮平和主要代谢产物N-去甲氯氮平(N-CLZ)的血清浓度的影响尚不清楚。目标:调查联用氯硝西泮(CLNAZ)对精神分裂症患者氯氮平(CLZ)血药浓度的影响。方法:应用高效液相色谱技术(HPLC)测定341例服用CLZ的住院精神分裂症患者CLZ及N-CLZ的血药浓度;并运用逐步回归法分析年龄、性别、CLZ剂量、联用CLNAZ等因素对于CLZ血药浓度的影响。结果:(1)联用CLNAZ可影响CLZ血药浓度和N-CLZ血药浓度(p=0.010, p=0.020);(2)性别、CLZ剂量、联用CLNAZ三因素可影响CLZ血药浓度(p=0.010,p=0.009, p<0.001)。CLZ血药浓度与女性、CLZ剂量呈正相关;与联用CLNAZ呈负相关;(3)CLZ剂量、联用CLNAZ两因素可影响N-CLZ血药浓度(p=0.020,p<0.001)。N-CLZ血药浓度与CLZ剂量呈正相关;与联用CLNAZ呈负相关。结论:联用CLNAZ可对精神分裂症患者的CLZ、N-CLZ血药浓度产生影响;而性别和CLZ剂量等因素对精神分裂症患者的CLZ、N-CLZ血药浓度亦有一定程度的影响。临床上应加强联用氯硝西泮的精神分裂症患者CLZ血药浓度监测,及时调整氯氮平剂量。
- 上海精神医学的其它文章
- Effects of transcranial direct current stimulation (tDCS) for auditory hallucinations: A systematic review
- Effect of repetitive transcranial magnetic stimulation on cigarette smoking in patients with schizophrenia
- Eye movement indices in the study of depressive disorder
- Comparative analysis of results from a cognitive emotion regulation questionnaire between international students from West Asia and Xinjiang college students in China
- Why is diagnosing MDD challenging?
- Psychogenic blepharospasm: a diagnostic dilemma

