环糊精对阿斯巴甜的甜感增强作用及二者相互作用热力学
朱甜甜 徐淑臻 葛炳强 陈忠秀
(浙江工商大学食品与生物工程学院,杭州310018)
环糊精对阿斯巴甜的甜感增强作用及二者相互作用热力学
朱甜甜 徐淑臻 葛炳强 陈忠秀*
(浙江工商大学食品与生物工程学院,杭州310018)
目前环糊精(CD)对阿斯巴甜(ASP)甜感强度的影响研究主要集中在环糊精对阿斯巴甜的稳定性研究。我们认为CD对ASP甜感强度的提升与其和ASP的结合常数有一定的关系。本文选择了五种环糊精,α-环糊精(α-CD)、β-环糊精(β-CD)、γ-环糊精(γ-CD)、羟丙基-β-环糊精(HP-β-CD)、甲基-β-环糊精(Met-β-CD),研究了这些环糊精存在下ASP的感官甜度的变化及二者的相互作用。结果表明,β-CD可以明显提升ASP的甜感强度。等温滴定量热(ITC)和荧光光谱对ASP与CDs结合过程亲和力的研究表明,ASP与β-CD的结合是自发的,并且具有最大的结合常数。差示扫描量热(DSC)、核磁共振(1H NMR)以及傅里叶变换红外(FT-IR)揭示了其结合过程的机制。本研究对理解甜味剂甜感强度与热力学结合常数的关系具有重要的意义,也为基于结合常数筛选风味保持剂的方法提供有益的基础。
阿斯巴甜;结合常数;热力学;甜感强度;环糊精
1 Introduction
Aspartame(α-L-aspartyl-L-phenylalanine l-methyl ester)is a high-intensity sweetener,commonly used in many diverse beverages and pharmaceuticals,especially soft drinks1.For some time past it has been widely accepted that ingestion of aspartame was non-genotoxicin vitro and in vivo studies2.However,the safety of aspartame,has long been in dispute,before,during,and after its approval by the Food and Drug Administration(FDA).Upon prolonged exposure to high temperatures and storage,part of the aspartame molecule may undergo breakdown leading to a loss of sweet taste.This limits its adequate use in applications3.On the other hand,one of the main safety concerns just derives from the products of the hydrolysis of aspartame,which comprise phenylalanine,aspartic acid and methanol,and from the degradation products such as 5-benzyl-3,6-dioxo-2-piperazine acetic acid and β-aspartame4.
There are several ways to solve the instability of aspartame. Decomposition of aspartame was found reduced in acid medium5. Accordingly,aspartame is stable in whey lemon beverage under refrigerated condition for 15 days6.Interestingly,at the same pH and buffer concentration,aspartame is more stable in citrate than in phosphate buffer.The reason lies in that buffering components can participate in chemical reactions depending upon their structural characteristics7.And,the stability of aspartame can be improved when it was parcelled with gluconic acid lactone8. Microencapsulating aspartame by double emulsion with sunflower oil,gum arabic and gelatin,followed by complex coacervation, was another way to avoid decomposition and to prolong its sweetness9.
Cyclodextrin(CD)is a cyclic oligosaccharide formed by glucopyranose joined by α-(1,4)bonds.It has a hydrophobic internal cavity and hydrophilic external circumference,forming a truncatedcone10.The most interesting characteristics of the CDs are their capacity of forming inclusion complexes with a great number of organic or inorganic compounds11.CDs are often used for encapsulation of lipophilic food ingredients to improve the stability of flavors,vitamins,colorants and unsaturated fats,leading to extended product shelf-life12,13.Therefore,cyclodextrins are one of the potential candidates to improve the stability of aspartame due to their complexation ability.It was reported that CDs can reduce the decomposition of aspartame solution14.Moreover, researchers tried to find suitable structure from its kinds of derivatives which showed stronger interaction with aspartame15,16. However,the relationship between the binding affinity of aspartame with CDs and the change of sensory sweetness intensity remains unknown.
It is known that thermodynamics governs the process of biomolecular recognition.Characterizing the thermodynamics of the binding will contribute to a reliable flavor-keeping design process. In our previous study17-21,we were interested in exploring the thermodynamic basis of the change of sweetness intensity.In this paper,we continue to conduct the sensory evaluation of aspartame in the presence of different CDs.Thermodynamic binding constants was further measured,with the aim to find if the CD with strong binding affinity to aspartame could protect the stability of aspartame well,and therefore to keep or enhance its sweetness intensity.The orientation of aspartame in CD cavity and the underlying mechanism were also disclosed by complimentary methods.
2 Materials and methods
2.1Materials
Aspartame(≥98%),α-cyclodextrin(≥98%),β-cyclodextrin (98%),γ-cyclodextrin(98%),methyl-β-cyclodextrin(98%),2-hydroxypropyl-β-cyclodextrin(97%),potassium bromide(≥99.5%),sodium chloride(≥99.5%)were all purchased from Sigma-Aldrich.Deionized water was used for preparation of all solutions.
2.2Methods
2.2.1 Sensory evaluation
Sensory evaluation was conducted to estimate the sweet intensity level of aspartame in different CDs.The concentrations of aspartame were selected to be approximately isointense with 3%, 6%,and 12%(w,mass fraction)sucrose solution at 25°C19.A series of concentrations of aspartame(0.125,0.250,0.500 g·L-1) in 0.1%different derivatives of CDs were fully mixed,stirring for 24 h before evaluation.Apanel of ten subjects(five males and five females,aged 22-24 years)was recruited from the graduate students of the College of Food&Biology Engineering,Zhejiang Gongshang University.Paired Comparison method was used in the experiment.Pure solution of aspartame was regarded as standard. Aspartame mixed with CDs were used as testing samples.The sweet intensity of solution was recorded in scores(0-100).All panelists were calibrated by giving them a pure solution of aspartame which was designated as 50 scores.Sample solution(5 mL)was put into a plastic beaker and all the samples were put in a predetermined sequence.Assessors were asked to place the test compound into the mouth for 2 s,and then expectorate with the mouth rinsed with distilled water 10 s of break was needed before each test.Each sample was assessed on three separate occasions. Data from 30 samples were averaged and the error was estimated.
2.2.2 Isothermal titration calorimetry(ITC)
An isothermal titration calorimeter(VP-ITC,Microcal Inc., Northampton,MA)was used to measure enthalpies from titration of aspartame into CDs at 25°C.The experiment cell(1.43 mL) was loaded with CDs(0.1 mmol·L-1)and the syringe with aspartame(30 mmol·L-1).The reference cell was filled with deionized water.All of the solutions were prepared in deionized water and were degassed before use.The first injection was set to 1µL,and then titration was performed in 28 injections of 10 μL at intervals of 360 s.The duration of each injection was 2 s,and the solution in the titration cell was stirred at a speed of 307 r·min-1throughout the experiment.Control experiments included the titration of aspartame into water and water into CD.The first titration point was removed from the data before fitting.The data were fitted by subtracting the dilution heat of aspartame in thecontrol experiment,which were found small and were negligible. Raw data(see Fig.S1 in Supporting Information)were obtained as a plot of heating rate against time and then were integrated to obtain a plot of observed enthalpy change per mole of injected CDs(ΔH,kJ·mol-1)against molar ratio(aspartame/CDs).Equilibrium binding constants and enthalpies were determined by fitting the data with a sequential binding site model by MicroCal Origin 7.0 software using a nonlinear least-squares approach (Levenberg-Marquardt algorithm).
2.2.3 Steady-state fluorescence spectra
Fluorescence measurements were performed on a Hitachi F-7000 fluorescence spectrophotometer.In the measurement of the intrinsic fluorescence of aspartame solution,the excitation and emission slits were both fixed at 5.0 nm.The excitation wavelength was set at 210 nm after the fluorescence 3D scanning experiment and the emission spectra were collected from 200 to 800 nm.The scan rate was set at 6000 nm·min-1.The fluorescence spectra of aspartame(0.05 mmol·L-1)with CDs at different concentrations(0,0.5,1,2,4,and 8 mmol·L-1,respectively)were measured at 25°C.
2.2.4 Preparation of the inclusion complex
Aspartame(14.7 g,0.05 mol)and β-CD(56.75 g,0.05 mol) were mixed in 10 mL of distilled water,stirred for 24 h at 35°C and then filtered.After cooling to room temperature,the mixture was stored at 4°C for 24 h.
2.2.5 Preparation of the physical mixture
Aspartame(14.7 g,0.05 mol)and β-CD(56.75 g,0.05 mol) were mixed and stirred in a small beaker at room temperature for 30 min until a uniform mixture was formed.The product obtained was the physical mixture of aspartame and β-CD.
2.2.6 Differential scanning calorimetry(DSC)
The DSC measurements of the samples(i.e.,aspartame,β-CD, the physical mixture and the complexes)were performed using a Mettler-Toledo 822 differential scanning calorimeter(Mettler-Toledo Inc.,Columbus,OH).Calibration was carried out using indium and zinc as reference materials.Samples weighed approximately 3 mg were analyzed in an aluminium pan in which a pinhole was punched into the pan lid.The samples were heated from 10 to 300°C at a rate of 10°C·min-1under a nitrogen purge.
2.2.7 Nuclear magnetic resonance(NMR)
The1H NMR experiments were performed on a BrukerAdvance 500 DMX spectrometer(Switzerland)with working frequencies of 500 mHz and equipped with a broadband observe probe.The1H NMR spectra were recorded in D2O solution at 25°C and all chemical shifts were measured relative to tetramethylsilane (TMS).For each1H NMR experiment,between 16 and 256 transients were collected into 65 K data points over a 5000 Hz spectral window,using a 2 s relaxation delay.
2.2.8 Fourier transform infrared(FT-IR)spectroscopy
Infrared spectra were recorded on a FT-IR spectrometer(Nicolet 380 model),equipped with deuterated triglycine sulphate (DTGS)detector,the spectra of Aspartame,CDs,their physical mixture and the inclusion complex were collected between 4000 and 400 cm-1with 16 scans at a resolution of 4 cm-1by the KBr method.The data was recorded and processed by the ezomnc32 software.
3 Results and discussion
3.1Sensory evaluation of the sweetness intensity of aspartame in cyclodextrin
Unpleasant tastes and odors can be masked or covered by CD at higher concentration or at lower temperatures.Most application of cyclodextrin in food was mainly concentrated on the improvement of stability of fruit juice.Andreu-Sevilla et al.22compared the effect of α-CD,β-CD,and γ-CD on the color,volatile composition,taste and aroma of juice and found that α-CD protest fruit juice best.Howard et al.23found that the stability of anthocyanins was improved in wild cherry juice with β-CD.Although lots of work has been reported on the dynamic binding process and the stability of the inclusion complex between aspartame and CDs, there is no report on the change of the sweetness intensity of aspartame by CDs.Herein,sweetness intensity of aspartame with CDs was evaluated.Three different concentrations of aspartame at 0.0125%,0.025%,0.05%(w)were selected.Paired comparison method results were adopted24.Pure aspartame solution was used as standard sample,giving sweetness score of 50 points.Aspartame solutions with CDs were the test samples.Statistical software SPSS Statistics16.0 and significance test(P(statistical significance)=0.001)were used to analyze all data for single factor variance.The results are shown in Fig.1.Each histogram in three groups represents sensory results under different concentrations of aspartame.Bar chart is the average sweetness score of aspartame,which is set to 50 points.As was shown in Fig.1,sweetness intensity of aspartame at 0.0125%and 0.025%(w)with β-CD had significant difference compared with other CDs(P=0.001 level of significance).Sensory evaluation disclosed that aspartame with β-CD and HP-β-CD has stronger sweetness intensity than with other CDs.It is noteworthy that CD itself is sweet and the sweetness intensity of 2.5%(w)β-CD is equivalent to 1.71%(w) sucrose solution25.In our experiment,we use 0.1%(w)cyclo-dextrin solution,which is equivalent to 3.4×10-6(w)of sucrose solution.Therefore,the additive effect of β-CD on sweetness can be neglected.The enhanced sweetness of aspartame is mainly from the increased stability which was offered by CDs.

Fig.1 Histogram for perceived sweetness intensity of aspartame in the presence of different cyclodextrins at 25°C
3.2Measurement of the thermodynamic binding constants for the interaction of CDs with aspartame
The thorough and precise characterization of the energies of molecular complex formation,including its dependence on relevant environmental variables,is important in molecular science research.To find the relationship between the enhanced sweetness of aspartame and its binding affinity with CDs,thermodynamic binding constants for the interaction of CDs with aspartame were further explored.Isothermal titration calorimetry(ITC)is an effective way to reveal the thermodynamics over the course of an interaction.Therefore,the observed enthalpy changes and the association strength,which may arise because of changes in hydrogen bonding and(or)hydrophobic interactions can be determined by this technique.Herein we selected a series of CDs to investigate the difference of binding affinity during their interaction with aspartame.Fig.2 is the thermograph of the ITC for CDs binding to aspartame at 25°C.It shows the integration of each peak(kilojoules per mole injectant)as a function of the molar ratio of aspartame to CDs after subtracting the heat of dilution.As can be seen from Fig.2(a),heat flow of each CD goes in the similar way.The negative value of ΔH throughout the entire binding processes suggests an exothermic process.Heat release from of the interaction of β-CD with aspartame is larger than those of other CDs.To elucidate the contribution of enthalpy or entropy to the association between CD and aspartame,ITC data were fit with one-site binding model.After the subtraction of the heats of dilution to the titration data,a curve typically associated with 1: 1 binding was obtained which was shown in Fig.2(b),giving a binding constant(K)of(1070.0±86.4)L·mol-1along with the exothermic heat of 7.666 kJ·mol-l.β-CD has maximum constant when binding with aspartame.The binding constant was larger than that reported in the earlier work of Moelands15and Garbow26et al. in which the equilibrium constant for the β-cyclodextrin-aspartame interaction from titrations was 128 L·mol-1by flow microcalorimeter and 149.2 L·mol-1by1H NMR technique respectively.The negative enthalpy and a significant favorable entropy change demonstrate that the spontaneous binding of aspartame with β-CD is from the non-specific hydrophobic effect.As the inclusion of guest molecule in CD cavity consists of basically the substitution of the included water molecules by the less polar guest,herein the process is energetically favored by the interactions of aspartame molecule with the solvated hydrophobic cavity of CD.Noteworthy to say that heat release from the interaction of other CDs and aspartame is so small that the thermodynamic parameters cannot be obtained from data fitting.Therefore,additional elucidation of thermodynamic parameters of the formation of the complex between other CDs and aspartame is necessary.
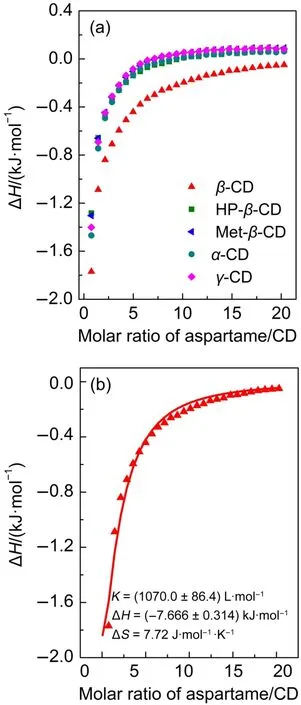
Fig.2 (a)Titration of aspartame(30 mmol·L-1)with five different CDs(0.1 mmol·L-1)at 25°C;(b)binding isotherm of aspartame(30 mmol·L-1)with β-CD(0.1 mmol·L-1)fitted by one-site binding model
As the thermodynamic parameters can also be calculated from spectrophotometric data27-29,fluorescence spectroscopy is used to further investigate their binding behavior of CD and aspartame. Fig.3 depicts the fluorescence spectra aspartame with increasing concentrations of CDs at excitation wavelength 210 nm at 25°C. The fluorescence spectrum of aspartame in water shows maxima at 295 nm.The fluorescence intensity of aspartame is increased by changing the microenvironment polarity and acid-base balance. At the same time,each CD forms inclusion complexes with aspartame,which provided a non-polar environment for guest molecules.Therefore,the fluorescence intensity of aspartame was seen to grow with increasing concentration of CD in all the cases, probably because CD protected the excited singlet and triplet phosphorescence of aspartame,so as not to be quenched by external factors.Fig.3(a)shows that the fluorescence intensity of aspartame increased regularly with the increasing concentration of α-CD without changing the emission wavelength and shape of the peaks.Increasing the concentration of β-CD(160 mmol·L-1) results in increased intensity along with red shift(from 295 to 300 nm),which suggests the interaction between aspartame and the CDs when excited30.The spectrum shape and the position of the maximum emission at 360 nm remains unchanged(Fig.3(b)).Two derivatives of β-CD,HP-β-CD and Met-β-CD,display similareffect on the fluorescence spectrum of aspartame,which are shown in Fig.3(d)and Fig.3(e).In the case of γ-CD,increased concentration results in an increased intensity with a slight shift to 297 nm at a concentration of 160 mmol·L-1(Fig.3(c)).The fluorescence changes(which were evidenced by the emission spectra and the increased fluorescence intensity)indicate that aspartame was embedded into the inner apolar cavity of CDs with a more hydrophobic environment,but the inclusion differs with each other due to the different structure of CDs.
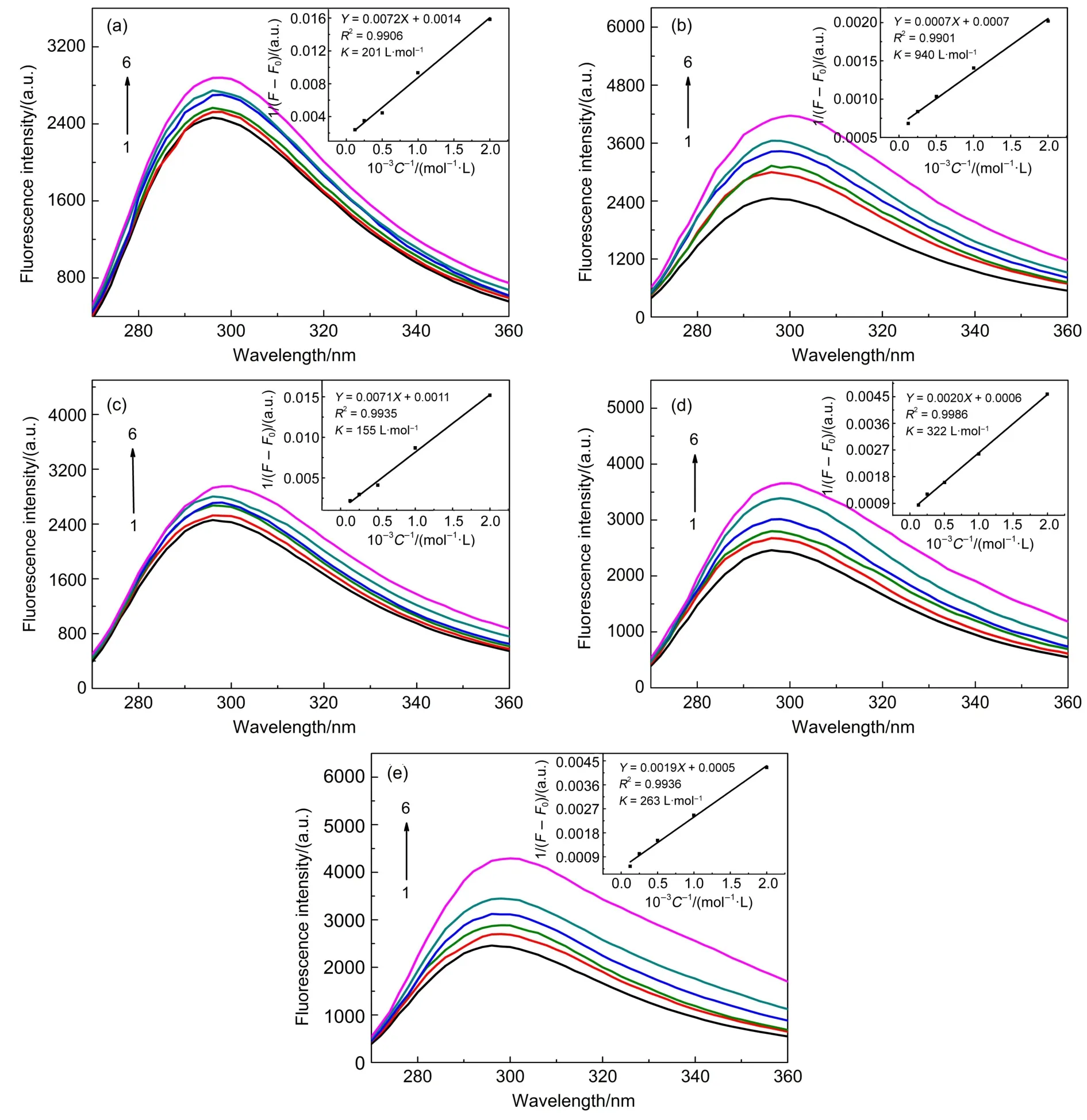
Fig.3 Fluorescence spectra of aspartame(0.05 mmol·L-1)with varying concentrations of CDs at 25°C
The fluorescence enhancement of aspartame with increasing CDs concentration was used for a quantitative study of interactions between aspartame and CDs.The Equation Hildebrand-Benesi is simplified as 1/(F-F0)=1/Fmax+1/(Fmax·Kb[β-CD])in the case of 1:1 stoichiometric ratio,where F0represents the initial fluorescence intensity of the substrate,F represents fluorescence intensity of inclusion complex and Fmaxrepresents the maximum fluorescence intensity.The straight line in the plot indicates the formation of a 1:1 molecular complex between aspartame and the studied CD.The linear fit of this kinetic model was quite acceptable with the correlation coefficients of greater than 0.99 in all cases.The calculated values of inclusion constant K are given in Table 1.
Noteworthy to say that in our results,the binding constant measured by fluorescence(940 L·mol-1)is slightly different from that measured by ITC(1070 L·mol-1).The reasons includes the different degree of accuracy and different mechanisms involved the microcalorimetry and fluorescence technique.In fact,among the reported aspartame-CD inclusion,the binding constant differsin value according to the pH and the technique used.The difference may also be attributed to the different equilibration time, temperature control and different solvents.For instance,the binding constant of aspartame and β-CD obtained by Sohajda et al.31was 123 and 111 L·mol-1using1H NMR or Capillary electrophoresis.Rajagopalan and Joy32reported that the binding constants of complexes of aspartame with β-CD from UVdata was 2974 L·mol-1at pH 2.5 and 338 L·mol-1at pH 12.5.It has been found that binding constants for the complexes of aspartame with α-CD at the both lower and higher pH values are almost the same and also the value is very low32.The α-CD comprises 6,the β-CD 7 and the γ-CD 8 glucopyranose units.Compared with α-CD and γ-CD,the stability of β-CD complex is high,which is attributed to a tighter fit inside the cavity.Smaller or larger cavity of CD is not favorable for the inclusion of aspartame.Hydroxypropyl ethers of β-cyclodextrin(HP-β-CD)and methyl-β-cyclodextrin(Met-β-CD)would result in steric hindrance on complex formation.At the same time the hydroxypropyl groups would provide extra protection of the aspartame molecules located within the complex against hydroxylions-catalyzed hydrolysis.This could explain the lower K of Met-β-CD in Table 1.β-CD and HP-β-CD has larger binding constants with aspartame than other α-CD and γ-CD. Therefore,we conclude the CD with larger complexing constant provides better stability for aspartame.Measuring binding constants between CDs with varied structures and aspartame could be an effective way to find a good protectant.

Table 1 Binding constants(K)of different cyclodextrins with aspartame at 25°C
3.3Characterization of the inclusion complexformation between aspartame and CDs
The above results show that different CDs have different effect on the sweetness of aspartame,and β-CD helps to keep the sweetness of aspartame most.In order to characterize the inclusion complex formation between aspartame and CDs,DSC and1H NMR were used for analysis.Fig.4 shows the DSC curves of individual aspartame or CDs,their physical mixture and the inclusion complex.DSC scan of aspartame displayed two sharp endothermic peaks at 186.3 and 246.7°C.The first peak of aspartame,with a transition temperature range usually from 167 to 190°C and with a maximum peak of transition at 185°C,represents the loss of the methyl ester and conversion to the dipeptide, as part L-phenylalanine.The second peak,with a transition temperature range from 234 to 254°C and with a maximum peak of transition at 240°C,represents the conversion to diketopiperatine33.The decomposition of aspartame was observed at 160°C with endothermic peak in DSC curve,which was attributed to the formation of diketopiperazine.The second endothermicevent observed at 230°C indicates the melting of diketopiperazine.Another endothermic effect at 235°C is for the melting of the decomposition compound34.
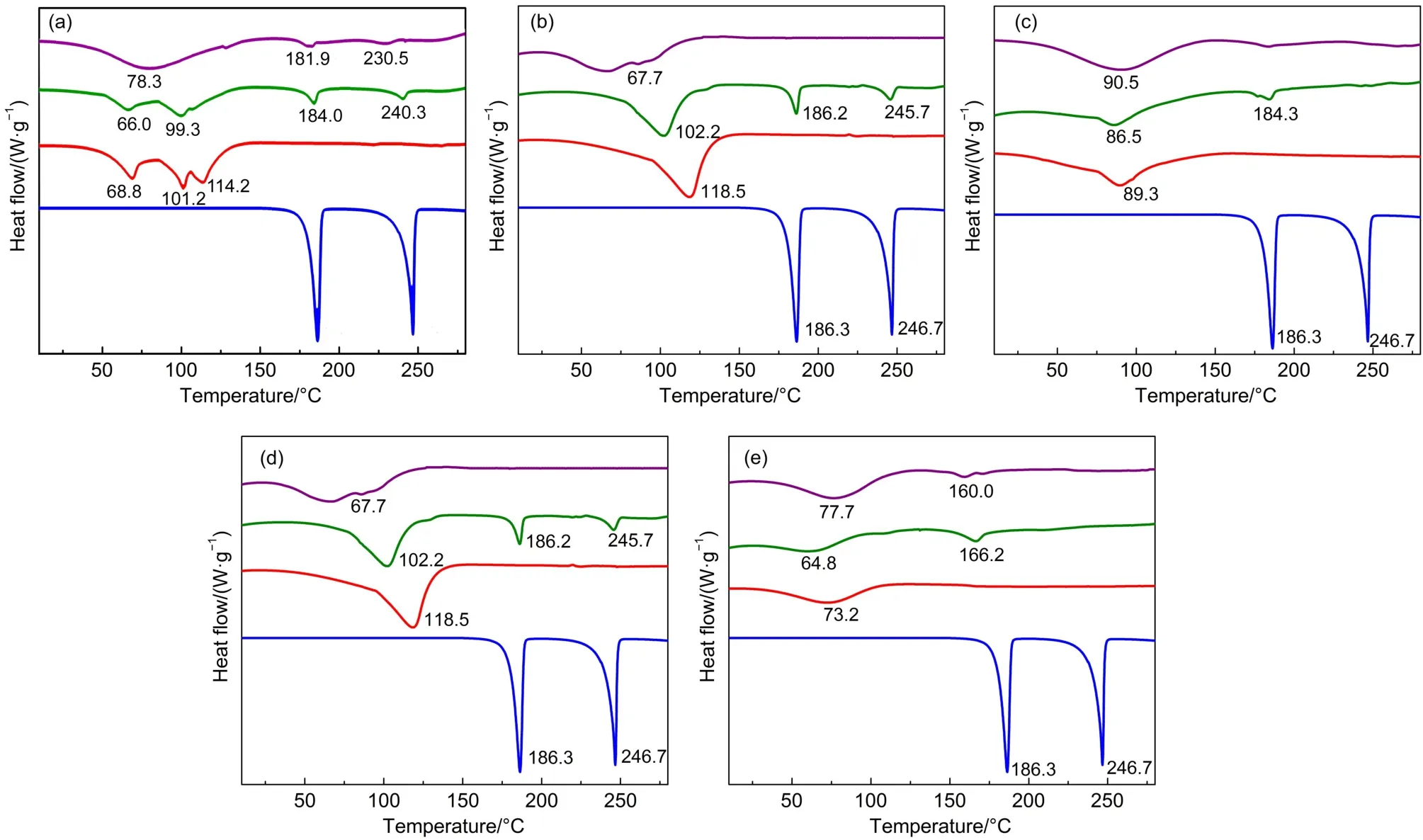
Fig.4 DSC thermograms of aspartame(blue line)with five CDs(red line),their physical mixture(yellow line)and inclusion complex(cyan line)
The DSC curves of pure aspartame and of the inclusion complex of aspartame with β-CD as a function temperature are given in Fig.4(b).The DSC thermogram of β-CD shows an endothermic peak at 118.5°C,possibly due to the dehydration.This is not consistent with the result reported by Karathanos et al.35,who observed an endothermic peak at 175°C of β-CD on the DSC thermogram.In the case of simple physical mixture of β-CD and aspartame,the melting enthalpy of aspartame results in a welldefined,strong exothermic peak,which is completely absent in the DSC-curves of a inclusion complex in the temperature range investigated.Similar DSC results were also found in the complexation between luteolin and CDs36.
DSC scans of the complex aspartame/β-CD confirmed that aspartame was located inside the cavity of β-CD.The disappearance of the endothermic peak at 186.3 and 246.7°C which was assigned to aspartame melting indicates that aspartame was located in the cavity of β-CD37.The new endothermic signal at 67.7°C,along with the disappearance of the endothermic peak assigned to aspartame,indicates that the inclusion complex did form.Similar results were also observed in the DSC curves of aspartame with other CDs(Fig.4(a,c,d,e))whether in their physical mixture or in complexed form.The only difference is that the signal of aspartame can still be observed more or less due to the ineffective inclusion,which is consistent with the results of binding constant and sensory evaluation.The results also get support from the research of Rajagopalan and Joy32which proved that the aspartame/cyclodextrin complex is not only complexation, but also inclusion of aspartame into β-CD cavity.
To further study the inclusion of aspartame in β-CD,1H NMR was used to characterize the chemical shift change of protons in aspartame and β-CD,in comparison with the chemical shifts of the same protons in the free components.Fig.5 shows the1H NMR spectra of pure components and aspartame/β-CD inclusion complex in a 1:1 molar ratio.The changes of the chemical shift of typical protons are organized in Table S1(Supporting Information).The complexation-induced chemical shift(Δδ)of H3 and H5 protons(Δδ=0.077,0.133)of β-CD are those expected as a result of interaction with those protons of the aspartame Ha,Hb,Hb′, Hc,Hc′,Hf(Δδ=0.014,0.025,0.021,0.008,0.008,0.188,respectively),which are oriented towards the β-CD cavity.This result is consistent with a previous investigation of aspartame and cyclodextrin by1H NMR and the interaction of benznidazole or albendazole with cyclodextrin derivatives32,38.FT-IR spectrum was further used to characterize the inclusion of aspartame by CDs (Fig.S2 in Supporting Information).The FT-IR spectrum of the physical mixture did not differ significantly from those of the single components.However,changes in the frequencies of the complex were found when compared with those of the pure substrate.The broad peak in the spectrum of aspartame/β-CD complex was increased in intensity owing to the increase in the number of hydroxyl groups.FT-IR results suggested a partial or complete shielding of chromophores in the CD cavity and are therefore rationalized as being indicative of complex formation.
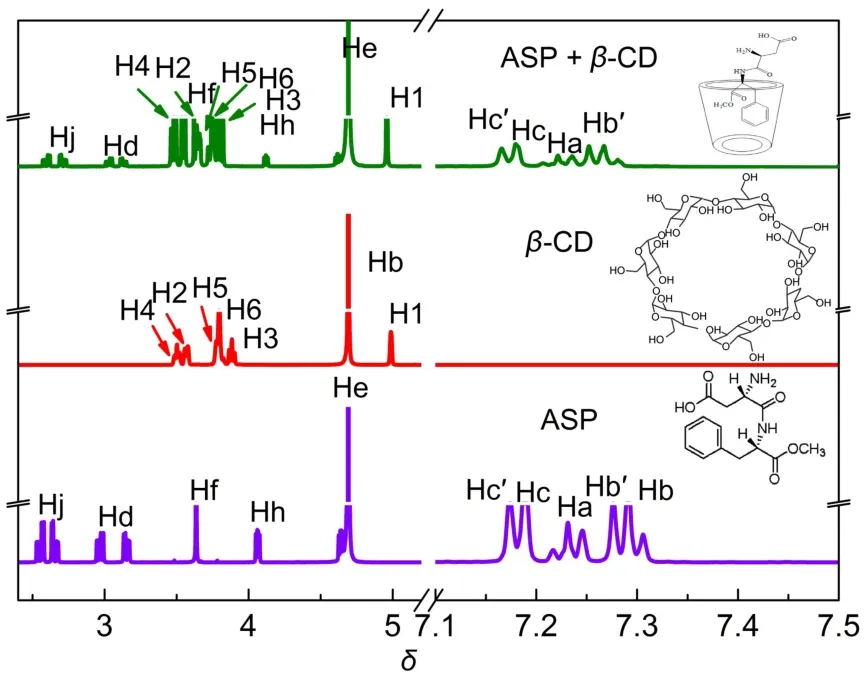
Fig.5 1H NMR spectra of aspartame(purple),β-CD(red)and inclusion complex(green)
4 Conclusions
General research of interactions between CDs and aspartame mainly focused on the complexation of aspartame by CDs and few reports were about the sensory sweetness of aspartame changed by CDs.We believe that there might be a relationship between the binding affinity of CDs with aspartame and sweetness intensity. Therefore,sensory evaluation was conducted and the results showed that β-CD significantly enhanced the sweetness intensity of aspartame in solution.Binding affinity of five CDs,α-CD,β-CD,γ-CD,HP-β-CD,and Met-β-CD,with aspartame was then investigated by ITC and fluorescence spectrum technique.Among the examined CDs,binding of aspartame with β-CD resulted in favorable free energy change with the biggest binding constant. These results suggested that sweetness intensity could be correlated to the binging affinity.DSC,1H NMR,and FT-IR further demonstrated the mechanisms behind the complexation.Considering that aspartame is included into the CD molecule,which is enwrapped into a hydrate shell,the interaction of the guest molecule with the taste receptors should be inhibited,resulting in reduced taste.However,guest molecule release depends on several factors such as association constant of the complex,temperature and concentrations of both components and etc.Herein,the fact that aspartame with β-CD(and HP-β-CD)displays stronger sweetness intensity can be attributed to the decreased degradation rate of aspartame in the presence of CDs.Release of more undecomposed aspartame from the hydrophobic cavity of cyclodextrins might help to keep the sweetness intensity.This research helps to understand the contribution of binding affinity to sweetness intensity of sweeteners.It also provides an approach of screening the protectors for flavor keeping by finding the thermodynamic signature.
Supporting Information:available free of charge via theinternet at http://www.whxb.pku.edu.cn.
(1 Buyukgoz,G.G.;Bozkurt,A.G.;Akgul,N.B.;Tamer,U.; Boyaci,I.H.Eur.Food.Res.Technol.2015,240,567. doi:10.1007/s00217-014-2357-y
(2) Marinovich,M.;Galli,C.L.;Bosetti,C.;Gallus,S.;La Vecchia, C.Food Chem.Toxicol.2013,60,109.doi:10.1016/j. fct.2013.07.040
(3) Kroger,M.;Meister,K.;Kava,R.Compr.Rev.Food Sci.Food Saf.2006,5,35.doi:10.1111/j.15 41-4337.2006.tb00081.x
(4) Wang,R.;Schroeder,S.A.J.Food Sci.2000,65,1100. doi:10.1111/j.13 65-2621.2000.tb10246.x
(5) Prankerd,R.J.;Stone,H.W.;Sloan,K.B.;Perrin,J.H.Int.J. Pharmaceut.1992,88,189.doi:10.1016/0378-5173(92)90316-T
(6) Arora,S.;Shendurse,A.M.;Sharma,V.;Wadhwa,B.K.;Singh, A.K.J.Food Sci.Technol.2013,50,770.doi:10.1007/s13197-011-0386-0
(7) Bell,L.N.;Wetzel,C.R.J.Agr.Food Chem.1995,43,2608. doi:10.1021/jf00058a010
(8) Wetzel,C.R.;Bell,L.N.Food Chem.1998,63,33. doi:10.1016/S0308-8146(97)00235-5
(9) Rocha-Selmi,G.A.;Bozza,F.T.;Thomazini,M.;Bolini,H.M. A.;Fávaro-Trindade,C.S.Food Chem.2013,139,72. doi:10.1016/j.foodchem.2013.0 1.114
(10) Qian,H.C.;Kanwal,S.;Jia,Q.Z.;Wang,Q.;Ji,H.F.;Zhu,Z. C.;Xia,S.Q.;Ma,P.S.Acta Phys.-Chim.Sin.2015,31,893. [钱海城,Kanwal,S.,贾青竹,王 强,计惠芬,朱志臣,夏淑倩,马沛生.物理化学学报,2015,31,893.]doi:10.3866/PKU. WHXB201503193
(11) Jiang,H.;Zhang,S.;Guan,Q.;Chen,C.;Gao,F.;Zhang,Y. Curr.Drug Discov.Technol.2007,4,295.doi:10.2174/ 157016307783220567
(12) Astray,G.;Gonzalez-Barreiro,C.;Mejuto,J.C.;Rial-Otero,R.; Simal-Gándara,J.Food Hydrocolloid 2009,23,1631. doi:10.1016/j.foodhyd.2009.0 1.001
(13) Shahidi,F.;Han,X.Q.Cri.Rev.In.Food.Sci.1993,33,501. doi:10.1080/10408399309527645
(14) Brewster,M.E.;Loftsson,T.;Baldvinsdóttir,J.;Bodor,N.Int. J.Pharmaceut.1991,75,R5.doi:10.1016/0378-5173(91) 90257-O
(15) Moelands,D.;Karnik,N.A.;Prankerd,R.J.;Sloan,K.B.; Stone,H.W.;Perrin,J.H.Int.J.Pharmaceut.1992,86,263. doi:10.1016/0378-5173(92)90205-G
(16) Budryn,G.;Zaczyńska,D.;Rachwał-Rosiak,D.;Oracz,J.Eur. Food Res.Technol.2015,240,1157.doi:10.1007/s00217-015-2419-9
(17) Chen,Z.X.;Guo,G.M.;Deng,S.P.J.Agr.Food Chem.2009, 57,2945.doi:10.1021/jf803302g
(18) Chen,Z.X.;Wu,W.;Zhang,W.B.;Deng,S.P.Food Chem. 2011,128,134.doi:10.1016/j.foodchem.2011.03.0 08
(19) Dong,W.R.;Chen,G.;Chen,Z.X.;Deng,S.P.Food Chem. 2013,141,3110 doi:10.1016/j.foodchem.2013.05.160
(20) Han,X.;Xu,S.Z.;Dong,W.R.;Wu,Z.;Wang,R.H.;Chen,Z. X.Food Chem.2014,164,278.doi:10.1016/j. foodchem.2014.05.040
(21) Xu,S.Z.;Han,X.;Tian,J.N.;Wu,Z.;Chen,Z.X.Acta Phys.-Chim.Sin.2014,30,1134.[徐淑臻,韩 雪,田俊楠,吴 寨,陈忠秀.物理化学学报,2014,30,1134.]doi:10.3866/ PKU.WHXB201404251
(22) Andreu-Sevilla,A.J.;Mena,P.;Martí,N.;Viguera,C.G.; Carbonell-Barrachina,Á.A.Food Res.Int.2013,54,246. doi:10.1016/j.foodres.2013.07.007
(23) Howard,L.R.;Brownmiller,C.;Prior,R.L.;Mauromoustakos, A.J.Agr.Food Chem.2013,61,693.doi:10.1021/jf3038314
(24) Cook,D.J.;Hollowood,T.A.;Linforth,R.S.T.;Taylor,A. J.Food Qual.Prefer.2002,13,473.doi:10.1016/S0950-3293 (02)00066-6
(25) Szejtli,J.;Szente,L.Eur.J.Pharm.Biopharm.2005,61,115. doi:10.1016/j.ejpb.2005.05.006
(26) Garbow,J.R.;Likos,J.J.;Schroeder,S.A.J.Agr.Food Chem. 2001,49,2053.doi:10.1021/jf001122d
(27) Górnas,P.;Neunert,G.;Baczyński,K.;Polewski,K.Food Chem.2009,114,190.doi:10.1016/j.foodchem.2008.09.048
(28) Guo,Q.L.;He,H.;Pan,L.L.;Liu,Y.Acta Phys.-Chim.Sin. 2016,32,1383.[郭清莲,何 欢,潘凌立,刘 义.物理化学学报,2016,32,1383.]doi:10.3866/PKU.WH XB201603093
(29) Guo,Q.L.;Pan,L.L.;Yang,L.Y.;He,H.;Zhang,Y.Z.;Liu,Y. Acta Phys.-Chim.Sin.2016,32,274.[郭清莲,潘凌立,杨立云,何 欢,张业中,刘 义.物理化学学报,2016,32,274.] doi:10.3866/PKU.WHXB201511021
(30) López-Tobar,E.;Blanch,G.P.;Ruiz del Castillo,M.L.; Sanchez-Cortes,S.Vib.Spectrosc.2012,62,292.doi:10.1016/j. vibspec.2012.06.008
(31) Sohajda,T.;Béni,S.;Varga,E.;Iványi,R.;Rácz,A.;Szente,L.; Noszál,B.J.Pharmaceut.Biomed.2009,50,737.doi:10.1016/ j.jpba.2009.06.010
(32) Rajagopalan,P.;Joy,T.S.R.Int.J.Agr.Food.Sci.2013,3,28.
(33) El-Shattawy,H.H.;Kildsig,D.O.;Peck,G.E.Drug Dev.Ind. Pharm.1982,8,651.doi:10.3109/03639048209042694
(34) Guguta,C.;Meekes,H.;de Gelder,R.J.Pharmaceut.Biomed. 2008,46,617.doi:10.1016/j.jp ba.2007.11.044
(35) Karathanos,V.T.;Mourtzinos,I.;Yannakopoulou,K.; Andrikopoulos,N.K.Food Chem.2007,101,652.doi:10.1016/ j.foodch em.2006.01.053
(36) Liu,B.G.;Li,W.;Zhao,J.;Liu,Y.;Zhu,X.A.;Liang,G.Z. Food Chem.2013,141,900.doi:10.1016/j. foodchem.2013.03.097
(37) Buschmann,H.;Schollmeyer,E.J.Cosmet.Sci.2002,53,185.
(38) Priotti,J.;Ferreira,M.J.G.;Lamas,M.C.;Leonardi,D.; Salomon,C.J.;Nunes,T.G.Carbohyd.Polym.2015,131,90. doi:10.1016/j.carbpol.2015.05.045
Sweetness Enhancement of Aspartame in the Presence of Cyclodextrins and the Thermodynamics in Binding
ZHU Tian-Tian XU Shu-Zhen GE Bing-Qiang CHEN Zhong-Xiu*
(College of Food&Biology Engineering,Zhejiang Gongshang University,Hangzhou 310018,P.R.China)
Current research on the effects of cyclodextrins(CDs)on the sweetness of aspartame(ASP)focuses on the stability of aspartame as protected by CDs.We propose a relationship between the sweetness intensity of aspartame and its thermodynamic binding affinity with CDs.In this paper,we describe the sensory evaluation of aspartamewiththefiveCDs α-cyclodextrin(α-CD),β-cyclodextrin(β-CD),γ-cyclodextrin(γ-CD),hydroxypropylβ-cyclodextrin(HP-β-CD),and methyl-β-cyclodextrin(Met-β-CD).β-CD was found to significantly enhance the sweetness intensity of aspartame.The binding affinity of CDs with aspartame was then investigated using isothermal titration calorimetry(ITC)and fluorescence spectroscopy.The binding of aspartame with β-CD resulted in a free energy change with the largest binding constant.Differential scanning calorimetry(DSC), nuclear magnetic resonance(1H NMR),and Fourier transform infrared(FT-IR)spectroscopy further revealed the mechanism behind the complexation.This research gives insight to the contribution of the thermodynamic binding affinity to the sweetness intensity of aspartame.It also provides an approach for screening flavorretention agents by measuring binding constants.
Aspartame;Binding constant;Thermodynamics;Sweetness intensity;Cyclodextrin
O642
10.3866/PKU.WHXB201609281
Received:July 25,2016;Revised:September 28,2016;Published online:September 28,2016.
*Corresponding author.Email:zhxchen@zjgsu.edu.cn;Tel:+86-571-28008980.
The project was supported by the Zhejiang Provincial Top Key Discipline of Food Science and Biotechnology,China(JYTSP20141012),National Natural Science Foundation of China(21673207),and Graduate Innovation Project of Zhejiang Gongshang University,China(CX201610053,20151216).
浙江省食品科学与生物技术“重中之重”基金(JYTSP20141012),国家自然科学基金(21673207)及浙江工商大学研究生创新课题基金(CX201610053,20151216)资助项目
——致癌