铜铁共催化烯烃串联加成/环化合成异喹啉二酮*
夏绿露,王良能,李增增,李荣杰,盛回香,吴亚琴,李 培,李 玲,唐 石
(化学国家级实验教学示范中心,湖南 吉首 416000)
1 合成路线
图1示出双重叔烷基化异喹啉二酮的合成路线.
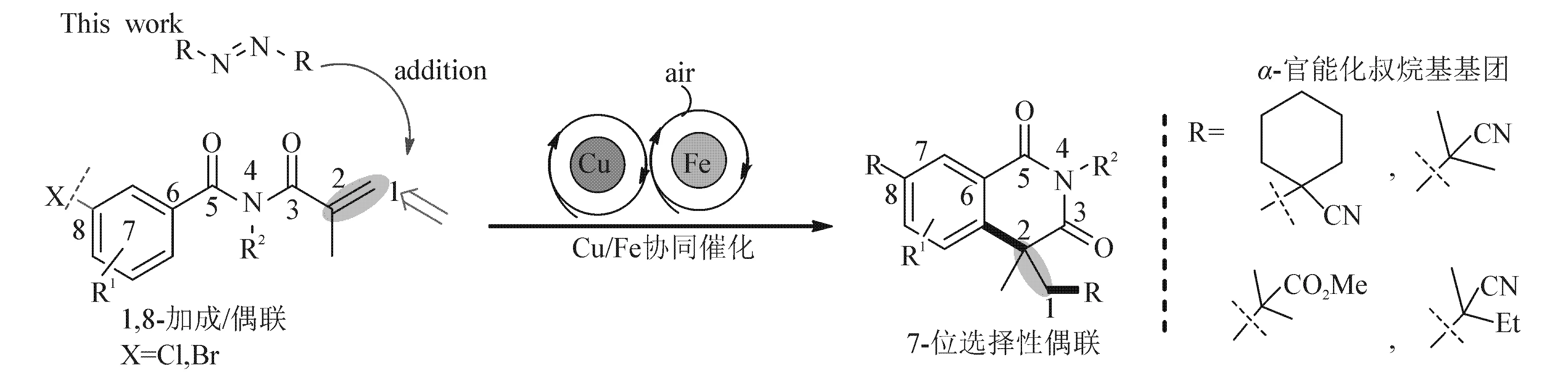
图1 双重叔烷基化异喹啉二酮的合成Fig. 1 Synthesis of Dual Tert-Alkylated Isoquinolinediones
2 实验部分
2.1 仪器和试剂
实验中所用的芳基酰氯、草酰氯、烷基胺、芳基胺和偶氮试剂购自阿拉丁试剂公司或百灵威试剂公司,其他试剂除特殊标明外均为分析纯.实验中所需的原料N-丙烯酰基-N-丁基苯甲酰胺(1a-1q)参照相关文献报道方法[11]合成得到.主要仪器:AV ANCE 400 MHz 超导傅里叶数字化核磁共振仪(瑞士Bruker公司);GC-MS-QP2010型质谱仪(日本岛津公司);RE-52AA型旋转蒸发仪(上海亚荣生化仪器厂);XT5A型显微熔点仪(北京市科仪电光学仪器厂).
2.2 目标产物3的合成
以产物3a合成为例,在反应管中依次加入CuI(摩尔分数10%),Fe(NH4)2SO4·6H2O(摩尔分数10%),KF(2 equiv),K3PO4(2 equiv),1,1-偶氮二异丁腈(2a)(4 equiv),N-丙烯酰基-N-烷基氯代苯甲酰胺(1a)(0.3 mmol),最后加入溶剂二氧六环 (2.0 mL),于空气环境下在90 ℃的油浴锅中反应 (通常18~20 h),经TLC检验反应完全后,经硅胶色谱柱分离,洗脱剂石油醚∶乙酸乙酯(7∶1)时,以78%产率得到最终目标产物3a.产物为无色固体.
2.3 产物的表征数据
产物 (3a).无色固体;m.p.77.2~78.6 ℃.1H NMR (400 MHz,CDCl3) δ:8.35 (d,J=2.0 Hz,1H),7.86 (dd,J=8.4,2.0 Hz,1H),7.56 (d,J=8.4 Hz,1H),4.05~4.01 (m,2H),2.77 (d,J=14.8 Hz,1H),2.34 (d,J=14.8 Hz,1H),1.81 (s,3H),1.79 (s,3H),1.66~1.58 (m,5H),1.47~1.38 (m,2H),1.16 (s,6H),0.97 (t,J=7.4 Hz,3H);13C NMR (101 MHz,CDCl3) δ:175.1,163.3,142.0,140.6,131.2,127.6,125.5,125.4,123.9,123.3,49.5,45.9,40.8,37.0,33.5,30.6,29.8,29.6,29.2,28.8,27.3,20.3,13.8;HRMSm/z(ESI) calcd for C23H30N3O2[M+H]+380.233 3,found:380.232 9.
产物 (3b).白色固体;m.p.75.0~76.7 ℃.1H NMR (400 MHz,CDCl3) δ:8.38 (d,J=2.4 Hz,1H),7.87 (dd,J=8.4,2.4 Hz,1H),7.56 (d,J=8.4 Hz,1H),7.48 (d,J=7.2 Hz,2H),7.33~7.25 (m,3H),5.32 (d,J=13.6 Hz,1H),5.16 (d,J=13.6 Hz,1H),2.73 (d,J=14.4 Hz,1H),2.34 (d,J=14.8 Hz,1H),1.80 (d,J=7.2 Hz,6H),1.62 (s,3H),1.12 (s,3H),0.96 (s,3H);13C NMR (101 MHz,CDCl3) δ:175.0,163.3,142.1,140.5,136.5,131.4,129.2,128.4,127.7,125.7,125.2,123.8,123.3,49.8,46.0,44.0,37.0,33.3,30.5,29.7,29.2,28.8,26.9;HRMSm/z(ESI) calcd for C26H28N3O2[M+H]+414.217 7,found:414.217 9.
产物 (3c).无色油状液体;1H NMR (400 MHz,CDCl3) δ:8.23 (s,1H),7.35 (s,1H),4.02~3.99 (m,2H),2.76 (s,4H),2.35 (d,J=14.8 Hz,1H),1.90 (s,3H),1.81 (s,3H),1.60~1.58 (m,5H),1.42~1.36 (m,2H),1.19 (s,3H),1.12 (s,3H),0.96 (t,J=7.3 Hz,3H);13C NMR (101 MHz,CDCl3) δ:175.3,163.4,143.3,140.2,138.8,131.0,125.8,123.9,123.5,122.9,49.0,45.7,40.7,34.6,33.4,30.6,29.9,29.6,28.2,28.1,26.8,21.6,20.3,13.8;HRMSm/z(ESI) calcd for C24H32N3O2[M+H]+394.249 0,found:394.248 8.
产物 (3d).无色油状液体;1H NMR (400 MHz,CDCl3) δ:8.36 (s,1H),7.59 (s,1H),4.04~3.99 (m,2H),2.77 (d,J=14.8 Hz,1H),2.30 (d,J=14.8 Hz,1H),1.96 (s,3H),1.90 (s,3H),1.66~1.60 (m,5H),1.41 (dd,J=14.2,7.2 Hz,2H),1.21 (s,3H),1.18 (s,3H),0.97 (t,J=7.2 Hz,3H);13C NMR (101 MHz,CDCl3) δ:174.5,162.6,142.0,139.7,137.8,130.2,127.9,123.8,123.1,122.6,49.4,45.8,40.9,35.5,33.3,30.5,29.9,29.5,27.5,27.3,27.1,20.3,13.8;HRMSm/z(ESI) calcd for C23H29ClN3O2[M+H]+414.194 3,found:414.194 1.
产物 (3e).无色油状液体;1H NMR (400 MHz,CDCl3) δ:8.41 (s,1H),7.38 (d,J=1.6 Hz,1H),4.05~4.01 (m,2H),2.80 (d,J=14.8 Hz,1H),2.24 (d,J=14.8 Hz,1H),1.89 (s,3H),1.84 (s,3H),1.63~1.56 (m,5H),1.46~1.39 (m,2H),1.21 (s,3H),1.18 (s,3H),0.98 (t,J=7.4 Hz,3H);13C NMR (101 MHz,CDCl3) δ:174.4,162.3,151.4,143.2,132.2,128.7,128.6,122.9,122.8,122.7,116.5,49.7,46.1,40.9,33.9,33.3,30.5,29.8,29.5,27.7,27.4,27.3,20.3,13.8;HRMSm/z(ESI) calcd for C24H29F3N3O3[M+H]+464.215 6,found:464.215 9.
产物 (3f).无色油状液体;1H NMR (400 MHz,CDCl3) δ:8.39 (d,J=8.0 Hz,1H),7.28 (d,J=6.4 Hz,1H),4.03~3.99 (m,2H),2.75 (d,J=14.8 Hz,1H),2.27 (d,J=14.4 Hz,1H),1.87 (s,3H),1.83 (s,3H),1.65~1.57 (m,5H),1.40 (dd,J=14.8,7.2 Hz,2H),1.22 (s,3H),1.16 (s,3H),0.97 (t,J=7.4 Hz,3H);13C NMR (101 MHz,CDCl3) δ:174.6,165.3,162.7,162.4,143.8,129.3,128.9,123.1,121.4,115.3,49.7,45.9,40.8,33.9,33.3,30.5,30.0,29.6,27.3,27.1,26.9,20.3,13.8;HRMSm/z(ESI) calcd for C23H29FN3O2[M+H]+398.223 9,found:398.224 1.
产物(3g).无色油状液体;1H NMR (400 MHz,CDCl3) δ:8.34 (d,J=2.0 Hz,1H),7.86 (dd,J=8.4,2.4 Hz,1H),7.55 (d,J=8.4 Hz,1H),4.05~4.01 (m,2H),2.74 (d,J=14.8 Hz,1H),2.33 (d,J=14.8 Hz,1H),2.21 (d,J=7.2 Hz,2H),1.89~1.85 (m,8H),1.64~1.57(m,12H),1.44~1.38 (m,5H),0.97 (t,J=7.3 Hz,3H);13C NMR (101 MHz,CDCl3) δ:175.3,163.4,141.8,140.9,131.6,127.4,125.9,125.2,122.2,121.4,49.7,45.6,44.1,40.7,38.3,37.5,36.9,36.5,35.8,33.7,29.6,24.8,24.7,23.5,23.4,22.7,22.5,20.3,13.8;HRMSm/z(ESI) calcd for C29H38N3O2[M+H]+460.295 9,found:460.295 7.
产物 (3h).无色油状液体;1H NMR (400 MHz,CDCl3) δ:8.24 (d,J=2.0 Hz,1H),7.54 (dd,J=8.4,2.4 Hz,1H),7.32~7.28 (m,1H),3.99 (td,J=7.2,3.2 Hz,2H),3.67 (s,3H),3.13 (s,3H),2.64 (q,J=14.4 Hz,2H),1.65~1.57 (m,8H),1.54 (s,3H),1.43~1.36 (m,2H),1.05 (s,3H),0.97 (t,J=7.4 Hz,3H),0.89 (s,3H);13C NMR (101 MHz,CDCl3) δ:177.0,176.6,176.0,163.9,144.0,140.2,131.1,127.0,125.7,124.7,52.3,51.4,50.5,46.4,45.8,41.6,40.5,33.7,29.6,29.2,26.4,26.3,23.2,20.4,13.8;HRMSm/z(ESI) calcd for C25H36NO6[M+H]+446.253 8,found:446.254 0.
产物 (3i).无色油状液体(d.r.= 1∶1);1H NMR (400 MHz,CDCl3) δ:8.34 (d,J=2.0 Hz,0.5H),8.29 (d,J=2.0 Hz,0.5H),7.85 (dd,J=8.4,2.4 Hz,0.5H),7.80 (dd,J=8.0,2.0 Hz,0.5H),7.57 (d,J=8.0 Hz,0.5H),7.53 (d,J=8.4 Hz,0.5H),4.06~4.01 (m,2H),2.85 (d,J=14.4 Hz,0.5H),2.67 (d,J=14.8 Hz,0.5H),2.43 (d,J=14.8 Hz,0.5H),2.23 (d,J=14.4 Hz,0.5H),2.06~2.00 (m,2H),1.80 (s,1.5H),1.76 (s,1.5H),1.66~1.60 (m,6H),1.45~1.39 (m,4H),1.05 (d,J=7.2 Hz,2H),1.00~0.96 (m,9H);13C NMR (101 MHz,CDCl3) δ:175.5,175.0,163.4,140.5,131.7,127.6,125.7,125.2,122.7,122.2,48.6,47.7,45.8,43.2,40.8,35.5,33.7,29.6,26.9,26.0,23.4,20.4,13.8,9.8,9.0;HRMSm/z(ESI) calcd for C25H34N3O2[M+H]+408.264 6,found:408.264 3.
3 结果与讨论
3.1 反应条件探索
以N-丙烯酰基-N-丁基-3氯代苯甲酰胺1a与偶氮二异丁腈2a的加成/环化反应为模板反应来探索最佳反应条件(表1).笔者使用CuI作为催化剂,dioxane(二氧六环)作为溶剂,K3PO4为碱对反应进行尝试,幸运的是,通过GC-MS检测发现有少量目标产物3a生成.为了进一步提升反应产率,笔者又进行了大量的尝试,发现以空气为氧化剂,且加入KF时更有利于目标产物异喹啉二酮的生成.因此,接着使用KF和空气在接下来的反应中来探索最佳条件.实验过程中尝试添加了不同种类的助催化剂(如LiBr,MnSO4,FeS,SnCl2·2H2O,Fe(NH4)2SO4·6H2O等),结果显示,不同的助催化剂对反应有不同程度的影响,尤其是在使用Fe(NH4)2SO4·6H2O作为助剂时,目标产物的产率上升至78%(entries 1~8).此外,笔者还试图通过使用一些含氮配体(如联吡啶,1,10-菲罗啉,三亚乙基二胺(DABCO))来提高产率,但未获得成功(entries 9~11).接下来对催化剂进行了优化,发现Cu(OAc)2,CuCl和Cu2O等催化剂的效果均不如CuI(entries 12~14).除此之外,还考查了其他无机碱(如KOAc,K2CO3,Li2CO3,Cs2CO3)等对反应的影响,发现K3PO4依然是最好的选择(entries 15~18).
综上所述,最佳反应条件为:N-丙烯酰基-N-丁基苯甲酰胺1a (0.3 mmol),偶氮二异丁腈2a (4 equiv),CuI(摩尔分数10%),Fe(NH4)2SO4·6H2O (摩尔分数10%),K3PO4(2 equiv),KF (2 equiv),dioxane (二氧六环)为溶剂,反应温度为90 ℃,空气环境中反应18~20 h.
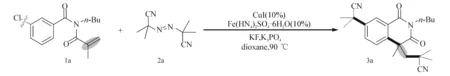
表1 优化反应条件摸索
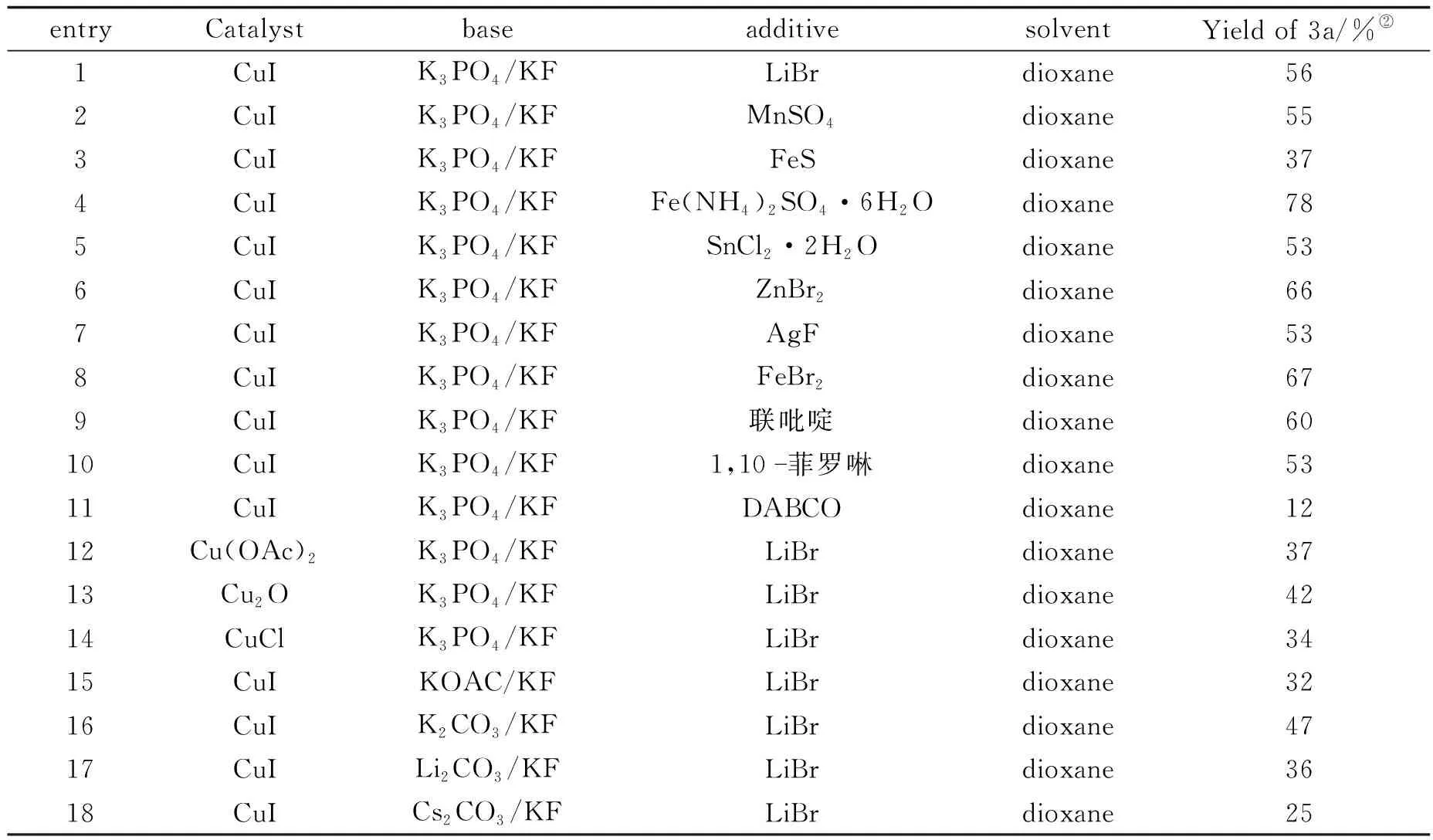
entryCatalystbaseadditivesolventYield of 3a/%②1CuIK3PO4/KFLiBrdioxane562CuIK3PO4/KFMnSO4dioxane553CuIK3PO4/KFFeSdioxane374CuIK3PO4/KFFe(NH4)2SO4·6H2Odioxane785CuIK3PO4/KFSnCl2·2H2Odioxane536CuIK3PO4/KFZnBr2dioxane667CuIK3PO4/KFAgFdioxane538CuIK3PO4/KFFeBr2dioxane679CuIK3PO4/KF联吡啶dioxane6010CuIK3PO4/KF1,10菲罗啉dioxane5311CuIK3PO4/KFDABCOdioxane1212Cu(OAc)2K3PO4/KFLiBrdioxane3713Cu2OK3PO4/KFLiBrdioxane4214CuClK3PO4/KFLiBrdioxane3415CuIKOAC/KFLiBrdioxane3216CuIK2CO3/KFLiBrdioxane4717CuILi2CO3/KFLiBrdioxane3618CuICs2CO3/KFLiBrdioxane25
注:①Reaction conditions:1a (0.3 mmol),AIBN 2a (4 equiv),CuI (10%),co-catalyst (10%),O2(1 atm),base (2 equiv),KF (2 equiv),and solvent (2 mL) at 90 ℃ under air atmosphere for 18 h.②Yield of the isolated product.
3.2 反应底物范围
得到最佳反应条件后,接着考察了反应底物的适用范围(图2).初步筛选表明:氮原子上带有n-Bu,Bn取代时,均能以适中产率得到目标产物(产物3a,3b).除了利用AIBN(偶氮二异丁基)为偶氮试剂之外,还尝试利用其他偶氮试剂为烷基自由基源,发现反应仍然保持较高的活性,分别以中等以上的产率得到产物(产物3g,3h,3i).
非常值得一提的是,此偶联反应表现惊奇的区域选择性.当丙烯酰基苯甲酰胺的苯环上3,4位上连有2个卤原子或者其他卤素时,反应总是在3位卤原子发生自由基偶联反应引入叔烷基基团.甚至当4位连有更活泼的卤元素(如F),而3位连有较惰性的卤元素(如Cl)的时候,反应依然遵循以上所述的区域选择性,即3位选择性发生烷基化偶联反应,以适中产率得到了一系列异喹啉二酮化合物(产物3c,3d,3e,3f).
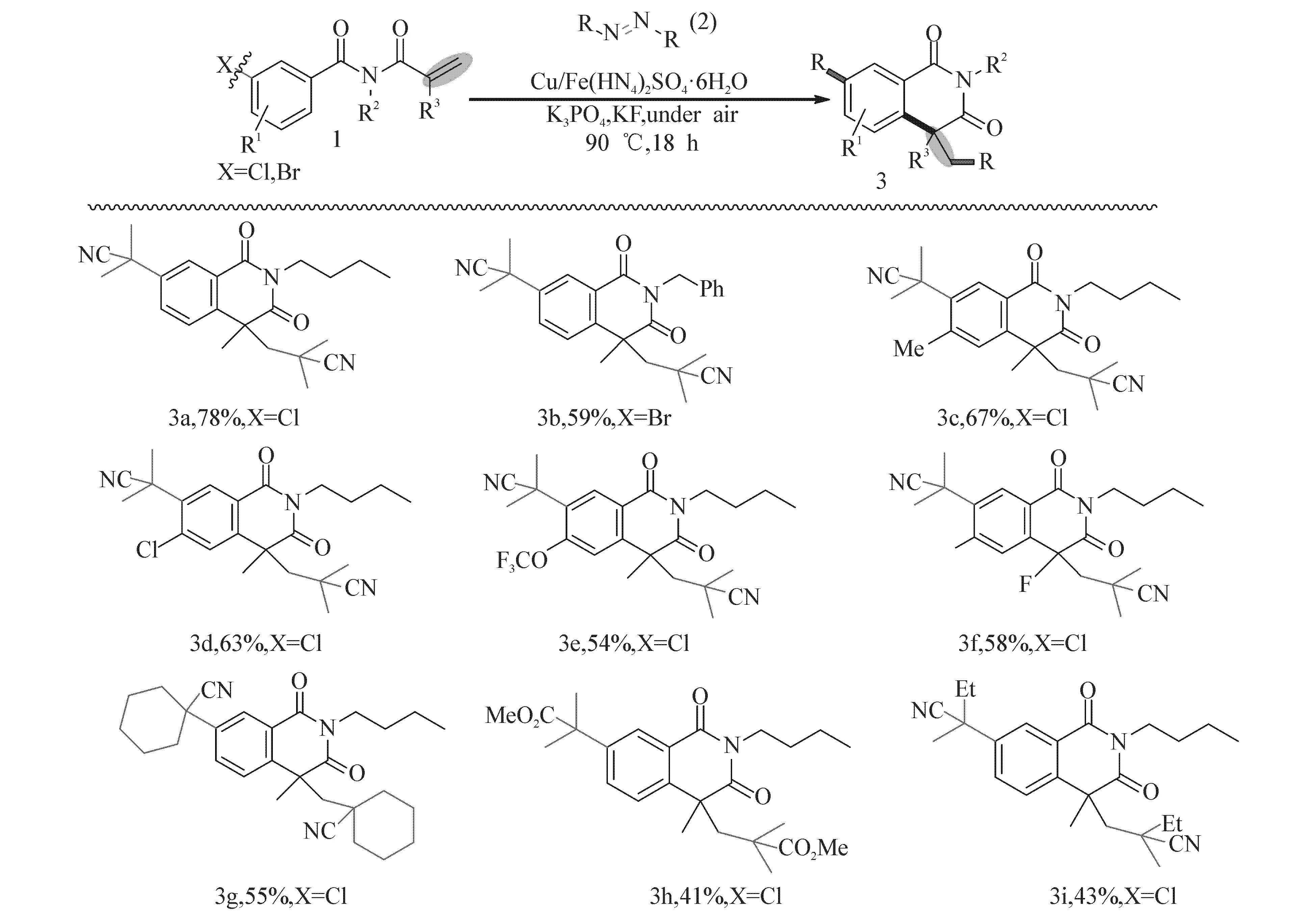
图2 底物N-烯丙酰基苯甲酰胺及烷基偶氮试剂的适应范围Fig. 2 Scope of Methacryloyl Benzamides and Alkyl azo Reagents
3.3 反应机理推测
为了探测反应机理,进行了如图3所示的控制试验.结果显示加入过量自由基清除剂TMEPO(2,2,6,6-四甲基哌啶-氮氧化物)时,此环化反应几乎不能发生,此现象表明此反应应该经历了一个自由基历程.
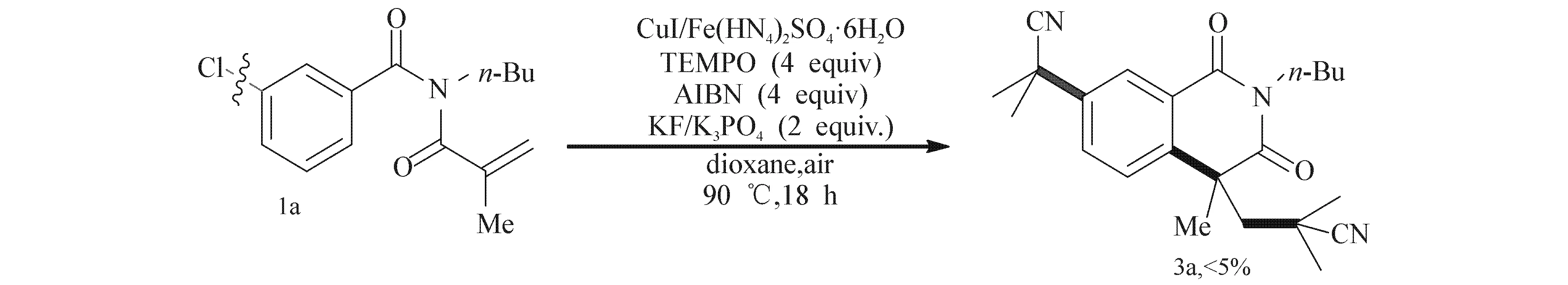
图3 有关产物3a形成的控制实验Fig. 3 Control Experiment for the Formation of 3a

图4 叔烷基化异喹啉二酮3a形成的可能机理过程Fig. 4 Proposed Mechanism for the Formation of Tert-Alkylating Isoquinolinedione 3a
4 结论
笔者首次发展了一种高效的CuI/Fe(NH4)2SO4·6H2O/air协同催化合成官能化异喹啉-1,3(2H,4H)二酮的新途径.此反应以烷基偶氮试剂为α-取代叔烷基自由基源物质,在廉价铜/铁/空气体系协同催化氧化下,N-丙烯酰基-N-丁基-3氯代苯甲酰胺经历一个由自由基加成引发的、继而去芳构化/自由基碳-碳偶联/重新芳构化的串联过程,一步成功构筑了多重α-官能化碳(叔)—碳键,得到了一系列7位叔烷基化异喹啉二酮衍生物.此反应底物适应范围广,反应操作简单、原料易得,为发现功能化异喹啉二酮类先导化合物提供了参考.

