Ablation of atrial fibrillation-patient selection,periprocedural anticoagulation,techniques,and preventive measures after ablation
Patient Selection
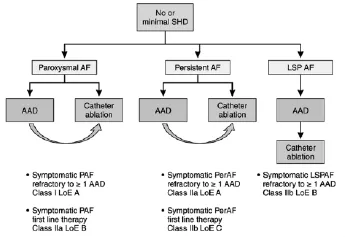
Figure 1 Selection of patients with no or minimal structural heart disease for atrial fibrillation(AF) ablation.AAD indicates antiarrhythmic drug;LoE,Level of Evidence;LSPAF,long-standing persistent atrial fibrillation;PAF,paroxysmal atrial fibrillation;and PerAF,persistent atrial fibrillation.
Patient selection for ablation is a shared decision by the patient and physician.The odds of a successful ablation not only are related to the technique of the procedure but also are critically to patient characteristics.The ideal patient with the highest likelihood of procedural success is one with paroxysmal AF,no underlying cardiac disease,and a nondilated left atrium(Figure 1).Paroxysmal AF is defined as AF <7 days in duration.Success rates with these patients approaches 80%.However,it has become clear in the last decade that even patients with persistent(>7 days)and long-standing persistent(continuous AF >1 year)AF may also benefit from AF ablation.In addition,patients with congestive heart failure or decreased left ventricular ejection fraction may be candidates for AF ablation(Figure 2).However,it is clear that the odds of a successful ablation are diminished with greater underlying heart disease.In particular,left atrial enlargement,mitral valve disease,and chronic heart failure are associated with poorer outcomes.It was once believed thatpatients need to failatleast1 antiarrhythmic agent before AF ablation.However,given the known toxicities of antiarrhythmic agents and the success of ablation,ablation is now acceptable as an initial rhythm control strategy,at least for paroxysmal AF.

Figure 2 Selection of patients with structural heart disease(SHD)for atrial fibrillation (AF)ablation.AAD indicates antiarrhythmic drug.
The latest AF ablation guidelines were updated in the United States in 2014 and in Europe in 2012(Table 1).For patients with symptomatic paroxysmal AF,the latest American Heart Association/American College of Cardiology recommendations give a ClassⅠrecommendation (is useful)for catheter ablation in patients with paroxysmal AF who have failed or are intolerant of classⅠorⅢantiarrhythmic agents.These same guidelines give a IIa recommendation(is reasonable)for those symptomatic patients with paroxysmal AF who wish to pursue ablation as an initial rhythm strategy and patients with symptomatic persistent AF who have failed or are intolerant to a classⅠorⅢantiarrhythmic agent.

Table 1 P atient Selection for AF Ablation According to US and European Guidelines
Repeat ablations are necessary in many patients,particularly in those with underlying heart disease.In general,a younger age,greater severity of symptoms,and contribution of AF to heart failure/cardiomyopathy factor into1the decision for repeat ablations.In many of these individuals,repeat ablations should be performed.
Anticoagulation
The vitamin K antagonist warfarin has been the gold standard for anticoagulation in AF.Its ability to lower the risk of thromboembolism has been established by large randomized,controlled trials (RCTs) in the 1980s and early 1990s.However,warfarin therapy is complicated bytheneed fordietarycompliance,sensitivity to multiple medications,frequent blood draws for monitoring, and often unexplained INR(international normalized ratio) fluctuations.Thus,there has been a widespread desire to develop warfarin substitutes that are safer and easier to administer.
Dabigatran,a direct thrombin inhibitor,and the direct factor Xa inhibitors rivaroxaban,apixaban,and edoxaban are currently available. All of these newer/direct anticoagulants were tested against warfarin;they have not been directly compared with one another in a clinical trial.Although the clinical results of the trials are similar,there are differences in trial design,patient thromboembolic risk,and end points that prevent their direct comparison.
The CHA2DS2-VASc score has largely supplanted the CHADS score for the estimation of stroke risk in patients with AF.The CHA2DS2-VASc score includes 7 clinical characteristics (congestive heart failure,hypertension,age,diabetes mellitus,stroke/transient ischemic attack,vascular disease,female sex)to stratify stroke risk.In the US guidelines,individuals with a CHA2DS2-VASc score of≥2 are recommended to undergo anticoagulation.ESC guidelines recommend anticoagulation for those with a CHA2DS2-VASc score of≥1(IIa;should be considered for those with a score of 1).The data on the risk of stroke in individuals with AF with a CHA2DS2-VASc score of 1 are conflicting and thus underlie the different recommendations in the United States and Europe.
Periprocedural prescription of anticoagulation is evolving.Initially,warfarin was held either with or without heparin bridging.Subsequent data emerged that showed that bleeding complications were actually lower with uninterrupted warfarin compared with a bridging strategy.In addition,uninterrupted strategies reduce both clinical and silent thromboembolic events.This pattern of withholding anticoagulation around the time of the ablation also applied to the direct anticoagulants,with some studies advocating bridging and others not.There is more concern about the treatment of bleeding complications with the direct anticoagulants because of the previous lack of reversal agents.Registry data have suggested that ablation can be performed without discontinuation of these agents,although often 1 or 2 doses are held before the procedure.In addition,the developmentofreversalagents forthese direct anticoagulants should provide more justification for uninterrupted anticoagulation at the time of the ablation.Continuation of anticoagulants should persist for at least 2 months after the procedure.Continuing anticoagulation for an extended time period depends on the CHA2DS2-VASc score.The 2012 ESC guidelines state that long-term anticoagulation is recommended in all patients with a CHA2DS2-VASc score of≥2,and the US guidelines agree that the decision for continued anticoagulation largely hinges on the CHA2DS2-VASc score.In the US guidelines,individuals with a low CHA2DS2-VASc score and successful ablation can likely stop anticoagulation after 2 to 3 months.Current guidelines do not recommend AF ablation for the sole purpose of discontinuation of anticoagulation.
Techniques
Techniques for AF ablation are maturing,and differences are not completely resolved.Techniques for the ablation of AF vary by energy source and location of atrial lesions(ie,lesion sets).
Energy sources available for AF ablation include radiofrequency,cryoablation,and laser.Radiofrequency and cryoablation are currently most commonly used.Radiofrequency creates a lesion with heat(typically up to 60℃)and can be delivered with or without saline irrigation at the tip of the catheter.Irrigated-tip catheters reduce the risk of char formation and improve lesion depth and size;in general,they are the most commonly used catheters for radiofrequency ablation.New catheters with the ability to quantify the force of contact have recently been developed and appear to give more consistent lesions than prior catheters.Cryoablation is administered via a left atrial balloon that occludes each PV individually and freezes to-50℃.Laser ablation is performed with special catheters designed to create a circumferential lesion set around each PV.
The location of ablation lesions is where the major controversies in AF ablation lie.It is reasonably acknowledged that for individuals with paroxysmal AF and no underlying heart failure,electric isolation of the PVs at the antral level is associated with a high degree of elimination of AF.This isolation can be accomplished with radiofrequency,cryoablation,or laser energy.Randomized,clinical trials in this patient population generally compare an ablative technique with antiarrhythmic drug therapy.
Whether one ablative technique to isolate the PVs is superior to the other is not clear.Limited registry data have compared radiofrequency with cryoablation.In the German Ablation Registry,the recurrence rate at 1 year after a single procedure was≈45%in both groups.There have now been 2 RCTs comparing radiofrequency and cryoablation.In FREEZE AF,the reported success rates in the radiofrequency and cryoablation groups were comparable at 1-year follow up(63.1%versus 64.1%after a single procedure and 70.7%versus 73.6%after multiple procedures in the radiofrequency and cryoablation group,respectively).In the recently published FIRE AND ICE (Cryoablation or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation)trial comparing radiofrequency and cryoablation in 762 patients with paroxysmal AF,the 1-year recurrences were 35.9% and 34.6% ,respectively.
In patients with nonparoxysmal AF,there is currently no one clearly preferred approach.In these individuals,isolation of the PVs alone may not be adequate to prevent recurrences.These additional lesion sets can be anatomically based or electrically guided.Anatomic guidance creates ablation lines in particular locations.The most common location is the roof between the right and left superior PVs (roof line).Additional lines can be placed between the left and right inferior veins,left inferior PV,and mitral annulus(mitral line).A cavotricuspid right-sided isthmus line may also be placed.However,reports from RCTs documenting outcomesofadditionallinearlesionshave been contradictory.Some RCTs have demonstrated benefits of adjunctive linear lesions in increasing arrhythmia-free survival rates in patients with nonparoxysmal AF undergoing PV isolation.In contrast,other RCTs have reported no additional advantages of a linear ablation approach over PV isolation alone in patients with AF,and the lines required significantly more ablation time,higher radiation doses,and longer procedure durations.
Electric lesion sets can be guided electrically by complex fractionated electric potentials or focal/reentrant sources(also described as rotors or drivers).Complex fractionated electric potentials can be identified by individual operators or by special software analysis programs available in some commercial mapping systems.Focal/reentrant sources are currently mapped by an endocardial basket and specialized software FIRM(Focal Impulse and Rotor Mapping)or by surface mapping(Figure 6).FIRM-guided ablation of persistent AF has shown higher 3-year freedom from AF compared with conventional ablation(single-procedure freedom from AF at 890 days,75%versus 30%).
Relatively common periprocedural complications include groin hematomas,pericardial chest pain,and atrial irritability with atrial premature contractions and AF.Less common but more severe complications include cardiac perforation and tamponade(≈1%),stroke or transient ischemic attack(≈1%),vascular aneurysms or fistulas(≈1%),PV stenosis(common previously but rare currently),and phrenic nerve paralysis, particularly with cryoablation. Rare complications include mitral valve injury,myocardial infarction,air embolism,radiation injury,atrial esophageal fistula,gastric motility disorders,and death.
词 汇
odds n.机会、机运
candidates n.候选人、应试者、考生
pursue v.追赶、追求、追随、继续
compliance n.顺从、遵从、依从
fluctuation n.波动,起伏
substitute n.&v.&adj.替代物,替代者;替代,取代,接替;替代的,临时的
prescription n.&adj.规定,处方,书面医嘱;凭处方供应的
cryoablation n.冷冻消融
char n.&v.炭,烧焦物;烧成炭,打杂,做
antral adj.窦的,前庭的
rotor n.转子,旋筒,旋翼
motility n.能动
注 释
1.factor into指“成为(影响)…的”因素,factor可作为动词使用,但以这种主动形式出现的很少见,通常以“be factored into”形式出现,如The comsumer's viewpoint should be factored into decision making.消费者的观点应作为决策因素计入。
参考译文
第87课 心房颤动消融-患者选择、围手术期抗凝、技术及术后预防措施
患者选择
患者消融的选择由患者与医师共同决策。成功消融的概率不但与手术技术有关,也与患者的特征明显相关。手术成功率最高的理想患者是阵发性心房颤动,无基础心脏疾病和左心房扩大(见图1)。阵发性心房颤动的定义是病程<7d。这类患者的成功率达80%。不过,最近10年已明确即使持续性心房颤动(>7 d)和慢性心房颤动也可从心房颤动的消融中获益。另外,充血性心力衰竭或射血分数下降的患者也可选心房颤动消融(见图2)。但是,基础心脏疾病较明显者消融成功概率降低。特别是左心房扩大、二尖瓣病变和慢性心力衰竭患者手术结果较差。先前认为心房颤动消融前至少有一种抗心律失常药物无效。不过,鉴于抗心律失常药物的已知毒性和消融的成功,消融术已成为初始节律控制的方案,至少适合阵发性心房颤动。
最近的心房颤动消融指南更新是2014美国和2012欧洲指南(见表1)。对于有症状的阵发性心房颤动患者,最新的AHA/ACC建议Ⅰ类或Ⅲ类抗心律失常药物治疗无效或不能耐受的阵发性心房颤动患者为Ⅰ类(有用的)建议,对于追求消融术作为首选节律控制方案的有症状的阵发性心房颤动患者、Ⅰ类或Ⅲ类抗心律失常药物无效或不能耐受的有症状的持续性心房颤动患者为Ⅱa类(有理由的)建议。
许多患者,特别是那些有基础心脏病者需要再次消融手术。总之,年轻的、症状较严重的、心房颤动导致心力衰竭或心肌病的,成为再次消融手术决策的因素。这些个体中的多数,应实施再次消融手术。
抗凝
维生素K拮抗剂华法林一直是心房颤动抗凝的金标准。80年代和90年代早期的大规模随机、对照试验已确立它能降低血栓栓塞的风险。然而,华法林治疗的复杂性在于需要饮食顺从、对多种药物敏感、需频繁抽血监测以及经常出现不能解释的国际标准化比值(INR)波动。这样,普遍希望发展一种对使用者既安全又方便的华法林替代物。
直接凝血酶抑制剂达比加全,直接Xa抑制剂利伐沙坦、阿哌沙坦和依度沙班现已面市。所有这些新型或直接的抗凝剂都与华法林做对比测试。它们相互之间未做直接比较的临床试验。尽管试验的临床结果类似,但试验的设计、患者血栓栓塞的风险和预防的终点存在差异,这妨碍了它们之间的直接比较。
CHA2DS2-VASc评分较大程度上取代了CHADS评估心房颤动患者中风风险。CHA2DS2-VASc评分纳入7个临床特征(充血性心力衰竭、高血压、年龄、糖尿病、脑卒中/短暂脑缺血发作、动脉疾病和女性)对脑卒中风险进行分层。美国指南建议对CHA2DS2-VASc评分≥2分的个体进行抗凝治疗。ESC指南建议对CHA2DS2-VASc评分≥1分的人群进行抗凝治疗(而CHA2DS2-VASc=1分的定为Ⅱa类推荐)。有关CHA2DS2-VASc=1分患者的中风风险资料存在冲突,从而导致美国与欧洲指南的不同。
围手术期抗凝方案不断演变中。最初是停用华法林,桥接或不桥接肝素。随后的资料显示,与桥接方案比较,不停用华法林的出血并发症竟然较低。此外,不停用方案减少临床的和隐匿的血栓栓塞事件。这种围消融手术期停用抗凝剂的方式也应用到直接抗凝剂,有些研究倾向于桥接,而另一些不是。更多关注的是直接抗凝剂的出血并发症,因为此前缺乏相应的逆转制剂。注册资料表明可以在不停用这些抗凝剂的情况下进行消融手术,虽然在消融前常常停1~2次。另外,这些直接抗凝剂逆转制剂的出现将为消融手术时不停用抗凝剂提供更为充分的依据。
消融手术后连续抗凝治疗应持续至少2个月。持续抗凝延长时间决定于CHA2DS2-VASc评分。2012年ESC指南建议所有CHA2DS2-VASc评分 2分的患者应长期抗凝治疗,美国指南同意持续抗凝的决策基本上依据CHA2DS2-VASc评分而定。对CHA2DS2-VASc评分低且成功消融的个体,美国指南建议2~3个月后停用抗凝剂。当前指南推荐心房颤动消融手术的理由并非只是为了停用抗凝剂。
消融技术
心房颤动的消融技术处在成熟的过程中,差异没有完全解决。心房颤动的消融技术因能源和心房病变(病灶)部位而异。
用于心房颤动消融的能源包括射频、冷冻和激光。目前极大多数使用射频和激光。射频作用源于热损伤(通常达60℃),可使用有或无头端盐水灌注的导管实施。灌注导管减少积碳形成的风险、提高损伤深度和范围,总之,这是最为常用的射频消融导管。近期生产出的新导管能够确定接触的力度,似乎较此前的导管产生更为一致的损伤。冷冻消融通过左心房球囊而实施,球囊分别堵塞每一个肺静脉冰冻至-50℃。激光消融通过特殊的导管实施,环绕每个肺静脉产生环形损伤。
消融损伤的部位是心房颤动消融的主要争论所在。普遍公认的是对于阵发性心房颤动且无心力衰竭者,于前庭水平电隔离肺静脉能极大地消除心房颤动。这种隔离可通过射频、冷冻或激光完成。这一人群的随机临床试验通常将消融技术与抗心律失常药物治疗作比较。
在隔离肺静脉上,尚不明确某种消融技术是否优于其他技术。有限的注册资料对射频和冷冻消融做了比较。在德国的消融注册中,单次手术1年后复发率两组均接近45%。目前有两项随机对照临床试验对射频消融与冷冻消融作了比较。FREEZE AF研究报告,射频与冷冻消融后随访1年的成功率接近(单次消融后为63.1%比64.1%,多次消融后为70.7%比73.6%)。最近出版的FIRE AND ICE(阵发性心房颤动的冷冻或射频消融治疗)试验对762例阵发性心房颤动患者的射频和冷冻消融进行了比较,1年的复发率分别为35.9%和34.6%。
对于非阵发性心房颤动患者,目前缺乏明确优选的方法。在这些人群,单纯肺静脉隔离不足以防范复发,可根据解剖或电学引导消融这些外加部位。特殊部位由解剖引导产生消融线。最常见的部位是位于左右上肺静脉之间的顶部(顶线),附加线可位于左右下静脉,左下肺静脉,和二尖瓣环(二尖瓣线)之间。还有腔静脉三尖瓣右侧峡部线。然而,附加线消融预后的随机临床对照研究报告存在矛盾。有些随机对照研究证实附加线消融有益于非阵发性心房颤动肺静脉隔离患者的无心律失常生存率提高。相反,其他随机对照研究报告显示对于心房颤动患者,在单纯肺静脉隔离的基础上加线性消融方法并无额外获益,却明显增加消融时间,射线量较大,手术时间较长。
可由复杂的碎裂电位或局部/折返源(又称转子或驱动器)从电学方面来探查电机能障碍部位。复杂碎裂电位由具体术者或某些商业标测系统中的特别软件分析程序识别。局部/折返源当前通过心内膜蓝(蓝状电极-译者注)和特殊软件FIRM(局部冲动和转子标测)或体表标测来标测。FIRM引导的持续性心房颤动消融表明,与常规消融比较,3年无心房颤动比例较高(单次手术后890天的无心房颤动率从30%提高到75%)。
较常见的围手术期并发症包括股部血肿、心包胸痛和伴随心房期前激动与心房颤动的心房易激。少见但更严重的并发症包括心脏穿孔和填塞、中风或短暂脑缺血发作、动脉瘤或瘘、肺静脉狭窄和膈神经麻痹,特别是冷冻消融。少见的并发症包括二尖瓣损伤、心肌梗死、气栓、放射损伤、心房食道瘘、胃动力障碍和死亡。
图1无或轻微结构性心脏疾病患者心房颤动消融的选择。AAD:抗心律失常药物了;LoE:依据水平;LSPAF:慢性持续性心房颤动;PAF:阵发性心房颤动;PerAF:持续性心房颤动。
图2结构性心脏病患者的心房颤动消融选择。AAD:抗心律失常药物。
表1基于美国和欧洲指南的心房颤动消融患者选择