Liver involvement in the drug reaction,eosinophilia,and systemic symptoms syndrome
Sylvia A Martinez-Cabriales,Neil H Shear,Emmanuel I Gonzalez-Moreno
Abstract
Key words: Drug reaction,eosinophilia,and systemic symptoms syndrome;Severe cutaneous drug reactions;Drug-induced hypersensitivity syndrome;Drug-induced liver injury;Acute liver failure
INTRODUCTION
Adverse drug reactions commonly involve the liver,the main organ in which drug metabolism occurs.It has been estimated that more than 600 medications have been related to significant liver injury[1].Furthermore,herbal,complementary,and alternative medications,as well as illicit drugs such as anabolic steroids and amphetamines,have also been related to this problem[2,3].Drug-induced liver injury(DILI) is one type of adverse drug reaction,which was ranked as one of the major causes of acute liver failure[4-6].It is classified into two main types:intrinsic or idiosyncratic[7].The former is all the predictable adverse drug reactions that are dosedependent and manifested shortly after the drug was ingested[8].In this case,the culprit is direct-chemical damage to the drug or its metabolite[9].The idiosyncratic drug reaction,which is unpredictable,is characterized by a delayed onset of symptoms once the drug was taken[10].It is subdivided into two categories:nonallergic and allergic reaction.In the non-allergic,the liver frequently is the only organ involved;however,in the allergic reaction,multisystemic organ involvement may be observed[11,12].
Drug reaction,eosinophilia,and systemic symptoms syndrome (DReSS),also widely known as drug-induced hypersensitivity syndrome (DiHS),corresponds to a hypersensitivity drug reaction[13].This syndrome was recognized in 1981 when Speilberg and Shear identified drug hepatotoxicity along with fever and a rash,which they called anticonvulsant hypersensitivity syndrome[14].Several diagnostic criteria have been proposed for DReSS/DiHS,which is characterized by the presence of a maculopapular erythematous skin eruption,fever,lymphadenopathy,eosinophilia,and visceral involvement such as hepatitis,pneumonitis,myocarditis,pericarditis,nephritis,and colitis,and the liver is the most common organ involved[15].Importantly,DReSS/DiHS might present with acute liver failure,which increases its mortality[16].In this context,liver failure is usually classified in the group of druginduced liver injuries[17].This review focuses on the liver involvement present in DReSS/DiHS reported in the literature in case series.
LITERATURE REVIEW
We reviewed the literature and summarized all reported case-series of DReSSassociated with liver involvement obtained from MEDLINE and EMBASE between January 1990 and July 2018 using the following terms:“DReSS syndrome,” “drug reaction with eosinophilia and systemic symptoms,” “drug rash with eosinophilia and systemic symptoms,” “drug hypersensitivity and eosinophilia,” “drug-induced hypersensitivity syndrome”.The search was limited to the English language.After gathering all articles,we described the number of patients included,those with liver involvement,type of presentation,blood work,drug involved,other associations,treatment received,mortality,and follow-up.
DEFINITIONS
In this review,we will use the acronym DReSS/DiHS instead of DRESS as a recent review highlighted the importance of clarifying that eosinophilia is not mandatory to confirm this syndrome[18].In 1996,Bocquetet al[19]established three criteria needed for diagnosis of DReSS/DiHS syndrome:skin eruption,eosinophilia (≥ 1.5 × 109/μL),and visceral involvement (transaminase elevation ≥ 2 times upper normal limit,lymphadenopathy > 2 cm in diameter,nephritis,interstitial pneumonia,or carditis).In 2006,Shioharaet al[20]proposed to include as diagnostic criteria the presence of human herpes virus 6 (HHV-6) reactivation,as they documented HHV-6 IgG titers and DNA 2-3 wk after the onset of the rash.The group suggested this virus to be a cause of this hypersensitivity syndrome.Finally,in 2007 the RegiSCAR group developed a new scoring system.Hospital admission as a result of the suspected drug-related reaction and at least three of the following findings:acute skin rash,fever,lymphadenopathy of at least two sites,the involvement of at least one internal organ,lymphocytosis/lymphocytopenia,peripheral eosinophilia,and thrombocytopenia.According to this scoring system,patients were classified into definite,probable,possible,or no diagnosis of DReSS/DiHS (Table 1)[21].
With regard of DILI,a previous definition set the following threshold for defining its diagnosis:elevation of AST and/or ALT or bilirubin or alkaline phosphatase > 2 upper limit of normal (ULN)[22,23].Subsequently,given the adaptation or tolerance that may occur in up to 20% of drugs,the levels of transaminases elevations were modified to > 5 ULN without symptoms,or rise in alkaline phosphatase > 2 ULN,or rise in bilirubin > 2 ULN with any transaminases increasing.Alternatively,AST or ALT < 5 ULN with symptoms also defines DILI[24].
Patients with acute hepatitis and elevated prothrombin time or international normalized ratio levels without mental status changes are frequently labeled as having a severe acute liver injury[25].
EPIDEMIOLOGY
Although liver involvement is the most common visceral manifestation of patients with DReSS/DiHS,it presents mainly as hepatocellular injury,sometimes cholestasis,or both and rarely fulminant hepatitis and death[26].Asymptomatic transaminasemia may occur in up to 20% of patients on drugs[27].An estimation of a severe cutaneous drug reaction is about 1 of every 1000 hospitalized patients[28].The DReSS/DiHS belongs to this category with an estimated incidence of one in 1000 to one in 10000 drug exposures[29,30]and mortality of approximately 5% to 10%[31].Liver injury is the most common organ damage seen in cases of DReSS/DiHS with rates ranging from 51% to 87%[15,31-34].Kardaunet al[35]reported that liver injury was the most common internal organ involvement seen in 75% (81/114) of DReSS/DiHS cases,in which 91%of the cases had visceral organ involvement.Shiohara[20]reported liver complications in up to 70% of drug-induced hypersensitivity syndrome patients.Cacoubet al[13]reported liver injury in 94% of DReSS/DiHS patients.One study reported β-lactams antibiotics,allopurinol,non-steroidal anti-inflammatory drugs,and sulfonamide as the most commonly associated drugs with DReSS/DiHS accompanied by liver dysfunction in 23 cases[36].Another study reported sulfonamides (13/14;92.9%),followed by antiepileptic drugs (19/22;86.3%),and allopurinol (15/19;78%) to have the highest risk of inducing liver injury in DReSS/DiHS[37].To put DILI in context,Russoet al[38]reported that drug hepatotoxicity was the cause in 15% of liver transplantation as a result of acute liver failure from 2291 transplants in the United States between 1990 and 2002.Even though acetaminophen either as a single treatment or combined with another drug,was the principal drug related in 133/270(49%) cases,idiosyncratic liver injury leading to 42% of liver transplants,was associated with four drugs:isoniazid,propylthiouracil,phenytoin,and valproate[38].
PATHOGENESIS
The pathogenesis of DReSS/DiHS is multifactorial including genetic polymorphisms and environmental factors.One hypothesis is based on the combination of a drugcovalently joined to a protein acting as a hapten,accompanied by a co-stimulatory trigger-virus infection or reactivation,bacterial infection,or inflammatory disorder in a genetically susceptible individual leading to T-cell responses to the antigen,which could be expressed on the hepatocytes surface[39].Studies have shown the presence of drug-specific cytotoxic T cells in the serum and liver of DILI patients and the skin of DReSS/DiHS patients[40,41].These cells,which release perforin,granzyme B,and Fas/Fas L-dependent cell death,are believed to induce cell death in both organs[40,42].

Table1 RegiSCAR scoring system for classifying drug reaction,eosinophilia,and systemic symptoms syndrome/drug-induced hypersensitivity syndrome
Another proposed mechanism involves the immune response to reactivation of latent viruses of the herpesvirus family[43,44],which is seen in DReSS/DiHS complicated cases.It is hypothesized that DReSS/DiHS triggers reactivation of latent viral infection,which may produce a viral exanthema of fevers and rash that may overlap with,or be difficult to distinguish from DReSS/DiHS.Tohyamaet al[43]compared 100 patients with or without an increase of anti-HHV-6 IgG titers and reported that the flare-up of symptoms such as fever and hepatitis was closely related to HHV-6 reactivation.In Eshkiet al[45]retrospective study,only seven patients were examined for an active HHV-6 infection.An active HHV-6 infection was found in six patients,including a patient with fulminant liver failure.Further tests confirmed that HHV-6 infection was a reactivation and not a primary infection.Furthermore,HHV-6 may also cause hepatitis,including fulminant liver failure that is rapidly reversed when antiviral treatment is promptly initiated[46].
Liver damage in patients with DReSS/DiHS could be caused by eosinophilic infiltration driven by interleukin IL-5[47-49].Hypereosinophilia,if persistent,can be toxic to endothelial cells and contribute to organ damage such as interstitial nephritis,pneumonitis,myositis,eosinophilic carditis,pancreatitis,thyroiditis or encephalitis,and possibly hepatitis[21].
CLINICAL PRESENTATION
The liver may be the first organ involved in a hypersensitivity drug reaction[37].It could range from a mild increase of liver enzymes to acute fulminant hepatic failure with the cholestatic type as the most common.The cholestatic pattern is characterized by increased serum transaminases and alkaline phosphatase with prolonged jaundice after drug withdrawal.The hepatocellular pattern presents with increased serum transaminases,minimal serum alkaline phosphatase elevation,and variable jaundice.A mixed pattern has combined features of hepatocellular and cholestatic injury(Figure 1).Peyrièreet al[15]reported liver involvement in more than 60% of 216 DReSS/DiHS cases with a hepatocellular necrosis more common than the cholestasis.Linet al[37]reported that atypical lymphocytosis was seen more frequently on DReSS/DiHS cases with liver injury than cases without liver involvement (74.2%vs30.0%,P= 0.010).One study reported that younger patients most commonly presented with a hepatocellular-type,and that the cholestatic-type was seen more often in older patients (P= 0.044).
Compared to other severe drug hypersensitivity reactions such as Stevens-Johnson syndrome,a study found a more severe hepatocellular pattern and a moderate to severe cholestatic-type liver injury along with longer liver recovery in DReSS/DiHS cases.They emphasized that the long duration of the liver involvement could last months after the rash resolved[36].Wanget al[50]reported a hyperbilirubinemia in 12(31.58%) patients,aspartate aminotransferase (AST) elevation (> 100 IU/L) in 19(50.50%) patients,and 9 (23.68%) patients developed hepatic failure.Several case reports have reported liver injury before skin eruption.Linet al[37]noticed this clinical presentation in 9.7% of cases.Leeet al[17]reported that renal dysfunction was more common in patients with liver dysfunction (39%vs1%,P= 0.001),and patients with liver dysfunction were more likely to have renal dysfunction (96%vs34%,P= 0.001).Lymphadenopathy was also commonly seen in patients with liver involvement (23%vs6%,P= 0.005).Mortality was significantly higher in patients with liver dysfunction(11%vs1%,P= 0.018).Ichaiet al[16]described the histological features on the liver from DReSS/DiHS cases.They reported acute hepatitis with cytotoxic phenotype.Eosinophils were found in five of seven cases.Kupffer cell hyperplasia with erythrophagocytosis was observed in six of seven cases.They also reported a diminished factor V level at admission (less than 40%),or a reduction at day 2 was predictive of death or liver transplant (Table 2).
SKIN BIOPSY
Linet al[37]did not find any difference in the eosinophils in the dermis between patients with or without liver injury (64.5%vs60%,P= 1).On the other hand,they reported that eosinophils in the dermis were present more frequently in patients with non-severe hypersensitivity hepatitis (88.9%vs30.8%,P= 0.002),concluding that the extreme group cases might be more related to the immunoallergic attack to the hepatocytes[37].Walshet al[51]reported that patients with clinical presentation of erythema multiforme-like were associated with higher elevations of AST (P= 0.01),concluding these patients have worse liver involvement[51].
TREATMENT

Figure1 Diagnostic algorithm of drug reaction,eosinophilia,and systemic symptoms syndrome / drug-induced hypersensitivity syndrome case series.
Although further studies are needed to evaluate the role of systemic corticosteroids in drug-induced systemic hypersensitivity and liver injury,it seems this therapy has a role in the treatment with DReSS/DiHS and liver involvement.A favorable outcome has been reported when fulminant hepatitis associated with DReSS/DiHS was treated with intensive corticosteroid therapy (methylprednisolone 1 g/d) for 3 d (3750 mg prednisone within 30 d)[52].On the other hand,the study by Leeet al[36]demonstrated that in patients with DReSS/DiHS associated with liver injury,the use of systemic corticosteroids did not confer additional benefits regarding disease duration and recovery of liver function.
MORTALITY
Concerning DReSS/DiHS,acute-stage mortality ranges from 5% to 10% and is mainly attributed to specific liver injury,myocardial or pulmonary lesions,and hemophagocytosis[19,26].Fifteen percent of liver transplantation cases in the United States are caused by DILI[38].The mortality of 10% in those patients with a combination of hepatocellular injury and jaundice,first described by Zimmerman,has been confirmed in several studies[53-55].In their case-series Ichaiet al[16]reported that 43.7% of patients (7/16) with DReSS/DiHS related acute liver injury/acute liver failure underwent transplantation (n= 5) or died (n= 2).
CONCLUSION
Although rare,DReSS/DiHS is considered a severe cutaneous drug reaction,which could potentially lead to death,especially in patients with delayed diagnosis,viral reactivation,the presence of systemic inflammatory response syndrome,and severe organ involvement.A better understanding of its pathophysiology is required to elucidate risk factors for severe visceral involvement,as it is demonstrated to be the main cause of mortality.Patients with ongoing deterioration of liver function must be tested for reactivation of latent viruses of the herpesvirus family.Furthermore,a multidisciplinary approach in patients with severe internal organ affection is of utmost importance.

Table2 Liver involvement reported in drug reaction,eosinophilia,and systemic symptoms syndrome/drug-induced hypersensitivity syndrome case series

Ang et al[58] 27 (M:12 F:15)0 8 (32) flared while SS tapering;17 completed SS treatment (7 to 160 d,mean of 50);Sequelae:RI 3;AT 1,and myocarditis 1 Um et al[47] 38 (M:18,F:20)26 (96.3) Liver enzymes > 10 UNL:13 (48);Eos 22 (%);Serology was not done Phenytoin 5;CBZ 4 RI 4 (15);RF 2 SS:25 (93);TS:2 (7);LT:0 38 (100) ALT (mean 383.39 IU/L,range 26-3633);AST(mean 382.73 IU/L,range 28-2360);Eos(> 500/μL) 35(91);Serology negative to CMV,EBV,or HSV Anticonvulsa nts 18 (47);Antibiotics 7(18);NSAIDs 5 (13)RI 6 (16);ATL 18 (47)TS + anti-H:22 (58);SS:16(42)1 (3) LF +opportunistic infection 36 (95)recovered completely;1(2.6) LI was lost at FU 5 (19) relapse of DReSS during tapering of SS;21 (77.8)recovered well;Sequelae:5(19) that recovered within 6 mo Kardaun et al[35]Wongkitisop hon et al[59]27 (M:14;F:13)26 (96.3) LI > 2 UNL;Hepatomegal y (7.4)ALT mean 188 IU/L (r 132-1708);AST 132 IU/L(r 89-857);TB 9 (33.3) mean 32.7 μmol/L(r 18.9-244.2 μmol/L);Eos(> 700/μL) 19(70)Phenytoin 9(33)Allopurinol 4(15)Nevirapine 4(15)RI 2 (7);ATL(19)Non-AT:4;SS(DMT/PDNL):23 (85.2)1 (4) died from MOF 117 (M:52;F 65)86/114 (75) Transiently disturbed;liver function tests;Hepatomegal y and coagulopathy Eos (≥ 1500 μL-1) 92 (81);(700-1499 μL-1) 16 (14);HIV 1;HHV-6 react 21/58(36)Anticonvulsa nts 41 (35);Allopurinol 21 (18);Sulfonamide 14 (12)RI 40/108(37);ATL 68/102 (67);SJS,TEN or AGEP features were seen in 8 patients NA 2 (2) 1 overlap with SJS/TEN;and 1 overlap with AGEP Walsh et al[51]27 (M:10;F:17)27 (100);TRC of HPB LI before rash 4 (14.8);Significant LI:20;Mild LI:7;Cholestatic pattern was associated with interface dermatitis (P= 0.036)AST mean 970 IU/L,median 250 (31-5183);GGT mean 522 IU/L,median 379(9-1903);ALP mean 295 IU/L,median 266 (57-819);Eos (> 0.4 ×109/ L) 25 (93)Anticonvulsa nts 12;Antimicrobial s 10;Antirheumatics 5 RI 2 (7);Pericarditis (1 patient);GA(1 patient)MOD 3 patients LT:2 3 (11) All had severe liver injured.Two after failed LT 18 patients completed FU and normalized liver function Lee et al[36] 23 (M:12;F:11)23 (100) Significant LI 23 (100)ALT 186 IU/L(114.0-458.0);AST 207 IU/L(90.0-766.0);ALP 147 IU/L(116.0-338.0);TB 1.1 (0.8-13.3);Eos 17(74)Beta-lactams 7 (54);Allopurinol 3(13);Sulfonamide 2 (15)RI 13 (56);If LI higher risk of RI (P <0.001);and of LN (P = 0.005)LT:(2 patients;1 died);IVIG:1(4);PDNL 4 (17.39) Duration of the disease in survivors on steroids:25.3± 14.8 d Uhara et al[60]12 (M:4;F:8) 11 (92) Peak of LI appeared 7 d after the rash(range 3-22);ALT mean 176 (range 91-311)Eos (>1.5 ×109 / L) 4;HHV-6-IgG 12 (100)CBZ 6;Salazosulfapy ridine 4 ATL 8 (66) Non-AT on the first weeks of examination Hydration:7;TS:5 PDN:1 patient had RA;DMT(single dose):1 0 All patients recovered;7 to 37 d(median,18)after withdrawal of the drug
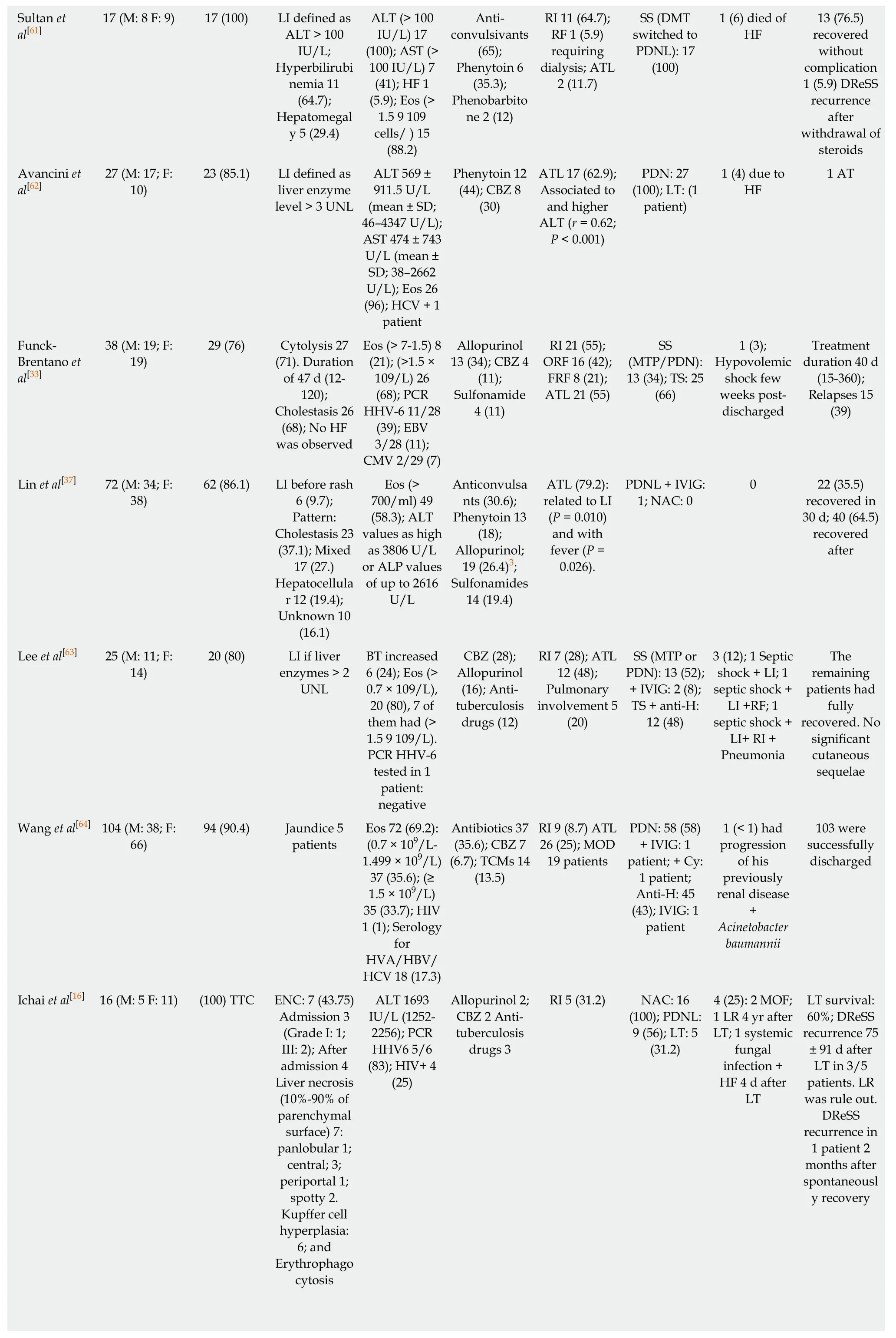
Sultan et al[61]13 (76.5)recovered without complication 1 (5.9) DReSS recurrence after withdrawal of steroids Avancini et al[62]17 (M:8 F:9) 17 (100) LI defined as ALT > 100 IU/L;Hyperbilirubi nemia 11(64.7);Hepatomegal y 5 (29.4)ALT (> 100 IU/L) 17(100);AST (>100 IU/L) 7(41);HF 1(5.9);Eos (>1.5 9 109 cells/ ) 15(88.2)Anticonvulsivants(65);Phenytoin 6(35.3);Phenobarbito ne 2 (12)RI 11 (64.7);RF 1 (5.9)requiring dialysis;ATL 2 (11.7)SS (DMT switched to PDNL):17(100)1 (6) died of HF 27 (M:17;F:10)23 (85.1) LI defined as liver enzyme level > 3 UNL ALT 569 ±911.5 U/L(mean ± SD;46-4347 U/L);AST 474 ± 743 U/L (mean ±SD;38-2662 U/L);Eos 26(96);HCV + 1 patient Phenytoin 12(44);CBZ 8(30)ATL 17 (62.9);Associated to and higher ALT (r = 0.62;P < 0.001)PDN:27(100);LT:(1 patient)1 (4) due to HF 1 AT Funck-Brentano et al[33]38 (M:19;F:19)29 (76) Cytolysis 27(71).Duration of 47 d (12-120);Cholestasis 26(68);No HF was observed Eos (> 7-1.5) 8(21);(>1.5 ×109/L) 26(68);PCR HHV-6 11/28(39);EBV 3/28 (11);CMV 2/29 (7)Allopurinol 13 (34);CBZ 4(11);Sulfonamide 4 (11)RI 21 (55);ORF 16 (42);FRF 8 (21);ATL 21 (55)SS(MTP/PDN):13 (34);TS:25(66)1 (3);Hypovolemic shock few weeks postdischarged Treatment duration 40 d(15-360);Relapses 15(39)Lin et al[37] 72 (M:34;F:38)62 (86.1) LI before rash 6 (9.7);Pattern:Cholestasis 23(37.1);Mixed 17 (27.)Hepatocellula r 12 (19.4);Unknown 10(16.1)Eos (>700/ml) 49(58.3);ALT values as high as 3806 U/L or ALP values of up to 2616 U/L Anticonvulsa nts (30.6);Phenytoin 13(18);Allopurinol;19 (26.4)3;Sulfonamides 14 (19.4)ATL (79.2):related to LI(P = 0.010)and with fever (P =0.026).PDNL + IVIG:1;NAC:0 0 22 (35.5)recovered in 30 d;40 (64.5)recovered after Lee et al[63] 25 (M:11;F:14)20 (80) LI if liver enzymes > 2 UNL BT increased 6 (24);Eos (>0.7 × 109/L),20 (80),7 of them had (>1.5 9 109/L).PCR HHV-6 tested in 1 patient:negative CBZ (28);Allopurinol(16);Antituberculosis drugs (12)RI 7 (28);ATL 12 (48);Pulmonary involvement 5(20)SS (MTP or PDN):13 (52);+ IVIG:2 (8);TS + anti-H:12 (48)3 (12);1 Septic shock + LI;1 septic shock +LI +RF;1 septic shock +LI+ RI +Pneumonia The remaining patients had fully recovered.No significant cutaneous sequelae Wang et al[64] 104 (M:38;F:66)94 (90.4) Jaundice 5 patients Eos 72 (69.2):(0.7 × 109/L-1.499 × 109/L)37 (35.6);(≥1.5 × 109/L)35 (33.7);HIV 1 (1);Serology for HVA/HBV/HCV 18 (17.3)Antibiotics 37(35.6);CBZ 7(6.7);TCMs 14(13.5)RI 9 (8.7) ATL 26 (25);MOD 19 patients PDN:58 (58)+ IVIG:1 patient;+ Cy:1 patient;Anti-H:45(43);IVIG:1 patient 1 (< 1) had progression of his previously renal disease+Acinetobacter baumannii 103 were successfully discharged Ichai et al[16] 16 (M:5 F:11) (100) TTC ENC:7 (43.75)Admission 3(Grade I:1;III:2);After admission 4 Liver necrosis(10%-90% of parenchymal surface) 7:panlobular 1;central;3;periportal 1;spotty 2.Kupffer cell hyperplasia:6;and Erythrophago cytosis ALT 1693 IU/L (1252-2256);PCR HHV6 5/6(83);HIV+ 4(25)Allopurinol 2;CBZ 2 Antituberculosis drugs 3 RI 5 (31.2) NAC:16(100);PDNL:9 (56);LT:5(31.2)4 (25):2 MOF;1 LR 4 yr after LT;1 systemic fungal infection +HF 4 d after LT LT survival:60%;DReSS recurrence 75± 91 d after LT in 3/5 patients.LR was rule out.DReSS recurrence in 1 patient 2 months after spontaneousl y recovery
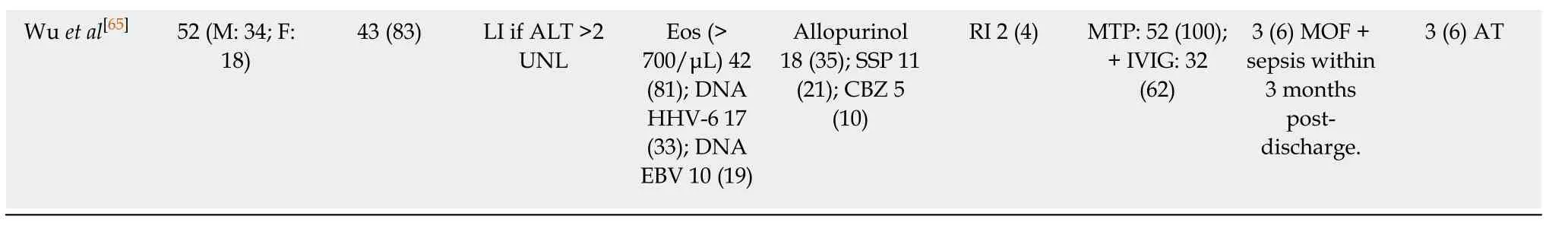
1Case series with Anticonvulsant hypersensitivity syndrome.2Authors reported that DReSS induced by phenytoin appeared sooner after the drug intake compared to carbamazepine (P = 0.01).3Allopurinol was related to cholestatic pattern.Hepatocellular-type pattern was seen in younger people while the cholestatic-type was seen in elderly (P =0.044).Patients treated with SS had more ATL and Eos than those treated with TS (P < 0.05),but no differences in liver involvement.AGEP:Acute generalized exanthematous pustulosis;Anti-H:Antihistamines;AHA:Autoimmune hemolytic anemia;AT:Autoimmune thyroid;ATL:Atypical lymphocytosis;BT:Bilirubin total;CBZ:Carbamazepine;CMV:Cytomegalovirus;CNS:Central nervous system;CSF:Cerebrospinal fluid;Cy:Cyclosporine;DIC:Disseminated intravascular coagulation;GA:Gastrointestinal;ICU:Intensive care unit;DMT:Dexamethasone;EBV:Epstein Barr virus;Eos:Eosinophilia;ENC:Encephalopathy;FH:Fulminant hepatitis;FU:Follow-up;HC:Hydrocortisone;HSV:Herpes simplex virus;HHV-6:Herpes virus type 6;HPB:Hepatobiliary disease;LN:Lymphadenopathies;LT:Liver transplant;LR:Liver rejection;MOD:Multi-organ damage;MOF:Multi-organ failure;MTP:Methylprednisolone;NA:Not-available;NAC:N-acetylcysteine;Non-AT:Non-active treatment;NSAIDs:Non-steroidal anti-inflammatory drugs;PCR:Polymerase chain reaction;PDN:Prednisone;PDNL:Prednisolone;RI:Renal injury;RF:Renal failure (ORF:Organic RF;FRF:Functional RF);SJS:Stevens-Johnson syndrome;SMX-TMP:Sulfamethoxazole-trimethoprim;SS:Systemic steroids;SSE:Sulfasalazine;SSP:Salazosulphapyridine;TEN:Toxic epidermal necrolysis;TMCs:Traditional Chinese Medicines;TRC:Tertiary Referral Center;TS:Topical steroids;TTC:Tertiary Transplant Center.
 World Journal of Clinical Cases2019年6期
World Journal of Clinical Cases2019年6期
- World Journal of Clinical Cases的其它文章
- Effects of apoptosis on liver aging
- Surgical method choice and coincidence rate of pathological diagnoses in transduodenal ampullectomy:A retrospective case series study and review of the literature
- lndividualized minimally invasive treatment for adult testicular hydrocele:A pilot study
- Successful totally transthoracic echocardiography guided transcatheter device closure of atrial septal defect in pregnant women
- Cardiac amyloidosis:A case report and review of literature
- Successful treatment with hysteroscopy for infertility due to isthmocele and hydrometra secondary to cesarean section:A case report
